Abstract
Background:
Easy accessibility to Medicare and better living conditions has increased life expectancy in recent years. There are over 60 million postmenopausal women above 55 years in India. Obesity, physical inactivity, and altered estrogen metabolism play an integrated role in contributing to the disease risk profile of postmenopausal women. These same risk factors also affect modulation of the autonomic nervous system. A study was undertaken to test the hypothesis whether there is indeed an alteration in autonomic functions in obese postmenopausal women.
Materials and Methods:
60 postmenopausal women without any gross systemic disease whose body mass index and waist/hip ratio were recorded. Subjects were divided into two groups of 36 Non-Obese and 24 Obese. The two groups were well matched for age and menopausal duration. The physical as well as physiological parameters like valsalva ratio, heart rate variation with deep breath test, heart rate response to postural change (30:15 R-R interval ratio), orthostatic tolerance test, and isometric handgrip test were recorded.
Results:
Results of valsalva ratio, deep breath test, and 30:15 R-R interval ratios and isometric handgrip test were significantly decreased and orthostatic tolerance values were significantly increased in Obese subjects.
Conclusion:
Findings show decreased sympathovagal activity with obesity in postmenopausal women.
Keywords: Autonomic functions, obesity and coronary heart disease, postmenopausal women
Menopause is the permanent cessation of menstruation due to loss of ovarian function. This transition is gradual which takes place over a period of time from the reproductive to non-reproductive phase of life. The transition from pre- to post-menopause is often associated with the emergence of metabolic syndrome features. These risk factors perse may be due to the direct result of ovarian failure or an indirect result of the metabolic consequences of central fat redistribution with estrogen deficiency.[1–3]
Menopause is associated with reduced physical activity and energy expenditure, an accelerated loss of fat free mass, alteration of adipose tissue metabolism, and fat oxidation. This deregulation of energy metabolism could induce an increase in total adiposity and a redistribution of fat in the abdominal region in postmenopausal women (PMW).[4–9] The distribution of adipose tissue in different anatomic depots also has substantial implication for morbidity. Specifically, intra-abdominal and abdominal subcutaneous fat has more significance than subcutaneous fat in the buttocks and lower extremities. Many of the complications of obesity, such as insulin resistance, diabetes, hypertension, hyperlipidemia, and hyperandrogenism in women, are linked more strongly to intra-abdominal or upper body fat than to overall adiposity.[9–12] Increased body fat percentage causes marked sympathetic activation and vagal inhibition leading to autonomic dysfunction.[13]
The altered sympatho-vagal activity has an additional adverse effect on health. As life expectancy increases, women in general experience a longer life after menopause. 60 million women in India are above the age of 55 years. Hence, there is a need to understand the effect of obesity and autonomic function in PMW. Early detection of subclinical autonomic dysfunction therefore will improve the quality of life by proper medication and lifestyle modification.[14,15]
MATERIALS AND METHODS
A total of 60 PMW without any gross systemic disease who were evenly matched for age and menopausal duration were selected. The physical and physiological parameters were recorded using weighing machine, height stands, 4 channel physiograph polyrite-D, timer, handgrip dynamometer, and sphygmomanometer.
Methods
Inclusion criteria
Only those subjects who were normotensive, without any gross systemic disease and non-smoker and who could abstain from caffeine-containing beverages, drugs, hormone replacement therapy, and alcohol during the study were taken.[9]
Exclusion criteria
Tachycardia, cardiac arrhythmias, presence of hypertension, diabetes, ischemic heart disease, retinopathy, nephropathy and the presence of a chronic disease or associated factors that may affect the autonomic reflexes were excluded.[9]
On first appointment, particulars of the subject and history were carefully recorded, thorough general physical examinations was done, and consent was taken. They were instructed to avoid smoking, drinking coffee, or consuming any drugs that may alter the autonomic functions (48 h prior to the test). The tests were performed in the research laboratory in the Department of Physiology of a tertiary care hospital in Pune.
Anthropometry
-
(a)
Body weight: A digital weighing scale was used to measure body weight with an accuracy of ± 100 g. Subjects were weighed without their shoes
-
(b)
Height: Standing body height was measured without shoes to the nearest 0.5 cm with the use of height stand with shoulders in relaxed position and arms hanging freely
-
(c)
Body mass index: Body Mass Index (BMI) was calculated as body weight in kilograms divided by square of body height in meters.[1] Those subjects with BMI greater than 25 were categorized as obese and those with BMI less than 25 as Non-Obese.[14] Among the 60 individuals, 24 were found to be Obese and 36 were Non-Obese
-
(d)
Waist/hip ratio: Waist/Hip Ratio (WHR) of these subjects were measured. Waist circumference was measured at the level of the iliac crest and hip circumference at the fullest point around buttocks. Waist circumference was divided by hip circumference in order to calculate the WHR. WHR > 0.9 was considered to be Obese.[15]
24 of our subjects were found to have BMI > 25, WHR > 0.9 and 36 were found to have BMI < 25, WHR < 0.9.
Determination of resting heart rate (RHR)
Apparatus: Polyrite-D.
Lead II of the ECG was selected for recording heart rate. Tracing speed used was 30 mm/s. Heart rate was recorded in the supine position by the conventional method during normal quiet breathing for a period of 1 min. The ECG tracings were screened for any suspected pathological waveform configuration.[15,16]
Tests for autonomic function
Tests reflecting cardiac parasympathetic functions
Heart rate response to postural change (30:15 ratio)
Heart rate variation during deep breathing
Heart rate response to valsalva maneuver (VR)
Tests reflecting sympathetic functions
Blood pressure response to postural change (orthostatic tolerance test (OTT))
Blood pressure response to sustained isometric handgrip (IHG)
The subjects were made to rest for 15 min in the supine position. The resting time given to subjects in between two tests was 5 to 10 min.
Heart rate response to postural change (30:15 ratio)
After a complete rest of 15 min in the supine position, the ECG recording was started. The subject was instructed to stand erect from the supine position as quickly as possible (within 3 s) with continuous ECG recording for at least 30 s.[15,16]
The ratio of the longest RR around 30th beat after standing to the shortest RR interval around 15th beat after standing were calculated for result of 30:15 ratio:[15,16] Normal ≥1.04, Abnormal ≤1.00
Heart rate variation during deep breathing
The subject was instructed to take deep inspiration over 5 s and followed by expiration over next 5 s completing six respiratory cycles in 1 min (six cycles were repeated in 1 min in this test). The inspiratory and expiratory timing were synchronized by looking at the observer's finger moving rhythmically up and down with the timer.[16]
The mean of the minimum RR intervals in the six inspiratory cycles was calculated and heart rate determined. Also the mean of the maximum RR interval in the six expiratory cycles of the same tracing were calculated for heart rate during expiration. The difference of the heart rate between the maximum in the inspiratory cycle and the minimum in the expiratory cycles was calculated and was used as result of the test Normal ≥15 beats/min.[17]
Heart rate response to valsalva maneuver
The subject was instructed to exhale forcefully through the mouth piece of a modified mercurial sphygmomanometer and to maintain pressure in the manometer up to 40 mmHg for 15 s. ECG recording were taken during the maneuver and continued for about 30 s after the performance.[15–17]
The ratio of the longest RR interval after blowing to the shortest RR interval during blowing was calculated: Normal ≥1.21, Abnormal ≤1.1[16]
Orthostatic tolerance test
Apparatus: mercurial sphygmomanometer, stethoscope.
Basal blood pressure was recorded. Then the patient was asked to stand up and the blood pressure was recorded immediately.[16]
The difference of the systolic blood pressures between the one recorded during lying supine and in erect posture was calculated. The fall in systolic pressure was used as the result of the OTT.
Normal: 10 mmHg or less, Borderline: 11–29, Abnormal: 30 mmHg or more.
Blood pressure response to sustained isometric handgrip
Apparatus: mercurial sphygmomanometer, stethoscope and handgrip dynamometer.
The subject was asked to perform maximum grip of the handgrip dynamometer with his dominant hand and the maximum capacity was noted down. After 5 min in the sitting position, the subject was asked to hold his grip with 30% of the maximum capacity for 5 min and the blood pressure was recorded just after release of the grip.
Calculation: The rise in diastolic blood pressure was calculated and taken as the result of IHG test.[16]
Normal: 16 mmHg or more, Borderline: 11–15 mm, Abnormal: 10 mmHg or less
All the mean values of all parameters of both groups were arranged in tabular form and spastically analyzed using Student's t-test and Pearson's correlation coefficient test (P value <0.05 * significant, P value <0.01** highly significant).[18]
No significant changes in resting pulse rate and casual blood pressure were found between the two groups.
RESULTS
Results showed significant differences in BMI, Waist/Hip Ratio, VR, Deep breath test, 30:15 R-R ratios, OTT & IHG Tests between the two groups. Markers of obesity were negatively correlated with autonomic function tests.
DISCUSSION
Women with the metabolic syndrome (central obesity, insulin resistance, and dyslipidemia) are known to be at especially high risk for cardiovascular disease (CVD). The prevalence of the metabolic syndrome increases with menopause and may partially explain the apparent acceleration in CVD after menopause.[1] The values of BMI for the Asian population have been reset as their body composition is different from that of the western world.[14] Recent comparative studies have shown that Indians have a higher percentage of body fat for a given BMI compared with the white Caucasians and African-Americans but have a lower muscle mass. Body composition of Indians is partly responsible for their higher insulin resistance. In the present study, subjects with BMI greater than 25 were categorized into Obese and those with BMI less than 25 as Non-Obese.[14]
WHRs > 0.9 in women and > 1 in men are considered abnormal. Many of the important complications of obesity, insulin resistance, diabetes, hypertension, hyperlipidemia, and hyperandrogenism in women are linked strongly to intra-abdominal or upper body fat than to overall adiposity. WHR has been found to be more predictive than BMI in most studies.[15] An increase in body fat percentage with menopause has been reported by many workers.[1,4,7–10,12] Heart rate variation is reported to be reduced in obese individuals. Human obesity is characterized by marked sympathetic activation. A 10% increase in body weight above an individual's usual weight is accompanied with a decrease in parasympathetic activity.[13] This effect of increased weight is one mechanism for cardiac alterations such as arrhythmias that accompany obesity.
Leptin is an anorexigenic hormone that influences the feeding and satiety centers in hypothalamus. At one time it was assumed that adipocytes are the sole site of leptin production and release. Evidence from studies in experimental animals and human now indicates that leptin is also derived from non-adipose sites, one of these being the brain.[10] Leptin and the sympathoadrenal system appear to be intimately linked. It has been suggested that there may be a two-way interaction between leptin and sympathetic nervous system, perhaps constituting a regulatory feedback loop, with leptin acting within the hypothalamus to cause activation of the central sympathetic outflow and stimulation of adrenal medullary release of epinephrine, and conversely, with the sympathetic nervous system inhibiting leptin release from white adipose tissue.[10,19,20]
In this study, obese subjects had significantly lower autonomic functions as compared to non-obese subjects. Similar results were reported by Tetsuya Kimura et al.[8] Epidemiological data shows that women below 50 years rarely develop CVD, but the incidence is equal in men and women around 70 years of age. Menopause may result in endothelial dysfunction and increase body weight and type II diabetes that causes increase sympathetic activation.[1]
Decreased baroreflex sensitivity also appears with increasing body mass. Obese humans have decreased responsiveness to sympathetic stimulation and down regulatedβ receptors in white adipose tissue. Gaining weight combines regularly with metabolic changes revealing adaptation processes towards “resistance” of feedback loops involved especially in organ systems ensuring supply and utilization of energy.[21]
Thus, this study implies that the altered sympatho-vagal activity has an additional adverse effect on health and ultimately on the quality of life in obese PMW [Figure 1 and Tables 1–3].
Figure 1.
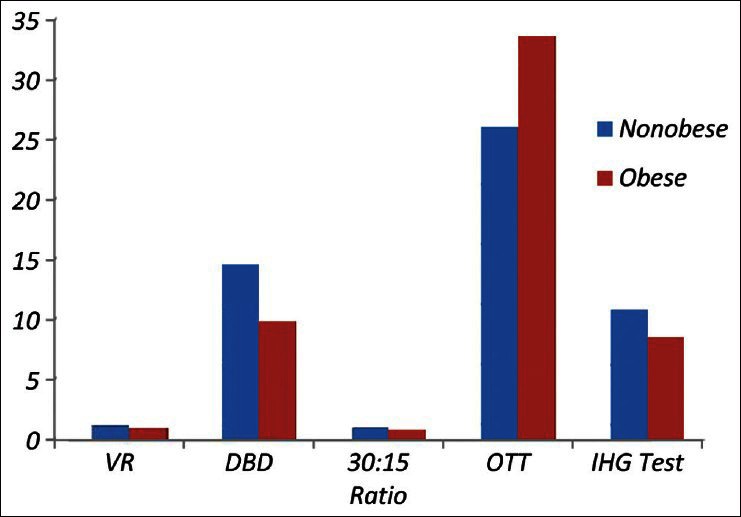
Shows the mean values of VR, DBD, 30:15 R‑R intervals ratio, OTT and IHG test in non‑obese and obese individuals
Table 1.
The average values of BMI, waist/Hip ratio, valsalva ratio, deep breath test, 30:15 R-R intervals ratio, OTT, IHG test of non-obese and obese postmenopausal subjects are shown
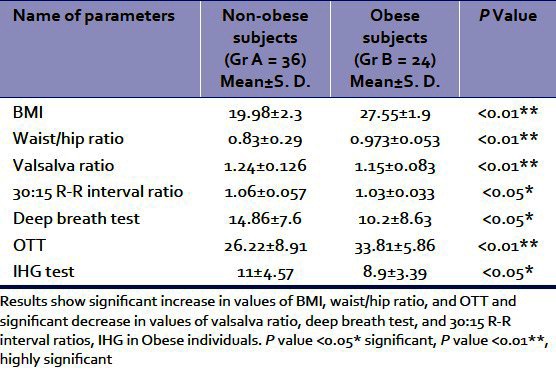
Table 3.
Shows of correlation of waist/hip ratio and autonomic function tests
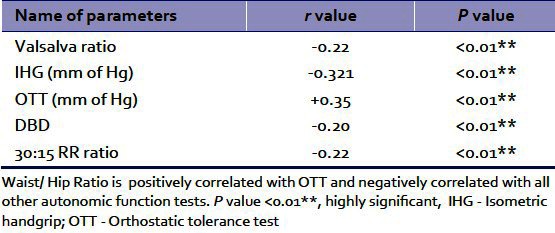
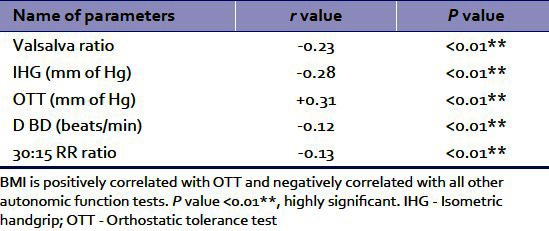
Table 2.
Shows of correlation of BMI and autonomic function tests
Dietary modifications, exercise, and yoga may improve autonomic functions in PMW by reducing body fat and weight.[3,21] Further studies with larger samples of population and using MRI or bioelectric impedance analyzer for measuring body fat percentage may prove useful.[7,8]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Carr MC. The emergence of metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 2.Fu CH, Yang CC, Lin CL, Kuo TB. Alteration of cardiovascular autonomic functions by vegetarian diets in postmenopausal women is related to LDL cholesterol levels. Chin J Physiol. 2008;51:100–5. [PubMed] [Google Scholar]
- 3.Pellizzer AM, Straznicky NE, Lim S, Kamen PW, Krum H. Reduced dietary fat intake increases parasympathetic activity in healthy premenopausal women. Clin Exp Pharmacol Physiol. 1999;26:656–60. doi: 10.1046/j.1440-1681.1999.03103.x. [DOI] [PubMed] [Google Scholar]
- 4.Phehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at Menopause: A controlled longitudinal study. Ann Intern Med. 1995;123:673–5. doi: 10.7326/0003-4819-123-9-199511010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Kirschner MA, Samojilk E, Drejka M, Sjmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990;70:473–9. doi: 10.1210/jcem-70-2-473. [DOI] [PubMed] [Google Scholar]
- 6.Nagpal S, Walia L, Lata H, Sood N, Ahuja GK. Effect of exercise on rate pressure product in premenopausal and postmenopausal women with coronary artery disease. Indian J Physiol Pharmacol. 2007;51:279–83. [PubMed] [Google Scholar]
- 7.Ferra CM, Lynch NA, Nicklas BJ, Ryan AS, Berman DM. Difference in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinal Metab. 2002;87:4166–70. doi: 10.1210/jc.2001-012034. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, Matsumoto T, Akiyoshi M, Owa Y, Miyasaka N, Aso T, et al. Body fat and blood lipids in postmenopausal women are related to resting autonomic nervous system activity. Eur J Appl Physiol. 2006;97:542–7. doi: 10.1007/s00421-006-0207-8. [DOI] [PubMed] [Google Scholar]
- 9.Pouliot MC, Despress JP, Lemicux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagital diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 10.Guízar JM, Ahuatzin R, Amador N, Sanchezg G, Romer G. Heart autonomic functions in overweight adolescents. Indian Pediatr. 2005;42:464–8. [PubMed] [Google Scholar]
- 11.Després JP, Moorjani S, Lupien PJ, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 12.Maser RE, Lenhard MJ, Irgau I, Wynn GM. Impact of surgically induced weight loss on cardiovascular autonomic function. Obesity(Silver Spring) 2007;15:364–9. doi: 10.1038/oby.2007.554. [DOI] [PubMed] [Google Scholar]
- 13.WHO/IASO/IOTF. Melbourne: Health Communications Australia; 2000. The Asia-Pacific perspective: Redefining obesity and its treatment. [Google Scholar]
- 14.Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. 16th ed. 1 and 2. New York: McGraw-Hill Inc; 2005. Harrison's principle of internal medicine. 28, 43, 44, 234, 422, 571, 1423, 2074, 2201, 2209, 2294-5 and 2428-32. [Google Scholar]
- 15.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–8. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torsvik M, Haggblom A, Eide GE, Schmutzhard E, Vetvik K, Winkler AS. Cardiovascular autonomic function tests in an African population. BMC Endocr Disord. 2008;8:19–28. doi: 10.1186/1472-6823-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park K. Jabalpur, India: Bhanot; 2009. Park's Textbook of preventive and social medicine; pp. 315–22. (742-56). [Google Scholar]
- 18.Nina E, Lambert G, Wiesner G, Kaye D, Schlaich M, Morris M, et al. Extra adipocyte leptin release in human obesity and its relation to sympathoadrenal function. Am J Physiol Endocrinol Metab. 2004;286:1–32. doi: 10.1152/ajpendo.00489.2003. [DOI] [PubMed] [Google Scholar]
- 19.Rayner DV. The sympathetic nervous system in white adipose tissue regulation. Proc Nutr Soc. 2001;60:357–64. doi: 10.1079/pns2001101. [DOI] [PubMed] [Google Scholar]
- 20.Laederach-Hofmann K, Mussgay L, Ru H. Autonomic cardiovascular regulation in obesity. J Endocrinol. 2000;164:59–66. doi: 10.1677/joe.0.1640059. [DOI] [PubMed] [Google Scholar]
- 21.Rashmi V, Raval KV, Nirupama D. Short communication effect of Raja Yoga Meditation on the lipid profile of postmenopausal women. Indian J Physiol Pharmacol. 2008;52:420–4. [PubMed] [Google Scholar]


