The GEF Tiam1 acts as a novel molecular link to the VE-cadherin–p67phox–Par3 polarity complex, leading to localized activation of Rac1 and NADPH oxidase in response to fluid flow.
Abstract
Hemodynamic forces regulate embryonic organ development, hematopoiesis, vascular remodeling, and atherogenesis. The mechanosensory stimulus of blood flow initiates a complex network of intracellular pathways, including activation of Rac1 GTPase, establishment of endothelial cell (EC) polarity, and redox signaling. The activity of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase can be modulated by the GTP/GDP state of Rac1; however, the molecular mechanisms of Rac1 activation by flow are poorly understood. Here, we identify a novel polarity complex that directs localized Rac1 activation required for downstream reactive oxygen species (ROS) production. Vav2 is required for Rac1 GTP loading, whereas, surprisingly, Tiam1 functions as an adaptor in a VE-cadherin–p67phox–Par3 polarity complex that directs localized activation of Rac1. Furthermore, loss of Tiam1 led to the disruption of redox signaling both in vitro and in vivo. Our results describe a novel molecular cascade that regulates redox signaling by the coordinated regulation of Rac1 and by linking components of the polarity complex to the NADPH oxidase.
Introduction
Shear stress, the frictional force produced by blood flow, is a critical regulator of cardiovascular development and function. Endothelial cells lining blood vessels are equipped with numerous mechanoreceptors that function to convert mechanical force into signaling cascades that regulate diverse EC responses, such as oxidative balance, gene expression, and alignment of cytoskeletal filaments (Hahn and Schwartz, 2009). The Rho family of small GTPases are master regulators of many cellular activities (Etienne-Manneville and Hall, 2002), and are critical for the shear stress response (Tzima, 2006). The family of small GTPases cycle between inactive GDP-bound and active GTP-bound states to regulate numerous EC responses to shear stress. In particular, Rac1 signaling regulates EC alignment and polarization (Tzima et al., 2002; Wojciak-Stothard and Ridley, 2003), NF-κB–dependent gene expression (Tzima et al., 2002), and reactive oxygen species (ROS) production (Yeh et al., 1999) in response to flow. However, the mechanisms by which hemodynamic forces activate Rac1 and orchestrate such diverse cellular responses remain unknown.
Work over the past few years has identified a mechanosensory complex at cell junctions consisting of PECAM-1, VE-cadherin, and VEGFR2, that is required for the activation of a number of shear-dependent signaling pathways. Interestingly, ECs lacking PECAM-1 or VE-cadherin exhibit impaired NF-κB activation and alignment in response to shear stress (Tzima et al., 2005). As NF-κB activation and EC alignment are both Rac1-dependent processes, we hypothesized that PECAM-1 and VE-cadherin may play a role in the regulation of flow-induced Rac1 activity.
Results
PECAM-1 is required for flow-induced Rac1 activation, whereas VE-cadherin is essential for polarization of active Rac1
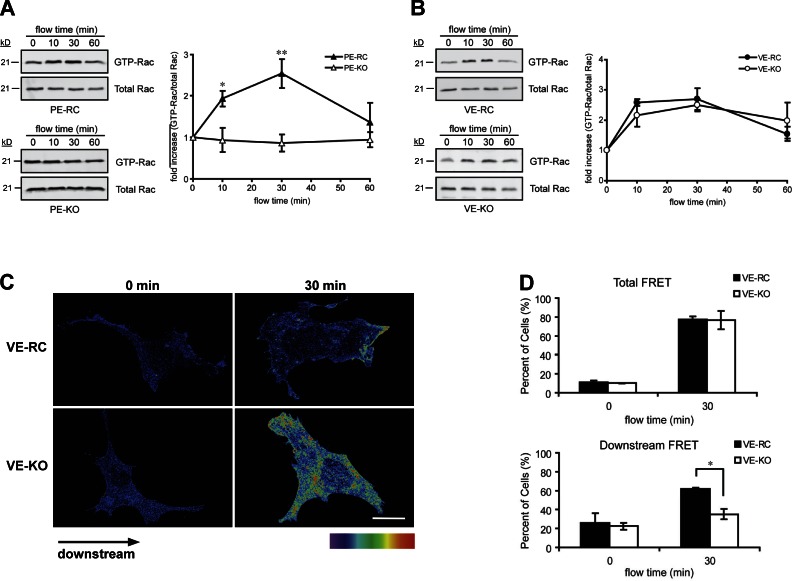
To gain insights into the pathway that regulates flow-induced Rac1 activation, we first tested the role of the junctional components of the mechanosensory complex. As flow-induced cell alignment and NF-κB activation are downstream of PECAM-1 and VE-cadherin (Tzima et al., 2005), and mediated by Rac1 (Tzima et al., 2002), we hypothesized that activation of Rac1 is dependent on these adhesion receptors. Pull-down assays were performed in which Rac1 GTP loading was determined by specific binding of the active GTPase to the p21-binding domain of PAK1 (PBD). PECAM-1−/− ECs (PE-KO) failed to show activation of Rac after onset of flow, whereas null cells engineered to reexpress PECAM-1 (PE-RC) showed an increase in Rac1 GTP loading in response to flow (Fig. 1 A), similar to that seen in wild-type ECs (Tzima et al., 2002; Wojciak-Stothard and Ridley, 2003). Surprisingly, despite defects in responses downstream of Rac (Tzima et al., 2005), VE-cadherin−/− ECs (VE-KO) showed normal levels of Rac1 activation, similar to those seen in VE-cadherin–expressing ECs (VE-RC; Fig. 1 B), suggesting that VE-cadherin is not required for flow-induced GTP loading of Rac1.
Figure 1.
PECAM-1 is required for flow-induced Rac1 activation, whereas VE-cadherin is essential for polarization of active Rac1. (A and B) Rac activation was assessed by Rac1-GTP pull-down assays in PE-RC vs. PE-KO (A) and VE-RC vs. VE-KO (B) ECs. The GTP-Rac1/total Rac1 ratio was quantified using ImageJ (National Institutes of Health). (C and D) ECs plated on fibronectin were transiently transfected with EGFP-Rac and mCherry-PBD. 24 h after transfection, cells were subjected to shear stress or kept as static control. FRET images were obtained using MetaMorph software (MDS Analytical Technologies) to show spatial Rac1 activation. Cells were first scored for total FRET signal. Cells showing a positive FRET signal were then scored for FRET directionality. Values are mean ± SEM, n = 3 and >100 cells were scored per condition. *, P < 0.05; **, P < 0.01. Bar, 10 µm.
Rho GTPase activation is highly spatially and temporally regulated to permit localized signaling responses. In particular, shear stress induces localized activation of Rac1, and Rac1 activity has to be spatially restricted in order for cells to align in the direction of flow (Tzima et al., 2002). To assess whether VE-cadherin is important for localized activation of Rac1 in response to flow, we used FLAIR (fluorescence activation indicator for Rho proteins; Kraynov et al., 2000). Fluorescence resonance energy transfer (FRET) images showed high Rac1 activity in both VE-RC and VE-KO ECs subjected to shear stress (Fig. 1 C). Quantitation revealed a substantial increase in the fraction of both VE-KO and VE-RC cells that scored positive for FRET (Fig. 1 D). However, when cells were scored for spatial distribution of Rac1-FRET (Fig. S1 A), we found that in the majority of VE-RC cells, Rac1 activity was strongly localized to the downstream edge, whereas Rac1 activity was randomly oriented in VE-KO ECs (Fig. 1 D). It is important to note that processing of our samples did not alter FRET observed in live ECs (Fig. S1 B). Taken together, these data demonstrate that PECAM-1 is required for global changes in Rac activation (GTP loading), whereas VE-cadherin is essential for providing the spatial information needed to polarize Rac1 activity in response to flow.
Vav2 mediates flow-induced Rac1 activation, whereas Tiam1 regulates spatial organization of activated Rac1
The activation of Rho proteins is mediated by specific guanine nucleotide exchange factors (GEFs) that catalyze the exchange of GDP for GTP (Hall, 2005). The mechanisms by which cells coordinate distinct sets of GEFs, GTPases, and effectors in response to a diverse array of biological stimuli remains an unanswered fundamental question (García-Mata and Burridge, 2007). We therefore sought to identify the specific GEFs that are involved in Rac1 activation in response to shear stress. We considered Vav2 and Tiam1 as candidate GEFs and examined their contribution to both GTP-loading and spatial localization of activated Rac. Tiam1 helps restrict the activity of Rac to dendritic spines via its interaction with the PDZ protein Par3 (Nishimura et al., 2005; Zhang and Macara, 2006), and Tiam1 scaffolding is also important for coupling upstream signals to downstream signaling events (Rajagopal et al., 2010). Vav2 mediates Rac activation in ECs in response to VEGF (Gavard and Gutkind, 2006) and is activated downstream of multiple receptor tyrosine kinases (Schiller, 2006). We therefore interrogated whether shear stress can also activate Vav2. Phosphorylation of Tyr172 revealed that shear stress induced rapid activation of Vav2 (Fig. 2 A). Interestingly, Vav2 activation was dependent on PECAM-1, as well as Src tyrosine kinase activity (Fig. 2 B; Fig. S2 C), suggesting that PECAM-1–mediated mechanotransduction and downstream Src activation (Tzima et. al, 2005) are required for shear stress–induced Vav2 activation. To study the role of Vav2 in shear stress–induced Rac1 activation, we used siRNAs against Vav2 to decrease its expression in ECs. Two siRNA sequences were used to rule out the possibility of the off-target effects (Fig. S2 A). As shown in Fig. 2 C, shear stress–induced Rac1 GTP loading was compromised in Vav2-depleted ECs, whereas nonspecific siRNA had no effect, suggesting that Vav2 mediates flow-induced GTP loading of Rac. Thus, Vav2 facilitates GTP loading of Rac1 in response to shear stress.
Figure 2.
Vav2 is required for flow-induced Rac1 GTP loading, whereas Tiam1 regulates spatial activation of Rac1. (A) VE-RC or VE-KO ECs were plated on fibronectin-coated slides and shear stress applied for the indicated times. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with phospho- and total Vav2 antibodies. Three experiments were performed and numbers under the gel panels represent the ratio of pVav2/total Vav2 normalized to t = 0. (B) PE-RC or PE-KO ECs were plated on fibronectin-coated slides and shear stress applied for the indicated times. Total Vav2 protein was immunoprecipitated from cell lysates and subjected to SDS-PAGE, followed by immunoblotting with phospho- and total Vav2 antibodies. Three experiments were performed and numbers under the gel panels represent the ratio of pVav2/total Vav2 normalized to t = 0 (top). Shear stress was applied to HUVECs on fibronectin-coated slides and shear stress was applied in the presence of DMSO or the Src family kinase inihibitor, SU6656. Total Vav2 was immunoprecipitated from cell lysates and immune complexes were subjected to SDS-PAGE. Western blots were performed to assess phospho-Vav2 levels (bottom). (C) ECs were transfected with control siRNA, siVav2#1 or siTiam1#1. 72 h after transfections, cells were plated on fibronectin-coated slides and shear stress applied for the indicated times. Rac1-GTP pull-down assays were performed to assess the Rac1-GTP loading. (D) HUVECs were transfected with indicated siRNAs and subsequently sheared or kept as static control. FRET images were obtained and cells scored for total FRET and downstream FRET. Values are mean ± SEM, n = 3 and >100 cells were scored per condition. *, P < 0.05; **, P < 0.01. Bar, 10 µm.
In contrast to Vav2, silencing Tiam1 with siRNAs (Fig. S2 B) did not affect the overall GTP loading of Rac1 by flow (Fig. 2 C; Fig. S2 D), suggesting that Tiam1 is not the GEF responsible for flow-induced Rac1 activity. To assess whether Tiam1 is important for localized activation of Rac1 in response to flow, we used the FRET-based Rac1 biosensor. As observed with the affinity-based pull-down assay, when we compared the average overall Rac1 activity levels using the average Rac1 FRET values in each cell, we could not detect a difference between the Tiam1-depleted cells and control siRNA-transfected cells. However, when scored for spatial distribution, Rac activity was randomly oriented in Tiam1-depleted cells, whereas control siRNA-transfected cells exhibited an obvious directionality of activated Rac1 relative to the flow direction (Fig. 2 D). Overall, these data demonstrate distinct yet synergistic roles for two Rac GEFs in shear stress signaling: Vav2 is required for GTP-loading of Rac1, and thus functions as a canonical GEF, whereas Tiam1 is important for localized activation of Rac1.
Spatial activation of Rac1 is mediated by VE-cadherin and is required for ROS production
Rac1 is a component of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Abo et al., 1991; Knaus et al., 1991) and is widely known to act upstream of ROS production in a variety of cell types, including ECs in response to shear stress (Chiu et al., 1997; Yeh et al., 1999). Interestingly, ROS production is temporally and spatially regulated to facilitate compartmentalization of redox signaling (Ushio-Fukai, 2009). As exposure of ECs to shear stress elicits directional cellular behaviors, including cell alignment and directional Rac activation, we hypothesized that ROS production may require both global and directional Rac1 activation in response to flow. To further test the idea that polarized Rac activity may be important for ROS production, we examined ECs transfected with a constitutively active Rac variant, V12Rac, which induces uniform high levels of Rac activity without detectable polarization toward the downstream edge (Tzima et al., 2002). Expression of V12Rac increased basal ROS production, consistent with previous reports (Sundaresan et al., 1996; Joneson and Bar-Sagi, 1998; Cheng et al., 2006). However, when generation of ROS was measured after shear stress, cells transfected with V12Rac showed a much smaller increase in ROS production compared with ECs transfected with wild-type Rac (Fig. 3 A). Thus, despite the presence of a constitutively active form of Rac1, loss of directionality of active Rac1 impairs flow-induced ROS production.
Figure 3.
Proper spatial activation of Rac1 is required for flow-induced ROS production. (A) ECs were transiently transfected with wild-type Rac (RacWT) or constitutively active Rac (RacV12) constructs. 24 h after transfection, ECs were loaded with 2,7-dichlorodihydrofluorescein diacetate (H2-DCFDA) at 37°C for 30 min before the onset of flow shear stress was applied to the cells and ROS production was assessed by measuring the fluorescence of the cell lysates at excitation 485 nm/emission 535 nm. (B) VE-RC or VE-KO cells grown to confluence were loaded with H2-DCFDAat 37°C for 30 min before the onset of flow. Shear stress was subsequently applied in continued presence of the H2-DCFDA dye for the indicated times. ROS production was assessed by measuring the fluorescence of the cell lysates at excitation 485 nm/emission 535 nm.
Our data suggest that although bulk Rac1 activation is normal, directional Rac activation is disrupted in the absence of VE-cadherin. As it appears that VE-cadherin can influence Rac1 localization, we next considered whether VE-cadherin regulates flow-induced ROS production downstream of Rac1. As shown in Fig. 3 B, onset of shear stress readily induced ROS production in VE-cadherin–expressing ECs (VE-RC). In contrast, markedly decreased ROS production was detected in VE-cadherin−/− cells (VE-KO). These data demonstrate that VE-cadherin is required for shear stress–induced ROS production, and suggest that flow-induced redox signaling requires both global and spatially regulated activation of Rac1.
Tiam1 is required for flow-induced ROS production in vitro and in vivo
Our results indicate that polarized Rac1 activation is required for flow-induced ROS production. In light of our data suggesting that Tiam1 is required for polarized Rac1 activation, we hypothesized that Tiam1 is also essential for flow-induced ROS production. Consistent with this hypothesis, ROS production was dramatically attenuated in Tiam1-depleted ECs (Fig. 4 A). Furthermore, our data indicate that Tiam1 is not required for GTP loading of Rac1, suggesting the hypothesis that Tiam1 GEF activity is not required for ROS production. We next tested the requirement for Tiam1 GEF activity in flow-induced ROS production. Rescue experiments were performed using siRNA-resistant wild-type Tiam1 and a GEF-deficient variant, 1047/1232ATiam1 (Fig. 4 B; Worthylake et al., 2000). As shown in Fig. 4 B, the wild type and a GEF-deficient Tiam1 mutant rescued flow-induced ROS production to a similar extent, suggesting that Tiam1 controls Rac1 activation and downstream redox signaling independent of its GEF activity.
Figure 4.
Tiam1 is required for flow-induced ROS production in vitro and in vivo. (A) ECs were transfected with control or Tiam1-specific siRNAs. 72 h after transfection, cells were incubated with the H2-DCFDA dye and sheared for indicated times. Shear stress was subsequently applied in continued presence of the H2-DCFDA dye for the indicated times. ROS production was assessed by measuring the fluorescence of the cell lysates at excitation 485 nm/emission 535 nm. (B) Human ECs were transfected with indicated combinations of siRNAs and rescue plasmids. 48 h after transfections, cells were lysed and lysates resolved on SDS-PAGE and analyzed by Western blotting using Tiam1-specific and actin-specific antibodies. 48 h after the transfections, cells were loaded with the H2-DCFDA dye, sheared for 30 min, or kept as static control and ROS production measured as described above. (C) Control or mouse Tiam1-specific siRNAs were introduced into C57BL/6 mice (>30 wk) through retro-orbital injection for two consecutive days. 72 h after the initial injection, the aortas were isolated and processed for en face staining. ROS production was visualized by incubating the tissue sections with H2-DCFDA dye at 37°C for 1 h. Images of aorta tissues were obtained immediately after processing the sections. Values are mean ± SEM, n = 4; *, P < 0.05; **, P < 0.01.
Our data suggest that Tiam1 controls ROS production by its ability to regulate spatial activation of Rac1. Similarly, in contrast to the in vivo situation (in which the levels of active GTPases are tightly regulated and occur in highly defined subcellular regions), when a Rho GTPase is globally activated, its effectors might aberrantly signal outside of the spatio-temporal context of their specific signaling modules (Pertz, 2010). To further evaluate the relevance of this pathway, we assessed the role of Tiam1 in ROS production in vivo. We developed a fluorescence-based assay to specifically monitor ROS production in the mouse aorta and verified that the measured fluorescence is solely due to ROS production, as it was absent in aortas treated with the ROS inhibitor diphenyleneiodonium (DPI; Fig. S3). As shown in Fig. 4 C and Fig. S4, in vivo knockdown of Tiam1 led to a marked decrease in ROS production. In this in vivo setting, we cannot distinguish between the role of Tiam1 in ECs and the contribution of other vascular cell types to ROS production. However, our in vitro data document that Tiam1 is required for the shear stress–mediated ROS production in ECs, thereby establishing that Tiam1 is an important mediator of the effect of shear stress in redox signaling.
Tiam1, Nox2, and Par3 polarize in response to shear stress
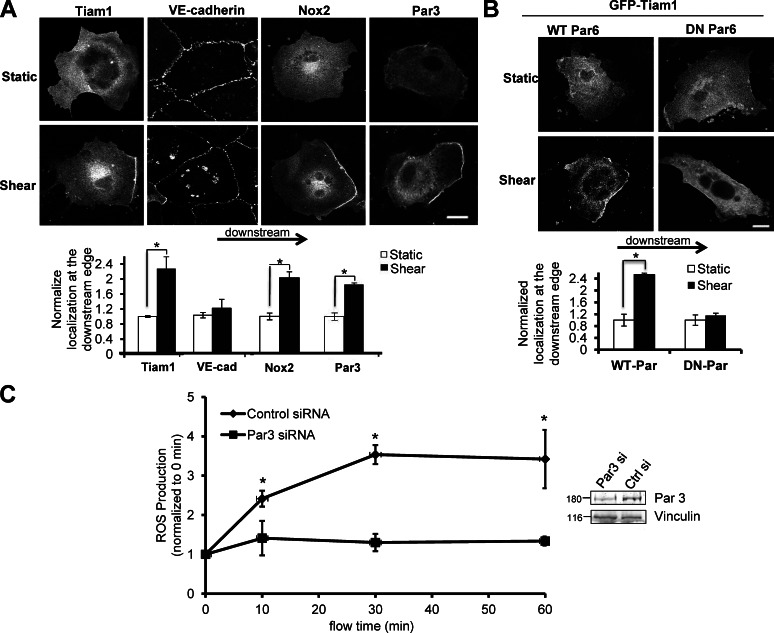
VE-cadherin and Tiam1 are clearly crucial for proper localization of active Rac1, which is instrumental for the subsequent activation of biological signaling events. Next, we considered whether the subcellular localization of Tiam1 itself may establish localized Rac1 activation. The onset of shear stress induced polarization Tiam1 to the downstream edge of the cell (Fig. 5 A), similar to the pattern of Rac1 activation induced by flow (Fig. 1 C). Interestingly, we did not observe polarization of VE-cadherin in response to shear stress (Fig. 5 A), suggesting that VE-cadherin itself does not redistribute under flow, but rather provides a platform for the recruitment of other proteins, such as Tiam1 and active Rac1 to the downstream edge of the cell. In light of our evidence suggesting that polarized Rac1 activation is required for flow-induced ROS production, we next tested if specific components of the NADPH oxidase also display redistribution in response to shear stress. The Nox2 component of the NADPH is highly expressed in ECs and is a major source of EC ROS production (Brown and Griendling, 2009). In agreement with our hypothesis, Nox2 was highly polarized to the downstream edge of the cell in ECs under flow (Fig. 5 A).
Figure 5.
Tiam1, Nox2, and Par3 polarize in response to shear stress. (A) ECs were transfected with myc-tagged Par3, GFP-Tiam1, or myc-Nox2. 48 h after transfection, shear stress was applied or cells were left as static controls. Cells were immediately fixed and immunostained with anti–VE-cadherin or anti-myc antibodies. Total fluorescence intensity was measured at the upstream and downstream regions of the cell. The ratio of downstream fluorescence to upstream fluorescence for each cell was calculated, and sheared values were normalized to static controls. n = 3 and >60 cells per condition were analyzed. Bar, 10 µm. (B) BAECs were cotransfected with WT-Par6 or DN-Par6 and wild-type GFP-Tiam1. 48 h after transfection, shear stress was applied or cells were left as static controls. Total fluorescence intensity was measured at the upstream and downstream regions of the cell. The ratio of downstream fluorescence to upstream fluorescence for each cell was calculated, and sheared values were normalized to static controls. n = 3 and >15 cells per condition were analyzed. Bar, 10 µm. (C) ECs were transfected with control or Par3-specific siRNAs. 72 h after transfection, cells were incubated with the H2-DCFDA dye and sheared for indicated times. Shear stress was subsequently applied in continued presence of the H2-DCFDA dye for the indicated times. ROS production was assessed by measuring the fluorescence of the cell lysates at excitation 485 nm/emission 535 nm. Bar graph is representative of three independent experiments. For all panels, values are mean ± SEM; *, P < 0.05.
A possible candidate for providing spatial cues for polarization of proteins, such as Tiam1, in response to shear stress is the polarity complex consisting of the partitioning-defective (Par) proteins (Par3 and Par6), which is necessary for establishment of polarity in various cells types and organisms (Macara, 2004). In this complex, Par3 acts as a scaffolding protein which, through direct interactions assembles Par6, aPKC, and Tiam1 in close proximity (Chen and Macara, 2005; Mertens et al., 2005; Nishimura et al., 2005). Importantly, the Par3/6 polarity complex is also required for polarization of the microtubule-organizing center in ECs in response to flow (Tzima et al., 2003). Indeed, we observed flow-induced polarization of Par3 to the downstream edge of the cell (Fig. 5 A); additionally, expression of a dominant-negative Par6 (DN Par6) abolished shear-induced Tiam1 polarization (Fig. 5 B), whereas Tiam1 polarization in ECs expressing wild-type Par6 (WT Par6) was unaffected. These data suggest that the flow-induced polarization of Par3/6 provides a spatial cue for the recruitment of other proteins (such as Tiam1) to the downstream edge of the cell that direct proper localization of activated Rac1 and subsequent redox signaling. Next, we hypothesized that depletion of Par3 would eliminate the polarity cue for the recruitment of other proteins, and thus disrupt flow-induced ROS production. In agreement with this hypothesis, ECs depleted of Par3 using specific siRNAs displayed blunted ROS production compared with ECs transfected with a control siRNA (Fig. 5 C).
A novel signaling complex that couples spatial Rac1 activation to NADPH oxidase
Par3/6 have been shown to complex with Tiam1 to provide polarity cues in numerous cell types and organisms (Chen and Macara, 2005; Mertens et al., 2005; Nishimura et al., 2005). Therefore, we investigated if these proteins also interact with each other to provide polarity cues in response to shear stress. Onset of flow induced the formation of a Par3–Tiam1 complex, which, unexpectedly, also contained VE-cadherin (Fig. 6 A). In contrast, flow did not promote increased association of Par3 with Tiam1 in the absence of VE-cadherin (Fig. 6 A). Surprisingly, shear stress also induced the association of Tiam1 and VE-cadherin with the p67phox subunit of NADPH oxidase (Fig. 6, B and C). Notably, the level of Tiam1 coimmunoprecipitation with p67phox was not increased by shear stress stimulation in the absence of VE-cadherin (Fig. 6 B), suggesting that VE-cadherin mediates ROS production by regulating Tiam1 association with p67phox. We next addressed the possibility of a direct interaction between Tiam1 and p67phox. In GST pull-down assays using recombinant proteins (Fig. 6 C), direct binding was observed between p67phox and the PHn-PHc Tiam1 variant, which encompasses the majority of the protein (Fig. 6 D). Moreover, the PHn-CC-Ex domain also associated with p67phox directly (Fig. 6 D), suggesting this region is primarily responsible for the direct interaction. Interestingly, the PDZ domain of Tiam1 did not bind to p67phox. Additionally, we performed NMR-based titration experiments where a VE-cadherin peptide comprising the last 16 amino acids was titrated into a sample containing 15N-labeled Tiam1 PDZ domain. Addition of the VE-cadherin peptide caused chemical shift perturbations in residues that are known to be important for ligand binding (Fig. S5; Shepherd et al., 2010; Shepherd and Fuentes, 2011), indicating a direct, but weak interaction. Importantly, a similar approach was used to show an interaction of the VE-cadherin C-terminal tail with the PDZ domain of Par3 (Tyler et al., 2010). Overall, these data show that Tiam1 binds to p67phox via its PHn-CC-Ex domain and that VE-cadherin binds the Tiam1 PDZ domain. Previous studies have shown that Par3 binds to the PHn-CC-Ex domain of Tiam1 through its CC domain and the C terminus of VE-cadherin through its third PDZ domain, both of which are direct (Nishimura et al., 2005; Iden et al., 2006; Tyler et al., 2010). These data suggest a direct association between components of the polarity complex and the NADPH oxidase. Some of these interactions are likely stabilized by the simultaneous interactions between multiple components of the VE-cadherin–Tiam1–p67 ternary complex.
Figure 6.
A novel signaling complex that couples spatial Rac1 activation to the NADPH oxidase. (A) VE-RC or VE-KO ECs were transiently transfected with HA-tagged Tiam1 expression construct. 24 h after transfection, cells were sheared for indicated times or kept as static control. Cell lysates were immunoprecipitated with an anti-HA antibody followed by immunoblotting with anti-Par3, anti–VE-cadherin, or anti-HA antibodies. Three experiments were performed for each cell type and numbers below the gel panels represent the fold of band intensity relative to t = 0 min. (B) VE-RC or VE-KO ECs were transiently transfected with HA-tagged Tiam1 and Flag-tagged p67phox. 24 h after transfection, cells were sheared for indicated times or kept as static control and cell lysates immunoprecipitated with anti-Flag M2 agarose. The immune complexes were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Flag antibodies. Three experiments were performed for each cell type and numbers below the gel panels represent the fold of band intensity relative to t = 0. (C) Tiam1 variants used in the in vitro direct binding assays. (D) Equal amounts of GST or GST-p67 fusions were incubated with indicated His-tagged Tiam1 variants in vitro. Bound proteins were detected by immunoblotting with an anti-6xHis monoclonal antibody. The white line indicates where the Western blot was cut to remove irrelevant lanes. (E) Shear stress regulates Rac1 activation through two distinct GEFs, Vav2 and Tiam1. Vav2 controls the global Rac1 GTP loading while Tiam1 links Rac1 to the flow-dependent polarity complex composed of Par3 and VE-cadherin. Tiam1 also couples the polarity complex to the NADPH oxidase for ROS production in a flow-dependent manner.
Discussion
In this study we identify a molecular mechanism by which shear stress activates Rac1 and regulates downstream ROS production. We reveal differential roles for two components of the mechanosensory complex in this pathway: PECAM-1 is required for GTP loading of Rac; in contrast, VE-cadherin is not required for GTP loading, but is essential for polarized activation of Rac1 in response to shear stress. This polarized activation of Rac1 is achieved via the formation of a VE-cadherin–Par3–Tiam1 complex, which allows its coupling to the p67phox subunit of the NADPH oxidase and downstream redox signaling. In support of this model, ECs depleted of VE-cadherin, Tiam1, or Par3 show impaired ROS production in response to shear stress. Our finding that knockdown of Tiam1 in the mouse aorta also reduces ROS production clearly points to the functional relevance of this pathway in vivo.
The mechanisms that determine polarity, including that of Rac1, in response to shear stress is a longstanding question in the field and is an intense area of study. Recent work modeling polarized Rac1 activation in ECs predicts that an endothelial cell in a confluent monolayer would experience compression at the upstream edge of the cell and tension at the downstream edge when exposed to flow (Allen et al., 2011). Therefore, mechanosensitive signaling, such as Rac activation, would occur at the downstream edge of the cell under mechanical stress. Other studies support the idea of a transfer of stress to the downstream edge of the cell, as rapid and directional rearrangement of intermediate filaments upon exposure to shear stress has been previously shown (Helmke et al., 2000). Furthermore, it has been shown that Rac activity is decreased at the upstream edge ECs under flow via a paxillin–p130Cas pathway. However, these experiments were performed in ECs subjected to a scratch wound (to reveal an upstream lamellipodium) and not in confluent monolayers (Zaidel-Bar et al., 2005). Our work identifies the molecular players required for polarized Rac1 activation in response to fluid shear stress. We further demonstrate that polarization of Rac1 signaling is a biologically meaningful event, as disruption of Rac polarity impairs flow-induced ROS production.
The establishment and maintenance of cell polarity is crucial for many biological functions and is regulated by conserved protein complexes. Interactions between Par complex proteins and Rho GTPases are essential for the proper regulation of cell and cytoskeletal polarity in a wide variety of polarized cell types. For example, Par3 functions to restrict Rac-GTP formation to dendritic spines by binding to Tiam1 (Nishimura et al., 2005; Zhang and Macara, 2006). Similarly, the Par3/6 polarity complex, in conjunction with Tiam1-mediated Rac1 signaling, controls apical–basal cell polarity in contacting epithelial cells (Mertens et al., 2005). A recent study reported that Tiam1 interactions with different scaffolding proteins couple distinct upstream signals to localized Rac1 activation and specific downstream pathways (Rajagopal et al., 2010). Our results now identify a novel role for Tiam1 as an adaptor in a VE-cadherin–p67phox–Par3 polarity complex that provides the spatial cues required for ROS production. We show that shear stress induces polarization of Par3/6, which mediates the polarization of Tiam1; Tiam1 binds to p67phox via its PHn-CC-Ex domain and to VE-cadherin via its PDZ domain. Surprisingly, VE-cadherin does not polarize under shear forces. These data suggest that VE-cadherin provides the membrane anchor that stabilizes other polarized proteins, such as the Par complex, to the downstream edge of the cell. Interestingly, VE-cadherin is required for flow-induced association of Tiam1with p67phox, suggesting that VE-cadherin mediates ROS production by regulating the association of these proteins. During the preparation of this manuscript, another group reported that VE-cadherin and Tiam1 mediate shear-induced Rac1 GTP loading and barrier enhancement in brain microvascular ECs (Walsh et al., 2011). Our data show that VE-cadherin and Tiam1 are not required for GTP loading of Rac1 but are important for polarized activation of Rac1. A possible reason for this discrepancy could be the origin of ECs used in that study and/or the approaches used to inhibit VE-cadherin and Tiam1. Here, we identify Vav2 as the critical GEF for shear-induced GTP-loading of Rac1. Our data indicate impaired Rac1 activation in Vav2-depleted ECs. Notably, Vav2 localization is not polarized under flow (unpublished data). This result agrees with our results indicating Tiam1 as the GEF required for Rac1 polarization. ECs depleted of Tiam1 (with Vav2 expression intact) globally activate Rac1 in response to shear, but lack downstream Rac1 polarity (Fig. 2 D). These results highlight the differential roles of the two GEFs in this system: Vav2 is required for global GTP loading and activation of Rac1, whereas Tiam1 is required for recruitment of activated Rac1 to the downstream edge of the cell.
The Nox family of proteins is essential in normal physiology (Brown and Griendling, 2009). Nox proteins and ROS have been shown to regulate many fundamental physiological processes, including cell growth, differentiation, apoptosis, angiogenesis, and cytoskeletal remodeling. In the vasculature, ROS are required for endothelial cell proliferation and migration during angiogenesis (Ushio-Fukai, 2007), as well as flow-mediated vascular remodeling via activation of MMPs (Castier et al., 2005). Within the endothelium, the membrane-bound Nox2 subunit of the NAPDH oxidase has been shown to be a major source to EC ROS production (Brown and Griendling, 2009). Our data suggest that Nox2 may also play a role in flow-mediated ROS production. In addition, NADPH oxidases are now recognized to have specific subcellular localizations, and compartmentalization of redox signaling is thought to involve the interaction of NADPH oxidase subunits with distinct signaling platforms associated with different subcellular sites (Ushio-Fukai, 2009). Thus, recruitment of p67phox to a VE-cadherin–Tiam1 scaffold in response to flow may reflect a general strategy of targeting oxidant production to particular subcellular sites as a means to gain signal specificity (Ushio-Fukai, 2009). Overall, these results indicate that VE-cadherin is required for the flow-induced Par3–Tiam1 complex formation and that these proteins might be components of a pathway that links spatial cues with redox signaling (Fig. 6 E). Importantly, E-cadherin was similarly required for establishing polarity during cell migration (Desai et al., 2009), indicating that this pathway may represent a general role for cell–cell adhesion in polarization.
The work described here identifies a novel signaling module that links cell–cell junctions, polarity cues, and redox signaling in response to blood flow. This signaling pathway constitutes a novel paradigm that demonstrates distinct yet synergistic roles for two Rac GEFs; one GEF regulates global Rac1 activation, while the second determines spatial activation of Rac1. Compartmentalization of Rac1 activity is achieved via formation of a VE-cadherin–Par3–Tiam1 complex and is required for coupling to the p67phox subunit of the NADPH oxidase and downstream redox signaling. The identification of a molecular cascade required for spatial mechanosignaling and NADPH oxidase activation provides new insights into mechanisms of ROS signaling in cellular responses related to signal transduction, inflammatory diseases, and atherosclerosis.
Materials and methods
Cell culture and shear stress
VE-cadherin null (VE-KO) and reconstituted (VE-RC) cells were prepared as described previously (Carmeliet et al., 1999). In brief, ECs were derived from undifferentiated mouse embryonic stem cells from VE-cadherin–null embryos and were transduced with a retrovirus expressing the Polyoma middle-T oncogene (VE-KO). VE-RC cells were obtained generated by reintroducing human VE-cadherin. VE-RC and VE-KO cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen) containing 10% FBS (Invitrogen), 5 µg/ml endothelial cell growth supplement (ECGS; Sigma-Aldrich), and 100 µg/ml heparin and penicillin/streptomycin (Invitrogen). PECAM-1 knockout (PE-KO) and reconstituted (PE-RC) cells were cultured in DMEM containing 10% FBS, 10 µM 2-mercaptoethanol (Invitrogen), 1× nonessential amino acids (NEAA; Invitrogen), and penicillin/streptomycin. Human umbilical vein endothelial cells (HUVECs) were cultured in EGM medium (Lonza) according to the manufacturer’s suggested procedure. Bovine aortic endothelial cells (BAECs) were cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin. For shear stress experiments, endothelial cells were plated on 10 µg/ml fibronectin and allowed to grow for 5 h in medium containing 10% FBS. Cells were then starved overnight in medium containing 0.5% FBS. Slides were loaded onto a parallel plate flow chamber in 0.5% FBS and 12 dynes/cm2 of shear stress was applied for indicated times. The starvation time was shortened to 1 h if HUVECs were used in shear stress.
Antibodies and inhibitors
Src inhibitors SU6656 and PP2 were purchased from EMD Millipore. Vav2, phospho-Vav2, and Tiam1 were purchased from Santa Cruz Biotechnology, Inc. The Vav2 antibody used for immunoprecipitation, anti-myc, and anti-HA antibodies were from Cell Signaling Technology. The Rac1 antibody was obtained from BD and the VE-cadherin antibody was from Enzo Life Sciences. The anti-FLAG and actin antibodies were purchased from Sigma-Aldrich and the anti-Par3 antibody was from EMD Millipore.
RNA interference and plasmid transfections
The siRNA sequences used in the study were as follows: siControl, 5′-UAAGGCUAUGAAGAGAUAC-3′; human siTiam1#1, 5′-GCGAAGGAGCAGGUUUUCU-3′; human siTiam1#2, 5′-GAACCGAAGCUGUAAAGAA-3′; human siVav2#1, 5′-GGAACAGCGAGCUGUUUGA-3′; human siVav2#2, 5′-AGUCCGGUCCAUAGUCAAC-3′; mouse siTiam1#1, 5′-UCAAGACUAUGAACAAGGU-3′; mouse siTiam1#2, 5′-GCGAAGGAGCAGGUUUUCU-3′; mouse siPar3, 5′-CGUUAAAUCCAUUAUCAAU-3′. The siRNAs were introduced into the cells as described previously using a calcium phosphate method (Liu et al., 2008). Plasmid constructs were transfected into VE-RC or VE-KO cells using Lipofectamine LTX (Invitrogen). To transfect HUVECs with plasmids or siRNA, the Amaxa Nucleofector kit and Nucleofector program A-034 were used. The wild-type pcDNA3-Tiam1 and the GEF-deficient mutant pcDNA3-Tiam1-1047/1232A were generous gifts from Dr. Channing J. Der (UNC-Chapel Hill, Chapel Hill, NC). The siRNA-resistant Tiam1 rescue plasmids were generated by changing the target sequence of human siTiam1#2 to 5′-GGACUGAGGCUGUAAAGAA-3′ (mutated nucleotides underlined) in the context of wild-type pcDNA3-Tiam1 or 1047A/1232A-Tiam1. Plasmid constructs (GFP-hTiam1-WT, myc-Par3, myc-Nox2, WT-Par6, and DN-Par6) were transfected into BAECs using the Effectene Transfection Reagent (QIAGEN) according the manufacturer’s protocol. Confluent monolayers were sheared 48 h after transfection.
Rac pull-down assays
Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in Rac pull-down buffer containing 50 mM Tris, pH 7.6, 150 mM NaCl, 0.5 mM MgCl2, 1% Triton X-100 supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml aprotinin, and 1 µg/ml leupeptin. The cell lysates were spun at 15,000 g for 5 min and the cleared supernatant incubated with 20 µg of purified recombinant GST-PBD for 30 min at 4°C. The beads were subsequently washed with Rac pull-down buffer and bound proteins eluted with SDS sample buffer and analyzed by Western blotting using a monoclonal anti-Rac antibody (BD). Whole-cell lysates were also analyzed for the normalization of protein loading.
FRET assays
EGFP-Rac and mCherry-PBD were validated as an appropriated fluorophore pair for FRET analysis. EGFP-tagged Rac and pcDNA3.1(+)-mCherry-PBD were cotransfected into endothelial cells using Lipofectamine LTX or Amaxa Nucleofector and subjected to shear stress for 0 or 30 min. Images of fixed cells were acquired using a laser scanning confocal microscope (710 LSCM; Carl Zeiss). Calculations to account for bleedthrough and background were performed as described previously (Kraynov et al., 2000; Tzima et al., 2002). In brief, after subtracting the background from all images, the EGFP-Rac images were thresholded and used to generate a binary image with values within the cell = 1 and outside the cell = 0. The FRET and mCherry-PBD images were multiplied by the binary image and a fraction of the EGFP-Rac and mCherry-PBD images was subtracted from the FRET image. The corrected 8-bit FRET images were displayed using pseudocolor, where blue is closest to 0 and red closest to 100.
ROS assays
Endothelial cells were preincubated with 10 µM of 2,7-dichlorodihydrofluorescein diacetate (H2-DCFDA) at 37°C for 30 min before the onset of flow. Shear stress was applied to the cells in continued presence of the dye for indicated times. Cells were rinsed with PBS and lysed in PBS containing 0.2% Triton X-100 and 1 mM N-acetylcysteine. Fluorescence was measured using the 485-nm excitation/535-nm emission filter in a Wallac Victor 1420 multilabel plate reader (PerkinElmer) and normalized to total protein concentrations in the lysates (Bradford assay; Thermo Fisher Scientific).
Immunoprecipitations and Western blotting
Cells were harvested in lysis buffer (20 mM Hepes, pH 7.4, 150 mM NaCl, 50 mM KCl, and 1% NP-40) supplemented with 1 mM aprotinin, 1 µg/ml leupeptin, 1 mM PMSF, 1 mM Na3VO4, 1 mM sodium pyrophosphate, and 1 mM β-glycerophosphate. Lysates were precleared with 50 µl protein A/G plus Sepharose beads (Santa Cruz Biotechnology, Inc.) for 1 h at 4°C. Supernatants were then incubated with 30 µl protein A/G plus Sepharose previously coupled to the primary antibodies for 2 h at 4°C with continuous agitation. The beads were washed three times with lysis buffer supplemented with protease and phosphatase inhibitors and the immune complexes were eluted in 2× SDS sample buffer. Associated proteins were subjected to SDS-PAGE and Western blotting using the appropriate primary antibodies and IRDye-conjugated anti–mouse or anti–rabbit antibodies (Rockland). Images of Western blotting were obtained with an Odyssey infrared scanner system (LI-COR Biosciences).
In vivo RNAi and en face staining of mouse aorta
50 µg of mouse Tiam1 or control siRNAs were mixed with jetPEI in vivo delivery reagent (Polyplus) and injected into C57BL/6 mice (>30 wk) through the retro-orbital veins for two or three consecutive days (Steel et al., 2008). 72–96 h after the initial injection, aorta arches were isolated and processed for en face staining as described previously (Chen and Tzima, 2009). In brief, aortas were perfusion-fixed and dissected out under dissection microscope. The aortic arches were cut longitudinally and pinned flat with endothelium facing up onto a Surperfrost/Plus glass slide (Thermo Fisher Scientific). ROS production was visualized by incubating the tissue sections with 10 µM of DCF dye at 37°C for 1 h. Images were obtained immediately after the DCF incubation to avoid decay of fluorescence.
In vitro binding assays
Human Tiam1 PDZ domain (841–930) and PHn-PHc (423–1406) were subcloned into a modified pET21a vector (EMD Millipore) that includes an N-terminal 6×His tag and a tobacco etch virus (rTEV) protease cleavage site. The PHn-CC-Ex domain (429–702) was subcloned into a modified pQE30 vector (QIAGEN) containing an N-terminal 6×His tag and rTEV sequence in frame with a GB1 fusion. To generate GST-p67 fusion, human p67phox was subcloned into the EcoRI and NotI sites of pGEX2T1 (GE Healthcare). The His-tagged Tiam1 PDZ and PHn-PHc were expressed from Escherichia coli Rosetta (EMD Millipore), and His-tagged PHn-CC-Ex purified from E. coli M15 (QIAGEN). GST or GST-p67 were expressed from E. coli BL21(DE3). To perform binding assays, GST or GST-p67 proteins were incubated with an excess (5 molar equivalents) of Tiam1 variants at 4°C for 1 h in binding buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 0.1 mM EDTA, 0.1 mM DTT, and 0.05% Tween 20) supplemented with protease inhibitors before loading onto Sepharose beads. After washing five times with binding buffer, bound proteins were eluted with binding buffer supplemented with 10 mM glutathione. Precipitated proteins were separated by SDS–PAGE and detected by Western blotting using a 6×His-specific monoclonal antibody (Covance).
NMR chemical shift perturbation experiments
Nuclear magnetic resonance spectra (1H-15N HSQCs) were recorded at 25°C on a 600-MHz spectrometer (Varian). The data were processed using NMRPipe and visualized in Sparky. NMR spectra were performed on a 15N-labeled Tiam1 PDZ sample (200 µM) in a buffer of 20 mM PO43−, 50 mM NaCl, 0.5 mM EDTA, and 10 mM NaN. Backbone (15N) resonance assignments were previously determined for the free Tiam1 PDZ domain. The human VE-cadherin peptide (654–669; MLAELYGSDPREELLY) was synthesized with an N-terminal dansyl chloride moiety and HPLC purified to >95% homogeneity (Genscript). For the chemical shift perturbation experiment, a reference 1H-15N HSQC experiment was first recorded on the free PDZ domain followed by serial addition of concentrated VE-cadherin peptide (10 mM) added in 8 titration steps to a molar ratio of 8:1, peptide to PDZ domain. An estimate of the dissociation constant (Kd) was determined to be 980 ± 150 mM by fitting peaks that had significant changes in chemical shift to a hyperbolic binding model.
Immunofluorescence and microscopy
BAECs were fixed in 2% formaldehyde, permeabilized in 0.2% Triton X-100 for 20 min, and then blocked in 10% goat serum for 1 h at RT. Cells were stained with anti–VE-cadherin (Enzo Life Sciences) or anti-myc (Invitrogen) antibodies (1:100). Slides were mounted in Vectashield mounting medium (Vector Laboratories). Images were obtained using the 60× 1.40 NA oil objective on a microscope (Eclipse E800; Nikon) equipped with a digital camera (ORCA-ER; Hamamatsu Photonics) and MetaMorph software (MDS Analytical Technologies). Confocal images were obtained using the 60× 1.40 NA oil objective on a microscope (FV500; Olympus) equipped with a digital camera (ORCA-ER; Hamamatsu Photonics) and were analyzed using ImageJ software (National Institutes of Health).
Quantification and statistical analysis
The intensities of immunoblotting or immunofluorescence staining were quantified using ImageJ. Data were analyzed by performing two-tailed Student’s t test (between two groups) and one-way ANOVA (among multiple groups) as appropriate. Statistical significance was defined as P < 0.05.
Online supplemental material
Fig. S1 provides additional information on FRET analysis. Fig. S2 provides additional siRNA and inhibitors studies that demonstrate shear-induced activation of Vav2 is required for Rac1 GTP-loading. Fig. S3 demonstrates specific detection of ROS production in vivo. Fig. S4 shows that successful knockdown of Tiam1 in vivo blunts ROS production in the mouse aorta. Fig. S5 demonstrates that Tiam1 binds to VE-cadherin. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201207115/DC1.
Supplementary Material
Acknowledgments
We thank Mauricio Rojas for in vivo RNAi and aorta isolation experiments; Robert Bagnell Jr. and the Lineberger Comprehensive Center Microscopy Core Facility for help with confocal microscopy; Kirk McNaughton for advice on en face preparation; Ian Macara for the Tiam1 expression construct; Keith Burridge for help with Rac1 pull-down assays; Dave Lambeth for the Flag-p67phox construct; Ajay Shah for the Nox2 construct; and Martin Schwartz for the mCherry-PBD. We also appreciate Channing Der and Keith Burridge for critical reading of the manuscript.
This work was funded by the NIH (grant no. HL088632 to E. Tzima), the National Science Foundation (grant no. MCB-0953080 to E.J. Fuentes), and the American Heart Association (grant no. 0835261N to E.J. Fuentes). C. Collins is an American Heart Predoctoral Fellow (12PRE119300000), M. Joshi is an American Heart Association Postdoctoral Fellow (0920148G), and T.R. Shepherd is supported by an NIH Predoctoral Fellowship (GM-008365) in Biotechnology. E. Tzima is an Ellison Medical Foundation New Scholar.
Author contributions: E. Tzima initiated and supervised the project; designed experiments and analyzed data. Y. Liu and C. Collins performed experiments and analyzed data. W.B. Kiosses performed FRET experiments and analyzed data. A.M. Murray, M. Joshi, T.R. Shepherd, and E.J. Fuentes performed experiments and analyzed data. Y. Liu, C. Collins, and E. Tzima wrote the manuscript.
Footnotes
Abbreviations used in this paper:
- BAEC
- bovine aortic endothelial cell
- EC
- endothelial cell
- FRET
- fluorescence resonance energy transfer
- GEF
- guanine nucleotide exchange factor
- H2-DCFDA
- 2,7-dichlorodihydrofluorescein diacetate
- HUVEC
- human umbilical vein endothelial cell
- NADPH
- nicotinamide adenine dinucleotide phosphate
- PBD
- p21-binding domain of PAK
- PECAM-1
- platelet endothelial cell adhesion molecule-1
- PE-KO
- PECAM-1 knockout
- PE-RC
- PECAM-1 reconstituted
- ROS
- reactive oxygen species
- VE-cadherin
- vascular endothelial-cadherin
- VE-KO
- VE-cadherin knockout
- VE-RC
- VE-cadherin reconstituted
References
- Abo A., Pick E., Hall A., Totty N., Teahan C.G., Segal A.W. 1991. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 353:668–670 10.1038/353668a0 [DOI] [PubMed] [Google Scholar]
- Allen R.J., Bogle I.D., Ridley A.J. 2011. A model of localised Rac1 activation in endothelial cells due to fluid flow. J. Theor. Biol. 280:34–42 10.1016/j.jtbi.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Brown D.I., Griendling K.K. 2009. Nox proteins in signal transduction. Free Radic. Biol. Med. 47:1239–1253 10.1016/j.freeradbiomed.2009.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Lampugnani M.G., Moons L., Breviario F., Compernolle V., Bono F., Balconi G., Spagnuolo R., Oosthuyse B., Dewerchin M., et al. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 98:147–157 10.1016/S0092-8674(00)81010-7 [DOI] [PubMed] [Google Scholar]
- Castier Y., Brandes R.P., Leseche G., Tedgui A., Lehoux S. 2005. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ. Res. 97:533–540 10.1161/01.RES.0000181759.63239.21 [DOI] [PubMed] [Google Scholar]
- Chen X., Macara I.G. 2005. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol. 7:262–269 10.1038/ncb1226 [DOI] [PubMed] [Google Scholar]
- Chen Z., Tzima E. 2009. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 29:1067–1073 10.1161/ATVBAHA.109.186692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Diebold B.A., Hughes Y., Lambeth J.D. 2006. Nox1-dependent reactive oxygen generation is regulated by Rac1. J. Biol. Chem. 281:17718–17726 10.1074/jbc.M512751200 [DOI] [PubMed] [Google Scholar]
- Chiu J.J., Wung B.S., Shyy J.Y., Hsieh H.J., Wang D.L. 1997. Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 17:3570–3577 10.1161/01.ATV.17.12.3570 [DOI] [PubMed] [Google Scholar]
- Desai R.A., Gao L., Raghavan S., Liu W.F., Chen C.S. 2009. Cell polarity triggered by cell-cell adhesion via E-cadherin. J. Cell Sci. 122:905–911 10.1242/jcs.028183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. 2002. Rho GTPases in cell biology. Nature. 420:629–635 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- García-Mata R., Burridge K. 2007. Catching a GEF by its tail. Trends Cell Biol. 17:36–43 10.1016/j.tcb.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Gavard J., Gutkind J.S. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8:1223–1234 10.1038/ncb1486 [DOI] [PubMed] [Google Scholar]
- Hahn C., Schwartz M.A. 2009. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 10:53–62 10.1038/nrm2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. 2005. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33:891–895 10.1042/BST20050891 [DOI] [PubMed] [Google Scholar]
- Helmke B.P., Goldman R.D., Davies P.F. 2000. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ. Res. 86:745–752 10.1161/01.RES.86.7.745 [DOI] [PubMed] [Google Scholar]
- Iden S., Rehder D., August B., Suzuki A., Wolburg-Buchholz K., Wolburg H., Ohno S., Behrens J., Vestweber D., Ebnet K. 2006. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep. 7:1239–1246 10.1038/sj.embor.7400819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneson T., Bar-Sagi D. 1998. A Rac1 effector site controlling mitogenesis through superoxide production. J. Biol. Chem. 273:17991–17994 10.1074/jbc.273.29.17991 [DOI] [PubMed] [Google Scholar]
- Knaus U.G., Heyworth P.G., Evans T., Curnutte J.T., Bokoch G.M. 1991. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 254:1512–1515 10.1126/science.1660188 [DOI] [PubMed] [Google Scholar]
- Kraynov V.S., Chamberlain C., Bokoch G.M., Schwartz M.A., Slabaugh S., Hahn K.M. 2000. Localized Rac activation dynamics visualized in living cells. Science. 290:333–337 10.1126/science.290.5490.333 [DOI] [PubMed] [Google Scholar]
- Liu Y., Sweet D.T., Irani-Tehrani M., Maeda N., Tzima E. 2008. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J. Cell Biol. 182:185–196 10.1083/jcb.200709176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I.G. 2004. Par proteins: partners in polarization. Curr. Biol. 14:R160–R162 [PubMed] [Google Scholar]
- Mertens A.E., Rygiel T.P., Olivo C., van der Kammen R., Collard J.G. 2005. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J. Cell Biol. 170:1029–1037 10.1083/jcb.200502129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Yamaguchi T., Kato K., Yoshizawa M., Nabeshima Y., Ohno S., Hoshino M., Kaibuchi K. 2005. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat. Cell Biol. 7:270–277 10.1038/ncb1227 [DOI] [PubMed] [Google Scholar]
- Pertz O. 2010. Spatio-temporal Rho GTPase signaling - where are we now? J. Cell Sci. 123:1841–1850 10.1242/jcs.064345 [DOI] [PubMed] [Google Scholar]
- Rajagopal S., Ji Y., Xu K., Li Y., Wicks K., Liu J., Wong K.W., Herman I.M., Isberg R.R., Buchsbaum R.J. 2010. Scaffold proteins IRSp53 and spinophilin regulate localized Rac activation by T-lymphocyte invasion and metastasis protein 1 (TIAM1). J. Biol. Chem. 285:18060–18071 10.1074/jbc.M109.051490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M.R. 2006. Coupling receptor tyrosine kinases to Rho GTPases—GEFs what’s the link. Cell. Signal. 18:1834–1843 10.1016/j.cellsig.2006.01.022 [DOI] [PubMed] [Google Scholar]
- Shepherd T.R., Fuentes E.J. 2011. Structural and thermodynamic analysis of PDZ-ligand interactions. Methods Enzymol. 488:81–100 10.1016/B978-0-12-381268-1.00004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd T.R., Klaus S.M., Liu X., Ramaswamy S., DeMali K.A., Fuentes E.J. 2010. The Tiam1 PDZ domain couples to Syndecan1 and promotes cell-matrix adhesion. J. Mol. Biol. 398:730–746 10.1016/j.jmb.2010.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel C.D., Stephens A.L., Hahto S.M., Singletary S.J., Ciavarra R.P. 2008. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim. (NY). 37:26–32 10.1038/laban0108-26 [DOI] [PubMed] [Google Scholar]
- Sundaresan M., Yu Z.X., Ferrans V.J., Sulciner D.J., Gutkind J.S., Irani K., Goldschmidt-Clermont P.J., Finkel T. 1996. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318:379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler R.C., Peterson F.C., Volkman B.F. 2010. Distal interactions within the par3-VE-cadherin complex. Biochemistry. 49:951–957 10.1021/bi9017335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E. 2006. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ. Res. 98:176–185 10.1161/01.RES.0000200162.94463.d7 [DOI] [PubMed] [Google Scholar]
- Tzima E., Del Pozo M.A., Kiosses W.B., Mohamed S.A., Li S., Chien S., Schwartz M.A. 2002. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 21:6791–6800 10.1093/emboj/cdf688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., Kiosses W.B., del Pozo M.A., Schwartz M.A. 2003. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J. Biol. Chem. 278:31020–31023 10.1074/jbc.M301179200 [DOI] [PubMed] [Google Scholar]
- Tzima E., Irani-Tehrani M., Kiosses W.B., Dejana E., Schultz D.A., Engelhardt B., Cao G., DeLisser H., Schwartz M.A. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 437:426–431 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. 2007. VEGF signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 9:731–739 10.1089/ars.2007.1556 [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. 2009. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 11:1289–1299 10.1089/ars.2008.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T.G., Murphy R.P., Fitzpatrick P., Rochfort K.D., Guinan A.F., Murphy A., Cummins P.M. 2011. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J. Cell. Physiol. 226:3053–3063 10.1002/jcp.22655 [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B., Ridley A.J. 2003. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J. Cell Biol. 161:429–439 10.1083/jcb.200210135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthylake D.K., Rossman K.L., Sondek J. 2000. Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature. 408:682–688 10.1038/35047014 [DOI] [PubMed] [Google Scholar]
- Yeh L.H., Park Y.J., Hansalia R.J., Ahmed I.S., Deshpande S.S., Goldschmidt-Clermont P.J., Irani K., Alevriadou B.R. 1999. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am. J. Physiol. 276:C838–C847 [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R., Kam Z., Geiger B. 2005. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J. Cell Sci. 118:3997–4007 10.1242/jcs.02523 [DOI] [PubMed] [Google Scholar]
- Zhang H., Macara I.G. 2006. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat. Cell Biol. 8:227–237 10.1038/ncb1368 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.