Abstract
Rationale: Diverse autoantibodies are present in most patients with idiopathic pulmonary fibrosis (IPF). We hypothesized that specific autoantibodies may associate with IPF manifestations.
Objectives: To identify clinically relevant, antigen-specific immune responses in patients with IPF.
Methods: Autoantibodies were detected by immunoblots and ELISA. Intrapulmonary immune processes were evaluated by immunohistochemistry. Anti–heat shock protein 70 (HSP70) IgG was isolated from plasma by immunoaffinity. Flow cytometry was used for leukocyte functional studies.
Measurements and Main Results: HSP70 was identified as a potential IPF autoantigen in discovery assays. Anti-HSP70 IgG autoantibodies were detected by immunoblots in 3% of 60 control subjects versus 25% of a cross-sectional IPF cohort (n = 122) (P = 0.0004), one-half the patients with IPF who died (P = 0.008), and 70% of those with acute exacerbations (P = 0.0005). Anti-HSP70 autoantibodies in patients with IPF were significantly associated with HLA allele biases, greater subsequent FVC reductions (P = 0.0004), and lesser 1-year survival (40 ± 10% vs. 80 ± 5%; hazard ratio = 4.2; 95% confidence interval, 2.0–8.6; P < 0.0001). HSP70 protein, antigen–antibody complexes, and complement were prevalent in IPF lungs. HSP70 protein was an autoantigen for IPF CD4 T cells, inducing lymphocyte proliferation (P = 0.004) and IL-4 production (P = 0.01). IPF anti-HSP70 autoantibodies activated monocytes (P = 0.009) and increased monocyte IL-8 production (P = 0.049). ELISA confirmed the association between anti-HSP70 autoreactivity and IPF outcome. Anti-HSP70 autoantibodies were also found in patients with other interstitial lung diseases but were not associated with their clinical progression.
Conclusions: Patients with IPF with anti-HSP70 autoantibodies have more near-term lung function deterioration and mortality. These findings suggest antigen-specific immunoassays could provide useful clinical information in individual patients with IPF and may have implications for understanding IPF progression.
Keywords: B cells, T cells, adaptive immunity, interstitial lung disease
At a Glance Commentary
Scientific Knowledge on the Subject
The processes that cause progression of idiopathic pulmonary fibrosis (IPF) remain enigmatic. The present findings show that an antigen-specific autoimmune response is highly associated with IPF outcomes. These data suggest that immune dysregulation may be linked to IPF progression.
What This Study Adds to the Field
The course of IPF is highly variable and unpredictable in individual patients. The findings here raise possibilities that specific immunoassays may be useful to identify a subpopulation of patients with IPF with particularly poor prognoses. These data imply that novel treatment(s) specifically focused to ameliorate autoantibody-mediated injuries might be beneficial for patients with IPF.
Abnormalities of humoral immunity are common in patients with idiopathic pulmonary fibrosis (IPF) (see, e.g., References 1–13). Unusual B-cell aggregates are present in IPF lungs (1, 2), along with immunoglobulin (antibody) gene overexpressions (3). Antigen–antibody complexes are found in sera and bronchoalveolar lavage fluid of subjects with IPF (4, 5). Myriad autoantibodies are present in more than 80% of these patients (6), and some particular autoantigen-specific antibodies are associated with IPF manifestations (7–9).
Autoimmunity can develop as a later, secondary process in diverse underlying diseases and may be irrelevant, epiphenomenal, or overtly pathological (14–16). If autoantibodies that have pathogenic effects are present in a disease population, mechanistically targeted biological response modifiers could be clinically beneficial, whereas most autoantibody-mediated lung syndromes are refractory to glucocorticoids and other nonspecific agents (17–20), as is IPF (21, 22).
Given many evidences of adaptive immune abnormalities in subjects with IPF (see, e.g., References 1–13, 23–25), we hypothesized that additional, yet-to-be-discovered autoantibodies in these patients might also have clinical relevance. Accordingly, we conducted investigations to discover and characterize novel autoantigen-specific immunologic responses in patients with IPF.
Some of the results of these studies have been previously reported as an abstract (26).
Methods
See online supplement E1 for additional methodological details.
Subjects
Clinical specimens were obtained from consecutive patients with IPF, normal volunteers, and subjects with interstitial lung diseases (ILD) other than IPF (6, 24, 25).
Diagnoses were established by expert clinicians, who analyzed all information, including conventional autoimmune assays (online supplement E1). All subjects with IPF fulfilled consensus diagnostic criteria (21). ILD was diagnosed by chest computed tomography scans. All subjects gave written informed consent. This study was approved by the University of Pittsburgh Institutional Review Board.
Lung Specimens
Pulmonary explant specimen processing has been detailed elsewhere (6). IPF lungs were obtained during therapeutic transplantations. Normal lungs were procured from cadaveric donors during harvests of other organs.
Autoantigen Discovery
Methods used to identify heat shock protein 70 (HSP70) and glucose-regulated protein 78 (GRP78) as putative IPF autoantigens are detailed in online supplement E1.
Autoantibody Determinations
Plasma anti-HSP70 IgG autoantibodies were detected by immunoblots (IB) (aka Western blots), and ELISA.
HLA Alleles
HLA Class II DR and DQ alleles were determined by polymerase chain reaction amplifications of leukocyte DNA, as detailed previously (25).
T-Cell Functional Assays
Peripheral blood mononuclear cells were cultured with and without recombinant HSP70 (rHSP70) or recombinant GRP78 (rGRP78). Hilar lymph node (HLN) cells were cultured with and without autologous lung protein fractions (6). Proliferation was measured by bromodeoxyuridine incorporation or, in selected cases, by 3H-thymidine incorporation. Intracellular IFN-γ and IL-4 production was quantified by flow cytometry (6, 24).
Immunohistochemistry
Intrapulmonary HSP70 expression was detected by incubating lung sections with mouse anti-human HSP70 followed by biotinylated goat anti-mouse IgG and avidin-HRP. Methods for examining in situ IgG-antigen (immune) complexes and complement were previously detailed (27).
Isolation of Anti-HSP70 Autoantibodies from Patients with IPF
IgG in plasma from patients with IPF was isolated by protein G. Anti-HSP70 IgG antibodies were then captured on rHSP70 columns, with yield estimated at approximately 10 μg anti-HSP70/ml of patient plasma.
Monocyte Stimulation Assays
Human CD14+ cells were treated with IPF patient-derived anti-HSP70 IgG or normal human IgG and incubated for 18 hours. CD69 expression was determined by flow cytometry (6, 24). IL-8 in the culture supernatants was analyzed using protein-suspended beads (24).
Statistical Analyses
Two- and three-group comparisons of continuous variables were made by Mann-Whitney or Kruskal-Wallis tests, respectively. Concurrent assays in the same specimens were compared by Wilcoxon rank tests. Dichotomous variables were analyzed by Chi-square. Logistic regression was used to determine odds ratios (OR) and 95% confidence intervals (CI) and perform multivariate analyses. Survival analyses were performed using product-limit estimation and adjusted for lung function and demographic variables using a Cox proportional hazards model. Hazard ratios (HR) and 95% CI were established by proportional hazard regression. P values less than 0.05 were considered significant. Data are depicted as means ± SE.
Results
Partial Characterization of IPF T-Cell Antigens
Previous investigations had shown that one or more proteins within aqueous extracts of IPF lungs induced proliferations of autologous hilar lymph node CD4 T cells (6).
Subsequent efforts to identify dominant intrapulmonary IPF T cell antigen(s) included fractionations of the complex lung protein extracts, with bioassays to measure antigen enrichment within the respective fractions based on the extent to which they induced proliferation among autologous CD4 T cells (6).
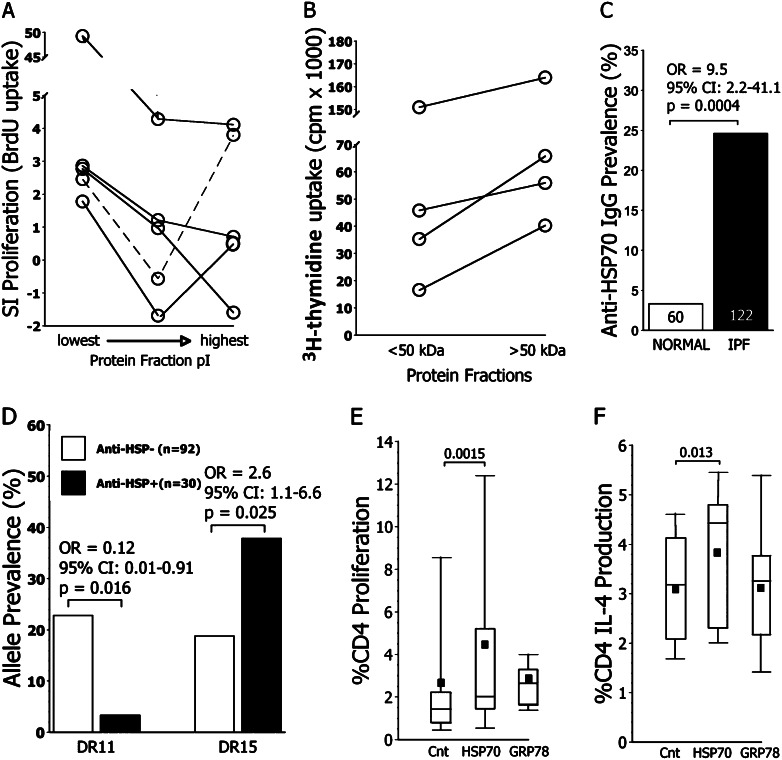
Aqueous protein lung extracts were first fractionated by ion exchange chromatography. The most acidic protein fractions (lowest isoelectric point [pI]) tended to be the most antigenic (Figure 1A). Subsequent size segregations of proteins within the lowest pI fractions indicated the greatest antigenicity in these preparations was among proteins greater than 50 kD (Figure 1B).
Figure 1.
Antigens of idiopathic pulmonary fibrosis (IPF). (A) Antigenicity of IPF lung explant extracts was greatest among the most acidic protein fractions (lowest pI). Proliferation was determined by bromodeoxyuridine (BrdU) incorporation among autologous hilar lymph node CD4 T cells (6). (B) Further fractionations of the acidic protein preparations by size filtration indicated the most immunogenetic antigens among the low pI proteins were greater than 50 kD. In these cases, proliferation was measured by 3H-thymidine because the preparations were limiting. (C) Prevalences of circulating anti–heat shock protein 70 (HSP70) IgG detected by immunoblots (IB) are depicted in healthy normal control subjects and patients with IPF. Respective subject numbers are denoted within columns. (D) HLA Class II DRβ1*11 alleles were underrepresented, whereas DRβ1*15 was overrepresented among the patients with IPF with anti-HSP70 autoantibodies. Prevalence is defined as the proportion of subjects with at least one allele copy. (E) Addition of rHSP70 to peripheral blood mononuclear cell cultures resulted in greater proliferation of CD4 T cells from patients with IPF, ascertained by BrdU incorporation, than in concurrent control cultures with no added antigens (Cnt), or cultures supplemented instead with glucose-regulated protein 78 (GRP78) (n = 24). Proliferation within GRP78-supplemented cultures did not significantly differ from those of control subjects. (F) IPF CD4 T cell IL-4 production was also more augmented by HSP70 than by the GRP78 (n = 15). The latter did not significantly differ from controls. CI = confidence interval; OR = odds ratio; SI = specific index.
Identification of Candidate Humoral Antigens
Given inherent difficulties with definitive purification of T-cell antigens from complex (but finite) tissue extracts, and the interdependence of B-cell and T-cell antigen specificities (28, 29), we hypothesized that circulating IgG antibodies isolated from subjects with IPF could affinity-purify candidate autoantigens. We further postulated a priori this approach would enable focusing on biologic validations of humoral autoantigens with physical characteristics that corresponded to the seemingly dominant CD4 T-cell antigen(s) (i.e., low pI and size > 50 kD). Additional unpublished findings in prior studies (6) had indicated the presence of autoantibodies against a cryptic approximately 50- to 80-kD antigen in patients with IPF was associated with more rapid clinical deterioration. Thus, our initial goal was to identify humoral autoantigens of subjects with IPF that were acidic proteins approximately 50 to 80 kD in size.
IgG antibodies isolated from pooled IPF plasma were used to immunoprecipitate cell lysate proteins, and these were subsequently identified by mass spectrometry. Two proteins with low pI and size approximately 50 to 80 kD were identified on replicate preparations: HSP70 and GRP78. Both are members of the HSP70 family of heat shock proteins (HSP), each has important biological functions, and they share extensive peptide sequence homology (30). Furthermore, both HSPs have been identified as autoantigens in other disorders (31–35). We also speculated a priori that concurrent study of these homologous HSP could provide useful comparative immunobiological insights.
Anti-HSP Autoantibodies Detected by IB
IB assays were performed in specimens from 122 subjects with IPF (Table 1) and 60 normal control subjects. Normal subjects had comparable ages (65 ± 1 yr) and sex distribution (67% men), and 54% were former smokers.
TABLE 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF SUBJECTS WITH IDIOPATHIC PULMONARY FIBROSIS TESTED FOR ANTI–HEAT SHOCK PROTEIN 70 IgG AUTOANTIBODIES BY IMMUNOBLOTS
| Anti-HSP70 Negative | Anti-HSP70 Positive | |
| n | 92 | 30 |
| Age, yr | 69 ± 1 | 69 ± 2 |
| Sex, % male | 74 | 67 |
| Lung biopsy, % | 54 | 50 |
| FVC % predicted | 62.7 ± 1.9 | 58.9 ± 4.1 |
| DlCO % predicted | 47.3 ± 1.9 | 45.4 ± 4.1 |
| Smoking history, % | 52 | 57 |
| Immunosuppressants, % | 21 | 10 |
Definition of abbreviations: DlCO = diffusing capacity for carbon monoxide; HSP70 = heat shock protein 70.
Lung biopsy describes the percentage of subjects in each population who had pulmonary histological assessments. In all cases these showed characteristic pathological features of IPF (21, 22). Smoking history denotes subjects with ≥5 pack-year history of cigarette smoking. Immunosuppressants denote subjects taking any single agent or various permutations of prednisone (5–20 mg/d), azathioprine, IFN-γ, mycophenolate, or tacrolimus. None of the intergroup differences were significant (the P value for the comparison of immunosuppressant use was 0.17). None of these clinical and demographic parameters were independently associated with subsequent mortality.
Nine subjects with IPF who fulfilled diagnostic criteria (21) had positive conventional serologic tests (online supplement E1) but did not exhibit clinical manifestations of autoimmune syndromes.
Anti-HSP70 IgG autoantibodies were detected by IB more frequently in IPF specimens than in normal control subjects (Figure 1C). Two of the nine subjects with IPF who had positive, albeit clinically occult, conventional autoimmune serologic tests were anti-HSP70positive. There were no differences of initial demographic or initial clinical characteristics between the anti-HSP70positive and anti-HSP70negative subjects with IPF (Table 1).
HLA Allele Biases
DRβ1*11 was underrepresented and DRβ1*15 was overrepresented among anti-HSP70positive patients (Figure 1D). Allele prevalences of DRβ1*11 and DRβ1*15 in a local normal population were previously found to be 21 and 23%, respectively (25).
IPF CD4 T-Cell Autoreactivity to HSP
Proliferation of IPF CD4 T cells was more frequently effected by rHSP70 than by rGRP78 (Figure 1E).
Profibrotic IL-4 production by CD4 T cells was previously noted to be increased and correlated with disease severity among subjects with IPF (6). CD4 T cells from patients with IPF increased their IL-4 production in rHSP70-supplemented cultures, unlike effects of rGRP78 (Figure 1F). Neither HSP augmented IFN-γ production (data not shown).
CD4 T cells from normal subjects (n = 15) were not stimulated by HSP70 (data not shown), and similar findings in other normal populations have been reported (32).
Lung Immunohistochemistry
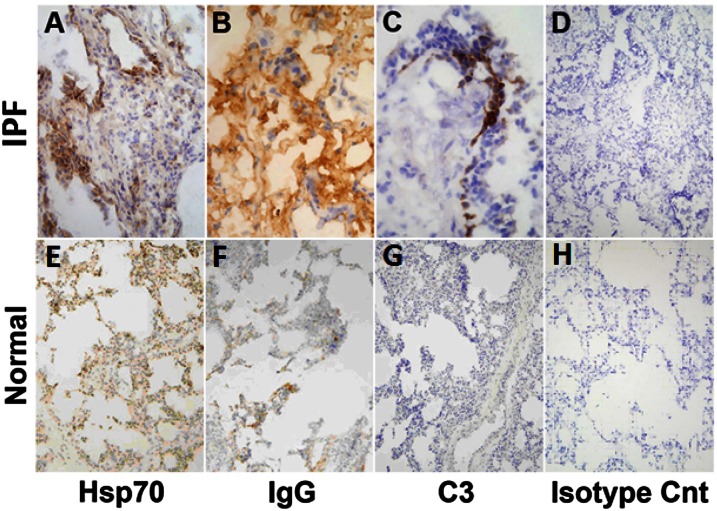
In situ HSP70 expression was present in all six IPF lung transplantation explants and more prominent than in the six normal lung sections (Figure 2). HSP70 expression was distributed in many different pulmonary cells, including airway and alveolar epithelia, macrophages, and endothelium, and appeared increased relative to normal lung specimens.
Figure 2.
Lung immunohistochemistry. Columns from left to right depict, respectively, expression of heat shock protein 70 (HSP70), IgG immune complexes (IgG), complement deposits (C3), and isotype controls. Rows, from top to bottom respectively, depict end-stage idiopathic pulmonary fibrosis (IPF) lungs explanted during therapeutic pulmonary transplantations and normal lungs harvested during multiorgan retrievals but not used in therapeutic transplantations (n = 6 each). IPF specimens were depicted at 20× to better illustrate anatomical localizations of the HSP70, IgG, and C3. Normal lung sections are shown at 10× to optimally depict the overall paucity of the expressions/depositions in these specimens.
IgG-antigen (immune) complexes were seen in four IPF lung explants and were equivocally present in another (Figure 2). Immune complexes were not evident in five of the six normal explants and were equivocally present in the other.
Complement was found in three IPF lung explants but was not evident in any normal lung, consistent with previous observations (27).
Effects of IPF Anti-HSP70 IgG on Monocytes
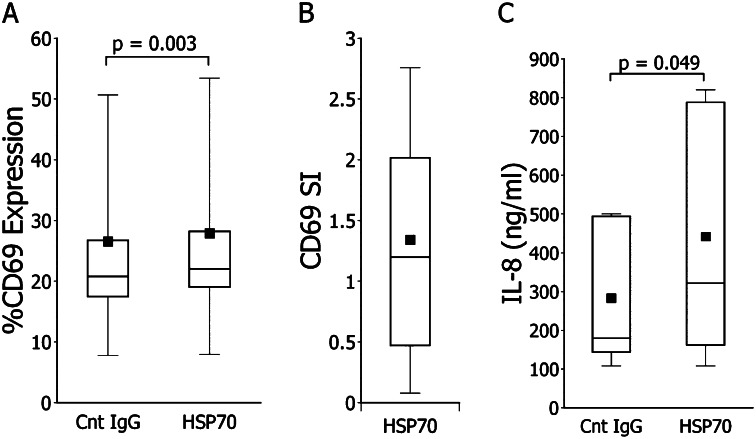
IL-8 expression is a correlate (and possible mediator) of IPF pathogenesis (36). Anti-HSP70 autoantibodies in other populations can increase monocyte productions of this mediator (33, 34). In comparison to treatments with control normal human IgG, IPF patient-derived anti-HSP70 autoantibodies also activated monocytes and increased their IL-8 production (Figure 3A–3C).
Figure 3.
Monocytes functional studies. (A) In comparison to concurrent, autologous control preparations treated with normal IgG, incubations with the idiopathic pulmonary fibrosis (IPF) autoantibody resulted in increased expression of CD69 in all but one specimen (n = 12). Aggregate mean increments induced by these single anti–heat shock protein 70 (HSP70) IgG treatments for 18 hours were 6 ± 1%. (B) Specific index (SI) (6) of CD69 expression, defined as percentages of CD69+ monocytes in IPF HSP70 IgG cultures minus that of the normal IgG cultures. (C) IPF anti-HSP70 increased IL-8 production of monocytes relative to normal IgG treatment.
Clinical Correlates of HSP Autoantibodies Detected by IB
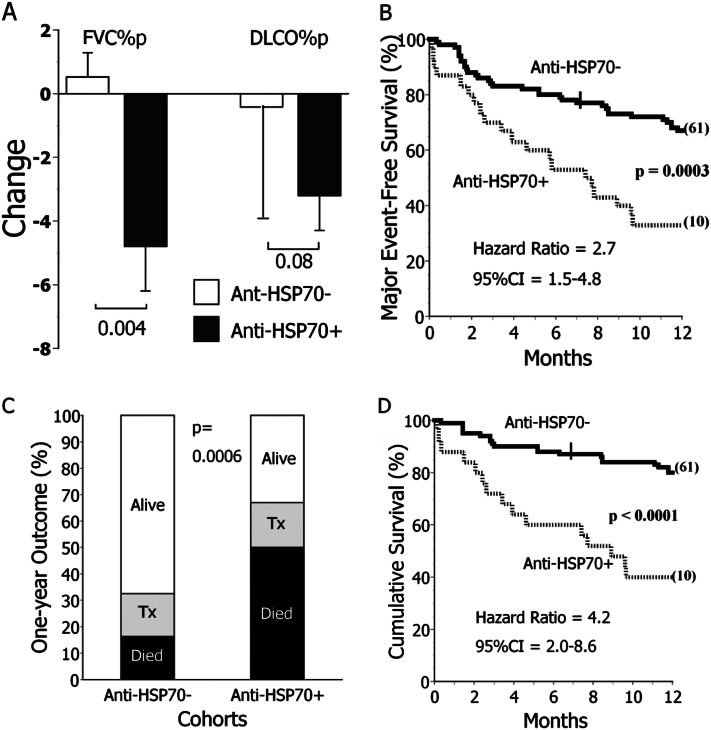
Pulmonary function tests were performed 5.5 ± 0.7 and 6.3 ± 0.3 months after specimen acquisitions from anti-HSP70positive and anti-HSP70negative subjects with IPF, respectively (P = 0.2). Anti-HSP70positive subjects had the greatest decrements of FVC (Figure 4A). Two of the three subjects with IPF who had greater than 10% decrements of FVC % predicted over this observation interval were anti-HSP70 autoantibody–positive (P = 0.03). Four of the 10 who had greater than 10% decrements of absolute FVC were similarly anti-HSP70positive (P = 0.07).
Figure 4.
Clinical correlates of heat shock protein 70 (HSP70) autoreactivity determined by immunoblot (IB). (A) Surviving subjects with idiopathic pulmonary fibrosis (IPF) with anti-HSP70 autoantibodies detected on IB testing (anti-HSP70+) (n = 14) had greater subsequent decrements of FVC, as % predicted values (FVC%p), and an insignificant tendency for greater decrements of percent predicted diffusing capacity for carbon monoxide (DlCO%p), compared with the subjects without this autoantibody (anti-HSP70−, n = 59). Pulmonary function determinations were made approximately 6 months after the plasma sample acquisitions (see text). (B) Patients with IPF with anti-HSP70 autoantibodies detected by IB (n = 30) had worse prognoses than those who were autoantibody negative (n = 92). Major (adverse) events are defined as either deaths or lung transplantations. Cross hatches denote censored events, and numbers in parentheses denote subjects censored at the end of observation. (C) The proportion of subjects with IPF who died during the next year was threefold greater among those with anti-HSP70 autoantibodies (anti-HSP70+) compared with the anti-HSP70−. Tx = transplanted. (D) Among patients with IPF who did not have lung transplantations during the observation period, those with anti-HSP70 autoantibodies (n = 25) had worse prognoses than the autoantibody-negative subpopulation (n = 77). CI = confidence interval.
One-year major adverse event–free survival of anti-HSP70positive patients with IPF was significantly decreased (Figures 4B and 4C). Respiratory failure accounted for 14 of the 15 deaths in anti-HSP70positive subjects, and nine of 15 deaths among the anti-HSP70negative. Aside from a lethal pulmonary embolus among the latter, other deaths in both subpopulations occurred without proximate clinical assessments. Findings of anti-HSP70 IgG autoantibodies by immunoblot had 40% sensitivity, 86% specificity, and 67% positive and negative predictive values for major adverse events in the subjects with IPF. Adjustment for age, sex, tobacco burden, FVC, and diffusing capacity for carbon monoxide in a Cox proportional hazards model did not significantly alter these findings (HR, 2.1; P = 0.03). Multivariate analyses also showed the demographic/clinical characteristics of Table 1 were not significantly associated with mortality of these subjects independently of the anti-HSP70 autoreactivity.
Anti-HSP70 autoantibodies were present in four of the six subjects who presented with acute IPF exacerbations, defined as increasing dyspnea and/or hypoxemia within the preceding 30 days, new radiographic infiltrates, and no other attributable cause of lung dysfunction (22) and three of four who subsequently developed acute exacerbations within 12 months (P = 0.0005). Six-month mortality of acute exacerbation patients was 100% among those with HSP70 autoantibodies versus 33% among the anti-HSP70negative (P = 0.016).
One-year major adverse event–free survival of anti-HSP70positive subjects was also diminished in post hoc analyses limited to the 116 patients who did not present with acute exacerbations (39 ± 10%) compared with 68 ± 5% for anti-HSP70negative subjects (P = 0.006). Another post hoc analysis, limited to patients with negative conventional serologic tests, similarly showed lesser major adverse event–free survival of anti-HSP70positive subjects (36 ± 9%) compared with 67 ± 5% among anti-HSP70negative (P = 0.003).
To exclude cryptic confounding by vagaries of lung transplantation selections, we also performed post hoc analysis limited to those 102 subjects with IPF who did not have these procedures. The 12-month mortality of the nontransplanted anti-HSP70positive subjects remained greater than that of the nontransplanted anti-HSP70negative cohort (Figure 4D). These findings remained significant (HR, 4.1; P = 0.002) after adjustment for age, sex, tobacco burden, FVC % predicted, and diffusing capacity for carbon monoxide % predicted.
Although autoantibodies with specificity for GRP78 were also present in several subjects with IPF, there was little concordance with anti-HSP70 autoreactivity, and there was no other evidence that anti-GRP78 responses were clinically meaningful in the IPF population (online supplement E2).
Anti-HSP70 ELISA
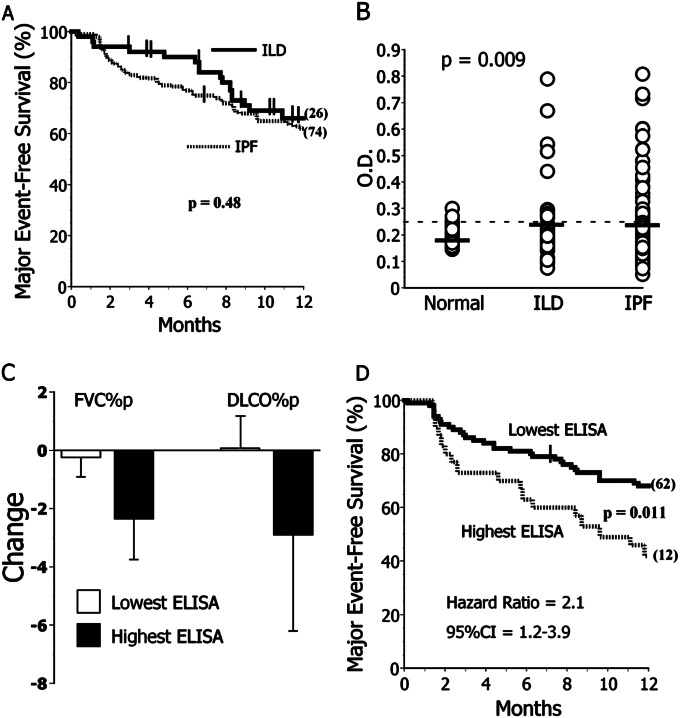
ELISA determinations were performed in the 114 remaining plasma specimens from this cohort, specimens recently acquired from seven additional subjects with IPF, as well as patients with ILD due to various etiologies (Table 2 and online supplement E3). One-year outcomes among the IPF and aggregate ILD populations were highly comparable (Figure 5A).
TABLE 2.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF SUBJECTS WITH LUNG DISEASE TESTED BY ANTI-HEAT SHOCK PROTEIN 70 ELISA
| IPF ELISA Cohort | ILD (Non-IPF) ELISA Cohort | |
| n | 121 | 51 |
| Age, yr | 69 ± 1 | 60 ± 2* |
| Sex, % male | 74 | 43* |
| Lung biopsy, % | 55 | 63 |
| FVC % predicted | 61 ± 2 | 71 ± 3* |
| DlCO % predicted | 47 ± 2 | 50 ± 3 |
| Smoking history, % | 53 | 52 |
| Immunosuppressants, % | 19 | 25 |
| Race, % African American | 0 | 8* |
Definition of abbreviations: DlCO = diffusing capacity for carbon monoxide; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis.
Lung biopsy describes the percentage of subjects in each population who had pulmonary histological assessments. In all cases these showed characteristic pathological features of IPF (21, 22). Smoking history denotes subjects with ≥5 pack-year history of cigarette smoking. Immunosuppressants denote subjects taking any single agent or various permutations of prednisone (5–20 mg/d), azathioprine, IFN-γ, mycophenolate, or tacrolimus.
P ≤ 0.002. Specific diagnoses of the subjects with ILD are detailed in online supplement E3.
Figure 5.
Heat shock protein 70 (HSP70) autoreactivity determined by ELISA. (A) Outcomes were near identical among subjects with idiopathic pulmonary fibrosis (IPF) (n = 121) and non-IPF interstitial lung disease (ILD) (n = 51). Post hoc analyses of absolute 1-year survival limited to those subjects who did not have transplantations during the observation period were also similar among these experimental cohorts (74 ± 4% vs. 78 ± 7% for IPF and ILD, respectively; P = 0.62). (B) Anti-HSP70 IgG ELISA optical density (OD) values were near identical in subjects with IPF and subjects with non-IPF ILD, and both were greater than among healthy control subjects (n = 59). The P value here is per Kruskal-Wallis (three-group) comparison. Values for both IPF and ILD were significantly greater than normal subjects in post hoc two-group analyses using Mann-Whitney tests. The dashed line denotes the mean plus SD of the values in normal subjects here. Thick horizontal lines denote the mean of each subpopulation. (C) Subjects with IPF with anti-HSP70 ELISA OD in the highest quartile (Highest ELISA) had nonsignificant trends for greater subsequent (∼ 6-mo) decrements of FVC % predicted (FVC%p) and percent predicted diffusing capacity for carbon monoxide (DlCO%p). (D) Subjects with IPF in the highest quartile of anti-HSP70 ELISA OD values (Highest ELISA) more frequently had major adverse events (deaths or lung transplantations) during the year after their specimen acquisitions. In contrast, anti-HSP70 autoantibody measures had no associations with clinical manifestations in the non-IPF ILD disease control cohort (see online supplement E3). CI = confidence interval.
ELISA optical density (OD) values in IPF and non-IPF ILD control subjects were near identical, and both were significantly greater than among normal subjects (Figure 5B). We hypothesized that the highest autoantibody concentrations in the disease populations, as approximated by semiquantitative ELISA OD values, may have the most clinical significance, and we used quartile distributions to stratify these cohorts for outcome analyses.
There were no clinical or demographic differences between subjects with IPF in the highest quartile of ELISA OD versus those with lesser values (online supplement E4). HLA DRβ*11 was underrepresented in patients with ELISA OD in the highest quartile compared with the other subjects with IPF (3 vs. 21%, P = 0.028), whereas DRβ*15 tended to be overrepresented in the former (41 vs. 25%, P = 0.097). Subjects with IPF in the highest ELISA quartile had nonsignificant trends for greater subsequent pulmonary function test deterioration (Figure 5C) and significantly worse 1-year outcomes (Figure 5D). Anti-HSP70 autoantibody ELISA OD values in the highest quartile had 38% sensitivity, 83% specificity, 55% positive predictive value, and 68% negative predictive value for major adverse events in the subjects with IPF.
In contrast to the anti-HSP70 ELISA assays in the IPF, these tests had no predictive value in the subjects with ILD (online supplement E3).
Discussion
The dysregulated immune response to HSP70 in patients with IPF has many characteristics of antigen-specific autoimmunity (28, 29). As a first principle, the extent of anti-HSP70 humoral and cellular autoreactivity in these patients is significantly greater than among healthy control subjects and is hence “abnormal” (Figures 1C, 1E, 1F, and 5B). The biologic plausibility of an autoimmune response is also conditional on finding the corresponding self-antigen within the diseased organ(s), and HSP70 is singularly abundant in IPF lungs (Figure 2). Frequency perturbations of HLA Class II alleles are another typifying feature of autoimmunity (25), and DRβ1*11 seems to confer “protection,” whereas DRβ1*15 is a risk factor for development of anti-HSP70 autoreactivity (Figure 1D). Most importantly, IPF outcomes were associated with results of two independent autoantibody assays (Figures 4 and 5D).
Autoimmune responses can develop as a consequence of many different disease processes (14–16, 28, 29). These “secondary” autoimmunities are often benign and irrelevant, having neither evident deleterious effects nor associations with disease manifestations. However, for reasons usually unknown, but which may in part be related to expressions of “permissive” HLA alleles and/or other regulatory gene polymorphisms, a small but important proportion are highly pathogenic (14–16, 28, 29). Among many examples, the developments of secondary autoimmune responses are implicated in the etiology of neurologic disorders among patients with cancer, carditis after various infections, and numerous other tissue-specific autoimmune syndromes (14–16, 37).
In addition to being associated with IPF clinical manifestations, several findings here indicate the anti-HSP70 autoreactivity may be pathogenic in these patients. IgG autoantibodies can cause cytotoxicities and promote neutrophil recruitment by the formation of antibody–antigen (immune) complexes and complement activation in target organs (27–29, 38), and both apoptosis and neutrophilia are prominent features of IPF (21, 22). The present data show pathognomonic immune complexes and complement are prevalent in IPF lungs (Figure 2).
Autoantibodies can also have function-altering effects (12, 33–35). HSP production and extracellular transport are up-regulated by diverse cellular injuries and stresses (Figure 2) (31–35, 39). The extracellular HSPs act as intercellular messengers by binding to specific cell surface receptors that transduce signals and modulate inflammatory responses (34, 39). HSP autoantibodies from other patient populations can augment productions of inflammatory mediators (33–35) by cross-linking the cell surface HSP-receptor complexes or after gaining access to intracellular autoantigens via lipid rafts (40). Data here show the anti-HSP70 autoantibodies of patients with IPF have similar proinflammatory functions (Figure 3). Furthermore, these effects were evident after only 18 hours of treatment with subphysiological autoantibody concentrations in a single, unstressed (i.e., low-level HSP expression) cell type. Insidious in situ autoimmune processes likely can exert considerable cumulative pathophysiological effects over protracted periods (28) and could probably do so too among the many other cell lineages that also express this ubiquitous autoantigen (Figure 2).
The HSP70 autoreactivity of IPF CD4 T cells also has considerable pathogenic potential (Figures 1E and 1F). T cells have exquisite antigen specificity and are inert to anatomically accessible autologous proteins in conditions of health. Findings of T-cell autoreactivity are distinctly abnormal (28, 29, 41). Antigen- (or autoantigen-) activated T cells can cause severe injuries, both directly and/or by production of mediators that activate and recruit other leukocyte, epithelial, and mesenchymal effectors, and these lymphocytes are implicated as the initiation of many disease-associated inflammatory cascades (28, 29, 41, 42). T-cell activation and profibrotic cytokine production induced by an autoantigen that is abundant in a diseased organ (i.e., HSP70, Figure 2) are unlikely benign.
If anti-HSP70 autoreactivity were the “sole cause” of the clinical abnormalities that define and distinguish IPF, autoantibodies with HSP70 specificity would be present in all (or nearly all) of these subjects and probably only in these subjects. Abnormal anti-HSP70 humoral autoreactivity is significantly overrepresented among the patients with IPF who are destined for poor outcomes (Figures 4 and 5) but was present in only approximately 25% of the initial, cross-sectional cohort. Thus, these findings cannot implicate humoral anti-HSP70 immune responses as the exclusive etiology of IPF.
Autoantibodies to GRP78 are also common in patients with IPF. However, there was no apparent IPF T-cell reactivity to this autoantigen (Figures 1E and 1F), nor were there any indications the anti-GRP78 humoral autoreactivity is associated with HLA alleles, pulmonary function deterioration, or outcome (online supplement E2). The concurrent presence of diverse, clinically relevant (e.g., anti-HSP70) and irrelevant (e.g., anti-GRP78) autoreactivities is a common feature of autoimmune diseases (14–16, 28, 29, 43). The striking discordances of the immune responses to these highly homologous HSPs lends further credence to the likelihood that the anti-HSP70 autoreactivity in subjects with IPF, in contrast to their development of anti-GRP78 autoantibodies, represents specific autoantigen-driven immunological dysregulation and is not simply due to globally increased immunoglobulin production (14–16, 28, 29).
Moreover, basic immunological processes are often shared among clinically distinct diseases, and relatively few autoantibodies are absolutely specific to a single clinical syndrome (14–16, 28, 29, 43). Despite this immunological promiscuity, however, assays that measure particular autoantibodies within a distinct patient population often do have relevance for understandings of disease mechanisms and/or provision of medical care (14–16, 43–45). Most autoantibody discovery methodologies, however, including the approach we used here, cannot inherently distinguish between the important and irrelevant immune responses. Thus, establishing the clinical relevance for any one of the many autoantibodies typically present in an autoimmune syndrome requires additional, painstaking demonstrations that concentrations or frequencies of that specific autoantibody are greater in the cohort of interest than in demographically matched healthy control subjects, it is associated with disease manifestations, and, ideally, has pathogenic effects (46).
Anti-HSP70 autoantibodies do not have clinical associations in patients with non-IPF ILD (online supplement E3). The ILD subjects here were not perfect case controls, being younger, somewhat less restricted, and composed of greater proportions of women and African Americans than the patients with IPF. The immunobiological significances of the 9-year age difference is arguable, however, and both sex and ethnicity biases here would likely increase autoantibody concentrations in the ILD, since autoimmune responses are generally more prevalent in women and African Americans (47). Furthermore, despite the disease heterogeneity among the ILD cohort, their aggregate prognoses were very similar to those of the subjects with IPF (Figure 5A). It is not evident how the intergroup demographic differences here could account for the absence of immunologic-clinical associations in the ILD. Instead, these differences seem more likely to be a reflection of dissimilar underlying (primary) pathogenic mechanism(s) and/or immunogenetics between these disease cohorts. It is also conceivable that HSP70 plays a unique and important (if still enigmatic) role in the pathogenesis of IPF, as implied by a recent finding that increased intrapulmonary expression of this HSP is a marker for poor prognoses among these patients (48).
Even if the potential pathogenicity, functional effects, and absolute uniqueness of the HSP70 autoreactivity in subjects with IPF are discounted, the evident clinical-immunological correlates in this population indicate autoantibody assays could ultimately have usefulness as biomarkers of impending progression among these patients. Various autoantibodies with prevalences among other highly specific disease populations that are similar to the IPF anti-HSP70 humoral autoreactivity (e.g., 5–40%) are widely used to predict clinical phenotypes and outcomes in those particular patients (44, 45).
However, the current anti-HSP70 IgG assays need further refinements, as well as corroborative validations, before their clinical application in patients with IPF. The imperfect sensitivity of the anti-HSP70 assays used here may reflect the disease contributions of other unmeasured deleterious autoantibodies or other even distinctly different pathological processes that are also involved in IPF progression versus technical limitations of the assays per se. Operating characteristics of these assays could likely be enhanced by use of HSP70 purified from human cell lines, delineation and use of specific HSP70 epitopes recognized by the most clinically relevant autoantibodies, as well as technical enhancements that could include luminescence-augmented readouts or multiplex (autoantigen epitope-linked) beads.
Perhaps more immediately important, the present data and other reports (7–9) show that IPF progression can be associated with antigen-specific autoimmunity. Thus, these findings may be an impetus for subsequent investigations to further define the characteristics and consequences of immune dysregulation in IPF (see, e.g., References 1–13, 23–25). The etiology of IPF, as well as the mechanism(s) by which this disease progresses, remain enigmatic despite extensive study (21, 22). Consequently, the rational selection of therapies that specifically target the causal biological process(es) has not yet been possible, and IPF remains a highly morbid disorder with a worse prognosis than many common malignancies. If humoral autoimmunity does play a pathogenic role in IPF progression, mechanistically focused therapies are available that could have greater efficacy than current nonspecific medical treatments (17–20, 49).
Acknowledgments
The authors thank Mr. Michael W. Stoner, Ms. Rebecca Mehl, and Ms. Emily K. Lindsay for technical assistance.
Footnotes
Supported in part by National Institutes of Health grants HL107172, HL084932, HL084948, HL007563, HL112711, and a generous donation from APT Pharmaceuticals.
Author Contributions: R.A.K., J.X., A.B., M.S., M.C.L., G.B., and Y.Z. performed immunoassays; E.C., L.O., and D.J.K. performed imaging studies; J.B., K.O.L., K.F.G., N.K., C.V.O., J.M.P., F.C.S., and M.D. enrolled subjects, procured tissue specimens, provided clinical data, or performed data interpretations; S.R.D. conceived and directed these studies. All authors were involved in writing and/or editing the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201203-0506OC on December 21, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Campbell DA, Poulter LW, Janossy G, du Bois RM. Immunohistological analysis of lung tissue from patients with cryptogenic fibrosing alveolitis suggesting local expression of immune hypersensitivity. Thorax 1985;40:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P. Cutting edge: non-proliferating mature immune cells form a novel type of organizing lymphoid structure in idiopathic pulmonary fibrosis. J Immunol 2006;176:5735–5739 [DOI] [PubMed] [Google Scholar]
- 3.Zuo F, Kaminski N, Eugui E, Allard J, Hakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall Aglio PP, Pesci A, Bertorelli G, Brianti E, Scarpa S. Study of immune complexes in broncholaveolar lavage fluids. Respiration 1988;54:36–41 [DOI] [PubMed] [Google Scholar]
- 5.Dobashi N, Fujita J, Murota M, Ohtsuki Y, Yamadori I, Yoshinouchi T, Ueda R, Bandoh S, Kamei T, Nishioka M, et al. Elevation of anti-cytokeratin 18 antibody and circulating cytokeratin 18: anti-cytokeratin 18 antibody immune complexes in sera of patients with idiopathic pulmonary fibrosis. Lung 2000;178:171–179 [DOI] [PubMed] [Google Scholar]
- 6.Feghali-Bostwick CA, Tsai CG, Valentine VG, Kantrow S, Stoner MW, Pilewski JM, Gadgil A, George MP, Gibson KF, Choi AM, et al. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol 2007;179:2592–2599 [DOI] [PubMed] [Google Scholar]
- 7.Ogushi F, Tani K, Endo T, Tada H, Kawano T, Asano T, Huang L, Ohmoto Y, Muraguchi M, Moriguchi H, et al. Autoantibodies to IL-1α in sera from rapidly progressive idiopathic pulmonary fibrosis. J Med Invest 2001;48:181–189 [PubMed] [Google Scholar]
- 8.Kurosu K, Takiguchi Y, Okada O, Yumoto N, Sakao S, Tada Y, Kasahara Y, Tanabe N, Tatsumi K, Weiden M, et al. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol 2008;181:756–767 [DOI] [PubMed] [Google Scholar]
- 9.Taillé C, Grootenboer-Mignot S, Boursier C, Michel L, Debray MP, Fagart J, Barrientos L, Mailleux A, Cigna N, Tubach F, et al. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:759–766 [DOI] [PubMed] [Google Scholar]
- 10.Grigolo B, Mazzetti I, Borzì RM, Hickson ID, Fabbri M, Fasano L, Meliconi R, Facchini A. Mapping of topoisomerase II alpha epitopes recognized by autoantibodies in idiopathic pulmonary fibrosis. Clin Exp Immunol 1998;114:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Fujita J, Bandho S, Ohtsuki Y, Yamadori I, Yoshinouchi T, Ishida T. Detection of antivimentin antibody in sera of patients with idiopathic pulmonary fibrosis and non-specific interstitial pneumonia. Clin Exp Immunol 2002;128:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace WHH, Howie SM. Upregulation of tenascin and TGF-β production in a type II alveolar epithelial cell line by antibody against a pulmonary auto-antigen. J Pathol 2001;195:251–256 [DOI] [PubMed] [Google Scholar]
- 13.Magro CM, Waldman WJ, Knight DA, Allen JN, Nadasdy T, Frambach GE, Ross P, Marsh CB. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum Immunol 2006;67:284–297 [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Kavanaugh A. Autoimmunity, vasculitis and autoantibodies. J Allergy Clin Immunol 2006;117:S445–S450 [DOI] [PubMed] [Google Scholar]
- 15.Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012;32:1119–1136 [PMC free article] [PubMed] [Google Scholar]
- 16.Pordeus V, Szyper-Kravitz M, Levy RA, Vaz NM, Shoenfeld Y. Infections and Autoimmunity: a panorama. Clin Rev Allergy Immunol 2008;34:283–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson SB, Kurtz SB, Donadio JV, Holley KE, Wilson CB, Pineda AA. Use of combined plasmapharesis and immunosuppression in the treatment of Goodpasture’s syndrome. Mayo Clin Proc 1979;54:714–720 [PubMed] [Google Scholar]
- 18.Sem M, Molberg O, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome: a retrospective case series. Rheumatology (Oxford) 2009;48:968–971 [DOI] [PubMed] [Google Scholar]
- 19.Martinu T, Howell DN, Palmer SM. Acute cellular rejection and humoral sensitization in lung transplant recipients. Semin Respir Crit Care Med 2010;31:179–188 [DOI] [PubMed] [Google Scholar]
- 20.Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Brezoni EA. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur Respir J 2012;40:641–648 [DOI] [PubMed] [Google Scholar]
- 21.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Lasky JA, Loyd JE, Noth I, Olman MA, et al. ; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotslanidis IE, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, Sotiriou I, Aidinis V, Margaritis D, Tsatalas C, et al. Global impairment of CD4+CD25+FoxP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:1121–1130 [DOI] [PubMed] [Google Scholar]
- 24.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS ONE 2010;5:e8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue J, Gochuico BR, Alawad AS, Feghali-Bostwick CA, Noth I, Nathan SD, Rosen GD, Rosas IO, Dacic S, Ocak I, et al. The HLA Class II allele DRB1*1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS ONE 2011;6:e14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue JM, Kahloon RA, Bhargava A, Banga G, Gochuico BR, Dacic S, Gibson KF, Kaminski N, Sciurba FC, Rosas IO, et al. Decreased survival of idiopathic pulmonary fibrosis patients with heat shock protein 70 autoreactivity [abstract]. Am J Respir Crit Care Med 2011;183:A4275 [Google Scholar]
- 27.Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, Zhang Y, Sciurba FC, Duncan SR. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun 2011;37:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Immunol 2001;7:899–905 [DOI] [PubMed] [Google Scholar]
- 30.Wisniewska M, Karlberg T, Lehtiö L, Johansson I, Kotenyova T, Moche M, Schüler H. Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70–2, HSPA6/Hsp70B, and HSPA5/BiP/GRP78. PLoS ONE 2010;5:e8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell AW, Todd A, Kinoshita G, Lynch TA, Keech CL, Gething MJ, Gordon TP. Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity. Clin Exp Immunol 2003;132:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abulafia-Lapid R, Gillis D, Yosef O, Atlan H, Cohen IR. T cells and autoantibodies to human HSP70 in Type 1 diabetes in children. J Autoimmun 2003;20:313–321 [DOI] [PubMed] [Google Scholar]
- 33.Yokota S-I, Chiba S, Furuyama H, Fujii N. Cerebrospinal fluids containing anti-HSP70 autoantibodies from multiple sclerosis patients augment HSP70-induced proinflammatory cytokine production in monocytic cells. J Neuroimmunol 2010;218:129–133 [DOI] [PubMed] [Google Scholar]
- 34.Yokota S-I, Seiji Minota S, Fujii N. Anti-HSP auto-antibodies enhance HSP-induced pro-inflammatory cytokine production in human monocytic cells via Toll-like receptors. Int Immunol 2006;18:573–580 [DOI] [PubMed] [Google Scholar]
- 35.Lu MC, Lai NS, Yu HC, Huang HB, Hsieh SC, Yu CL. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum 2010;62:1213–1223 [DOI] [PubMed] [Google Scholar]
- 36.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2012;185:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallwellis-Opara A, Dorner A, Poller WC, Noutsias M, Kuhl U, Schultheiss HP, Pauschinger M. Autoimmune features in inflammatory cardiomyopathy. Clin Res Cardiol 2007;96:469–480 [DOI] [PubMed] [Google Scholar]
- 38.Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation 2009;120:2012–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett 2007;111:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang JY, Jeong JG, Jun HR, Lee SC, Kim JS, Kim YS, Kwon MH. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol Life Sci 2009;66:1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monaco C, Andereakos E, Kiriakidis S, Feldman M, Paleolog C. T-cell-mediated signaling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr Drug Targets Inflamm Allergy 2004;3:35–42 [DOI] [PubMed] [Google Scholar]
- 42.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol. 2004;4:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon DH, Kavanaugh AJ, Schur PH. American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum 2002;47:434–444 [DOI] [PubMed] [Google Scholar]
- 44.Ho KT, Reveille JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther 2003;5:80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards TJ, Eggebeen A, Gibson K, Yousem S, Fuhrman C, Gochuico BR, Fertig N, Oddis CV, Kaminski N, Rosas IO, et al. Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum 2009;60:2183–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan SR. Perspective: Clues, not conclusions. Nature 2012;489:S15. [DOI] [PubMed] [Google Scholar]
- 47.Reveille JD. Ethnicity and race and systemic sclerosis: how it affects susceptibility, severity, antibody genetics, and clinical manifestations. Curr Rheumatol Rep 2003;5:160–167 [DOI] [PubMed] [Google Scholar]
- 48.Boon K, Bailey NW, Yang J, Steel MP, Groshong S, Kervitsky D, Brown KK, Schwarz MI, Schwartz DA. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF). PLoS ONE 2009;4:e5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 2009;119:1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]