Abstract
The Ocular Response Analyzer (ORA) (Reichert Ophthalmic Instruments, Buffalo, NY) allows direct measurement of corneal biomechanical properties. Since its introduction, many studies have sought to elucidate the clinical applications of corneal hysteresis (CH) and corneal resistance factor (CRF). More recently, detailed corneal deformation signal waveform analysis (WA) has potentially expanded the diagnostic capabilities of the ORA. In this review, the role of CH, CRF, and WA are examined in keratoconus (KC) and iatrogenic ectasia (IE). The PubMed database was searched electronically for peer-reviewed literature in July 2012 and August 2012 without date restrictions. The search strategy included medical subject heading (MeSH) and natural language terms to retrieve references on corneal biomechanics, CH, CRF, corneal deformation signal WA, IE, and KC. The evidence suggests that while CH and CRF are poor screening tools when used alone, increased sensitivity and specificity of KC and IE screening result when these parameters are combined with tomography and topography. Recent advances in WA are promising, but little is currently understood about its biomechanical and clinical relevance. Future studies should seek to refine the screening protocols for KC and IE as well as define the clinical applicability of WA parameters.
Keywords: Corneal hysteresis, corneal deformation signal waveform analysis, corneal resistance factor, keratoectasia, keratoconus, ocular response analyzer
Introduction
Since the introduction of the ocular response analyzer (ORA) (Reichert Ophthalmic Instruments, Buffalo, NY) as a means of measuring corneal biomechanical properties, many studies have sought to elucidate the clinical applications of such parameters as corneal hysteresis (CH) and corneal resistance factor (CRF). More recently, detailed corneal deformation signal waveform analysis (WA) has expanded the potential diagnostic abilities of the ORA. However, questions remain about the clinical usefulness of these parameters.
In this review, the role of CH, CRF, and WA is examined in keratoconus (KC) and iatrogenic ectasia (IE). The PubMed database was searched electronically for peer-reviewed literature in July 2012 and August 2012 without date restrictions. The search strategy included medical subject heading (MeSH) and natural language terms to retrieve references on corneal biomechanics, CH, CRF, corneal deformation signal WA, IE, and KC.
Cornea Anatomy and Structure
The cornea is the transparent anterior portion of the eye through which the iris and pupil can be visualized. The transparency and curvature of the cornea are essential to good vision. The cornea and lens make up the refractive elements of the eye, with the cornea providing approximately 3/4 of its refractive power (≈45 D).[1] The cornea is thickest at the limbus (0.6–0.8 mm) and thins centrally (0.5–0.6 mm), with an aspheric curvature.[2] The hydrated cornea is composed of ≈ 80% water. A hydrophilic proteoglycan extracellular matrix maintains corneal hydration by drawing water into the cornea from the tear film anteriorly and the aqueous posteriorly through a fluid pump mechanism.[1,3]
The cornea is made up of five layers and is mainly composed of collagen. Listed from anterior to posterior, the layers are: Epithelium, Bowman’s membrane, stroma, Descemet’s membrane, and endothelium.[4] The epithelial layer is very thin, with a basement membrane composed of type-IV collagen, and contributes little to the overall strength and curvature of the cornea.[4,5] Bowman’s layer is a thin (8–12 μm), tough layer of irregularly arranged type V collagen fibers that protects the stroma.[4,5] The corneal stroma makes up approximately 90% of the corneal thickness and is composed of highly organized collagen fibrils (type I > type III, type V) with their orientation maintained by a proteoglycan matrix.[4,5] Collagen fibrils are organized into bundles called lamellae which vary in number across the cornea from 300 centrally to 500 at the limbus.[6] The anterior lamellae of the stroma take random courses from the limbus, often interweaving with and terminating in the collagen of Bowman’s membrane.[7] Posterior lamellae have a more ordered organization, running from limbus-to-limbus along the superior-inferior or nasal-temporal meridians.[8–10] At the limbus, stromal and scleral collagen interweave in a circumferential manner, which accounts for an increase in corneal thickness in this region.[11] Descemet’s membrane is composed of type IV collagen and is flexible and extensible, preventing the transmission of stromal stress to the endothelium.[4,5]
The cornea is a dynamic tissue, with structural changes resulting from modified hydration, advancing age, and pathology. Changes in corneal hydration result in a loss of transparency due to changes in fibril spacing. The ability of the cornea to accommodate hydration through altered fibril spacing allows the cornea to swell rapidly. Collagen deposition and the formation of glycation-induced cross-links between fibrils occur throughout life.[12,13] These processes result in an increased cross-sectional area of fibrils and progressive corneal stiffening with age.[14] Since glycation-induced cross-linking is also increased in hyperglycemic states, diabetic patients can develop increased corneal stiffness.[15,16]
Material Properties of the Cornea
Freidenwald first described the viscoelastic properties of the cornea in 1937, followed by Nyquist and Woo.[17,18] Elasticity refers to the ability of a substance to deform reversibly under stress. Viscous materials, on the other hand flow when an external shear force is applied and do not regain their original shape when the force is removed. Viscoelastic materials exhibit characteristics of both viscosity and elasticity. The terms hysteresis, creep, and stress relaxation describe the viscoelastic properties of the cornea.[19] Hysteresis describes the biomechanical response of a viscoelastic material to stress and is a measure of the energy dissipated by the material.[19] Thus, CH represents the energy absorbed by the cornea during applanation. Viscoelastic creep refers to the elongation that occurs over time with prolonged exposure to stress.[19] A clinical application of this property may be the progression of corneal ectasia, in which viscoelastic creep results from the sustained stress of intraocular pressure (IOP).[19,20] Viscoelastic stress relaxation describes the slow relaxation of load when a material is exposed to constant strain.[19]
The elastic properties of the cornea can be quantified using extensiometry, an ex vivo method of measuring corneal stiffness which allows calculation of the elastic modulus (Young’s modulus). Young’s modulus is calculated using the slope of stress over strain.[19] Because corneal stiffness increases as the load increases, the stress versus strain relationship is non-linear. Thus, values of elastic modulus are expressed as a function of load. However, variability in experimental conditions between studies (tissue hydration, loading techniques, etc.) has resulted in the reported values of corneal elasticity spanning orders of magnitude.[19,21]
Corneal shear strength results from collagen interweaving and other interlamellar cohesive forces which prevent stromal sublayers from sliding and bending.[19,22] While corneal shear strength is much lower than corneal tensile strength, it is important for maintaining cornea shape after refractive surgery and may play a role in ectasia.[19,23,24]
The cornea also exhibits anisotropic qualities, meaning it behaves differently when stress is applied in various directions.[1] A lamella under stress will flatten in the direction of the applied load as well as both directions perpendicular to the load.[19] The relationship between transverse and axial strain is represented by Poisson’s ratio (v). Poisson’s ratio is commonly used as a constant in calculations, but it is also a physical property of the tissue which potentially plays a role in iatrogenic ectasia.[19]
Measurement of Biomechanical Properties
The ORA (ORA; Reichert Inc., Depew, New York), described by Luce, is the only commercially available medical device capable of measuring corneal biomechanics in vivo.[25] The ORA is an air-puff tonometer that uses bidirectional applanation to measure IOP. An infrared beam is used to track changes in the shape of the anterior cornea during inward and outward deviation, and the resulting waveform allows the assessment of biomechanical parameters.
The ORA measurement process gives the following results [Table 1]:
Table 1.
Description of optical response analyzer data
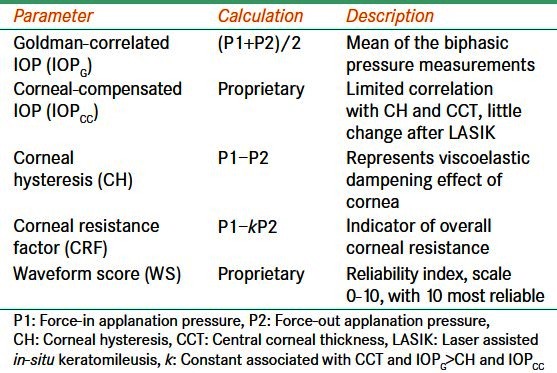
Goldmann-correlated IOP (IOPG): This value is the mean of the biphasic pressure measurements.[26,27]
Corneal-compensated IOP (IOPCC): This value is a more accurate indicator of true IOP than the IOPG because it is less affected by corneal properties such as CCT and CH.[26,27] It has little correlation with CCT in normal eyes and remains fairly unchanged after laser-assisted in situ keratomileusis (LASIK).[25,28,29]
CH: This value is calculated as the difference between force-in applanation (P1) and force-out applanation (P2) as in the formula (P1 − P2).[26,27] CH is related to the viscoelastic dampening effect of the corneal tissue and has a low correlation with CCT.[30]
CRF: This value is derived using the formula (P1 − k P2).[26,27] The constant, k, was developed through empirical evaluation of the relationship between P1 and P2 and CCT, such that the value of k is more strongly associated with CCT and IOPG, and not with IOPCC or CH.[26,27] Thus, CRF is an indicator of overall corneal resistance that is relatively independent of IOP or the combined viscous dampening and elastic resistance behaviors of the cornea.[25]
Deformation signal waveform: The biomechanical waveform produced by the corneal deformation signal provides a unique description of each eye.[27] Software available with newer versions of the ORA allows detailed analysis of the deformation signal waveform by providing 37 parameters, each describing a morphological feature of the waveform [Table 2].[27,31–34] Several studies have investigated the clinical relevance of these waveform parameters.[31–34] However, their clinical significance is currently unknown. In addition, the manner in which individual parameters represent biomechanical properties is unknown.
Waveform score (WS): Newer versions of the ORA software have also incorporated a reliability index known as a waveform score.[32] The WS is reported on a scale from 0 to 10 with higher numbers representing more reliable measurement data.[35] Up to four measurements can be stored at a time and the most reliable signal (the one with the highest WS) is highlighted as the best score value (BSV).[32] Several studies have sought to characterize the reproducibility of data at various WS cut-off values (ranging from 3.5 to 7.0).[32,35–37] Variations in the results of these studies can be explained by the use of differing models for threshold estimation and differences in study population, including age. Recently, Ayala et al., in a study of 266 eyes, reported that signals with a WS of seven or below render less reliable results, and that several measurements with a WS above seven should be obtained.[37]
Table 2.
Description of corneal deformation signal waveform parameters [Figure 1]
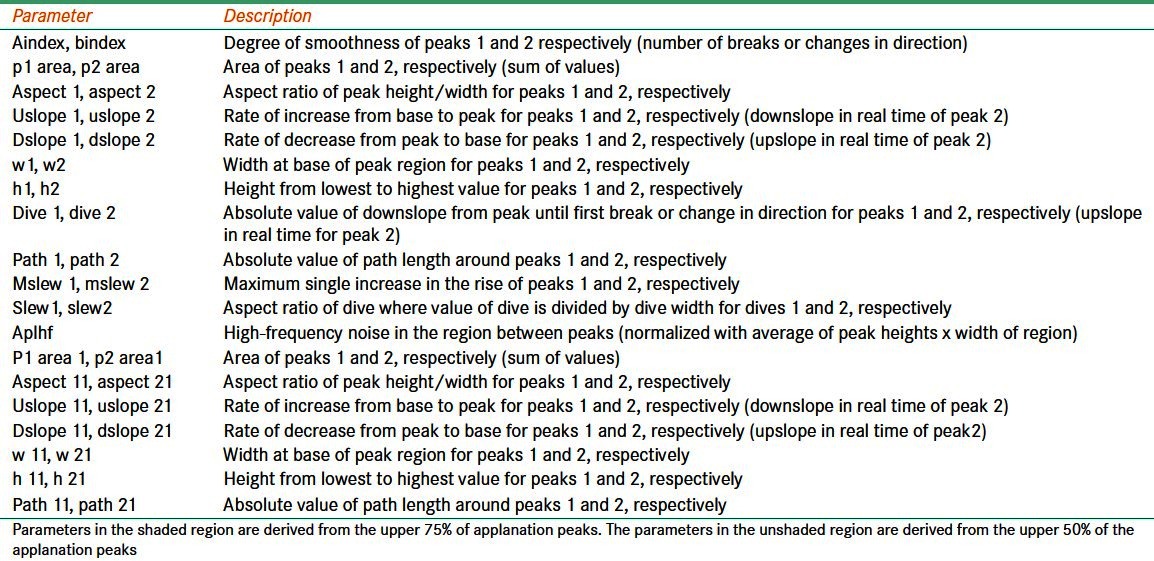
Figure 1.
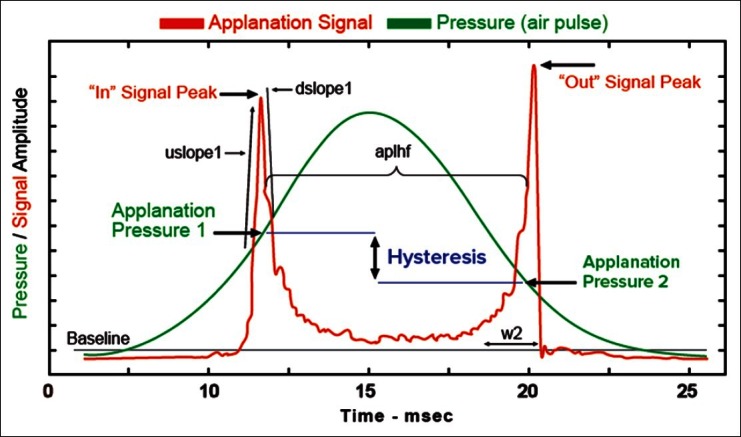
Corneal deformation signal waveform
Iatrogenic Corneal Ectasia
Iatrogenic corneal ectasia is a rare complication of refractive surgery with an incidence of 0.2-0.66%.[38,39] Corneal thinning caused by ablation and flap creation is thought to weaken the cornea leading to biomechanical degeneration from delamination and interfibrillar fracture.[40] The result is a very thin stromal bed (<300 μm) and progressive steepening with myopic shift, irregular astigmatism, and reduced visual acuity.[38,39] The main risk-factor for iatrogenic ectasia is irregular topography pre-operatively, including asymmetric inferior corneal steepening or asymmetric bowtie patterns with skewed steep radial axes above and below the horizontal meridian.[41,42] Additional risk-factors include, a thin residual stromal bed (225-250 μm), high myopia, young age at operation, and deep ablation.[41,42]
Forme fruste keratoconus (FFK), a latent biomechanical instability which can be activated by refractive surgery, is the main cause of corneal ectasia.[43–45] Effective pre-operative screening is essential because the incidence of FFK is higher among those seeking refractive surgery than the general population and the time course of disease progression is accelerated when induced by surgery.[46–48]
Studies demonstrate that CH and CRF are decreased in FFK, but the clinical usefulness of these parameters is diminished by low specificity and sensitivity.[49] Current ectasia risk assessment models utilize multiple diagnostic methods in an effort to increase screening effectiveness.
The ectasia risk score system (ERSS) utilizes placido-disk based corneal topography and CCT combined with patient age and the anticipated level of excimer correction to assess ectasia risk. Initially, ERSS was validated with a reported sensitivity and specificity of 92% and 94%, respectively, although, more recent external validation showed a significant proportion of missed at-risk patients using this method.[50,51] The addition of corneal biomechanical data to this model would likely increase its effectiveness.
Kozobolis et al. recently reported high accuracy in differentiating FFK eyes from normal eyes using a diagnostic model, which combined biomechanical and tomographic data. These findings are similar to the screening criteria of the Rio de Janeiro corneal tomography and biomechanical study group, which utilizes three-dimensional corneal tomography and biomechanical analysis in screening.[49]
In a recent study of WA by Toboul et al., a negative predictive value of 99.7 was reported if any one of six corneal biomechanical parameters found to be associated with KC was above threshold (parameters associated with KC were CRF, P1, P2, time 1, pmax, and tpmax).[52] If this report is accurate, the probability of a patient with one of these parameters above threshold presenting with KC is 3 in 1000 instead of the 9 in 1000 prevalence in a normal LASIK cohort.[52]
CH and CRF are important in the differentiation of healthy and diseased corneas. While the ability of CH and CRF to discriminate individual pathologies is limited by low specificity and sensitivity, evidence suggests that these parameters enhance the diagnostic capability of screening programs when combined with other studies. We suggest screening protocols utilize CH and CRF in addition to tomographic and topographic evaluation in order to maximize the sensitivity and specificity of ectasia risk assessment.[53,54] Deformation signal WA shows promise in detecting subtle changes associated with FFK and could be useful in pre-operative assessment of ectasia risk. However, WA is still poorly understood and requires validation before it can be broadly accepted. Future studies should focus on improving screening protocols and further developing the clinical applications of WA.
Keratoconus
Keratoconus is a progressive ectatic disorder of the cornea characterized by bilateral, asymmetric, non-inflammatory degeneration, which results in central and paracentral thinning and protrusion. Progression of the disease is thought to begin with focal degeneration of material properties followed by slippage of collagen fibrils, changes in the stromal extracellular matrix, and a cycle of thinning, increased strain, and redistribution of stress.[23,55] The native collagen network is mostly unorganized in keratoconus with decreased fibrillar interweaving, although collagen composition, distribution, and packing appear normal.[23] Irregularities in the corneal collagen network result in distortion of refractive function which can lead to high myopia and irregular astigmatism. Keratoconic corneas also exhibit increased levels of collagenolysis, loss of keratocytes, and reduced collagen cross-links.[56,57] Risk-factors contributing to the biomechanical degeneration of keratoconic corneas include genetics, contact lens microtrauma, eye rubbing, and atopy.[58,59]
Studies consistently report that CH and CRF are lower in keratoconus than in normal and post-LASIK corneas, although there is a considerable overlap with normal control eyes.[25,30,45,60–67] Higher grade keratoconus also correlates with lower CH and CRF values, and the difference of CH and CRF (CH-CRF) is more positive as disease severity worsens.[61]
Galletti et al. recently reported improved sensitivity and specificity of CRF in detecting keratoconus, even in the topographically normal fellow eyes of keratoconus patients, when the effect of CCT was eliminated through stratification.[68]
Kara et al. reported significantly lower CH and CRF in topographically normal relatives of patients with keratoconus.[69] Given the increased incidence of keratoconus among relatives, this study suggests that a decrease in CH and CRF may be an early indicator of biomechanical instability, preceding the subtle topographic changes commonly used in keratoconus screening.
Studies of WA have sought to establish parameters for distinguishing keratoconic from normal eyes. Zarei-Ghanavati et al. reported that CH and 6 corneal deformation signal waveform parameters (P1area, uslope1, dslop1, w2, aindex, and aplhf) had the best statistical ability to differentiate between post-femtosecond LASIK and keratoconic corneas.[34] Wolffsohn et al. also reported increased sensitivity and only a mild decrease in specificity (3%) of keratoconus detection and severity prediction by incorporating waveform parameters in addition to baseline pachymetric and keratometric assessment.[33] As discussed previously, the biomechanical correlation of these waveform parameters is currently unknown, however, these studies illustrate the potential role of WA in KC screening and its ability to distinguish between corneas altered by disease and surgery. With the emergence of treatments like collagen cross-linking, which can effectively suspend the progression of keratoconus, the ability to detect subtle biomechanical changes in very early keratconus will eliminate the need for advanced keratoconus treatments like penetrating keratoplasty.
The evidence suggests that CH and CRF, when used in combination with other parameters, are useful in assessing early biomechanical changes in KC. However, when used alone these parameters have low sensitivity and specificity. Corneal deformation signal WA is a new field with great potential in detecting early KC. However, the biomechanical significance of these parameters is undefined. Future studies should be directed at further developing the clinical application of WA parameters and their ability to assist in the diagnosis of keratoconus.
Limitations in Measuring Keratoconus
Comparison of keratoconus and normal eyes with the ORA has limitations. Keratoconus is associated with decentralized irregularities which may be missed by ORA since analysis occurs in the central 3-4 mm of cornea. The ORA can also confuse corneal tissue response with surface response since a specular reflection is required to measure applanation pressure. Central corneal surface irregularities, corneal scars, and epithelial ulceration can also cause light scatter which could interfere with the ORA’s infrared specular reflection beam, altering the waveform. Given these limitations, the importance of reliability cannot be over-emphasized. Future studies should examine the reliability and usefulness of WS in assessing keratoconic versus normal corneas.
Conclusion
The ORA allows direct analysis of corneal biomechanical properties including CH, CRF, and WA. While many studies have demonstrated significant changes in CH and CRF in various disease states, the clinical usefulness of these parameters is limited by low sensitivity and specificity. However, CH and CRF play an important role in screening protocols, where they improve the sensitivity and specificity of tomography and topography studies in assessing pre-operative ectasia risk. Corneal deformation signal WA is an exciting frontier. Early studies offer promising results in detecting subtle changes in FFK, post-LASIK, and KC corneas. Further research is needed to clarify the role of WA clinically, as well as elucidating the biomechanical significance of WA parameters.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Ruberti JW, Roy AS, Roberts CJ. Corneal biomechanics and biomaterials. Annu Rev Biomed Eng. 2011;13:269–95. doi: 10.1146/annurev-bioeng-070909-105243. [DOI] [PubMed] [Google Scholar]
- 2.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 3.Fischbarg J, Maurice DM. An update on corneal hydration control. Exp Eye Res. 2004;78:537–41. doi: 10.1016/j.exer.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 4.DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–98. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Ihanamäki T, Pelliniemi LJ, Vuorio E. Collagens and collagen-related matrix components in the human and mouse eye. Prog Retin Eye Res. 2004;23:403–34. doi: 10.1016/j.preteyeres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–58. [PubMed] [Google Scholar]
- 7.Müller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–43. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abahussin M, Hayes S, Knox Cartwright NE, Kamma-Lorger CS, Khan Y, Marshall J, et al. 3D collagen orientation study of the human cornea using X-ray diffraction and femtosecond laser technology. Invest Ophthalmol Vis Sci. 2009;50:5159–64. doi: 10.1167/iovs.09-3669. [DOI] [PubMed] [Google Scholar]
- 9.Meek KM, Blamires T, Elliott GF, Gyi TJ, Nave C. The organisation of collagen fibrils in the human corneal stroma: A synchrotron X-ray diffraction study. Curr Eye Res. 1987;6:841–6. doi: 10.3109/02713688709034853. [DOI] [PubMed] [Google Scholar]
- 10.Meek KM, Quantock AJ. The use of X-ray scattering techniques to determine corneal ultrastructure. Prog Retin Eye Res. 2001;20:95–137. doi: 10.1016/s1350-9462(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 11.Newton RH, Meek KM. The integration of the corneal and limbal fibrils in the human eye. Biophys J. 1998;75:2508–12. doi: 10.1016/S0006-3495(98)77695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM. Ageing of the human corneal stroma: Structural and biochemical changes. Biochim Biophys Acta. 1992;1138:222–8. doi: 10.1016/0925-4439(92)90041-k. [DOI] [PubMed] [Google Scholar]
- 13.Daxer A, Misof K, Grabner B, Ettl A, Fratzl P. Collagen fibrils in the human corneal stroma: Structure and aging. Invest Ophthalmol Vis Sci. 1998;39:644–8. [PubMed] [Google Scholar]
- 14.Elsheikh A, Wang D, Brown M, Rama P, Campanelli M, Pye D. Assessment of corneal biomechanical properties and their variation with age. Curr Eye Res. 2007;32:11–9. doi: 10.1080/02713680601077145. [DOI] [PubMed] [Google Scholar]
- 15.Seiler T, Huhle S, Spoerl E, Kunath H. Manifest diabetes and keratoconus: A retrospective case-control study. Graefes Arch Clin Exp Ophthalmol. 2000;238:822–5. doi: 10.1007/s004179900111. [DOI] [PubMed] [Google Scholar]
- 16.Kuo IC, Broman A, Pirouzmanesh A, Melia M. Is there an association between diabetes and keratoconus? Ophthalmology. 2006;113:184–90. doi: 10.1016/j.ophtha.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Nyquist GW. Rheology of the cornea: Experimental techniques and results. Exp Eye Res. 1968;7:183–8. doi: 10.1016/s0014-4835(68)80064-8. [DOI] [PubMed] [Google Scholar]
- 18.Woo SL, Kobayashi AS, Lawrence C, Schlegel WA. Mathematical model of the corneo-scleral shell as applied to intraocular pressure-volume relations and applanation tonometry. Ann Biomed Eng. 1972;1:87–98. doi: 10.1007/BF02363420. [DOI] [PubMed] [Google Scholar]
- 19.Dupps WJ, Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–20. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupps WJ., Jr Biomechanical modeling of corneal ectasia. J Refract Surg. 2005;21:186–90. doi: 10.3928/1081-597X-20050301-15. [DOI] [PubMed] [Google Scholar]
- 21.Bryant MR, McDonnell PJ. Constitutive laws for biomechanical modeling of refractive surgery. J Biomech Eng. 1996;118:473–81. doi: 10.1115/1.2796033. [DOI] [PubMed] [Google Scholar]
- 22.Smolek MK. Interlamellar cohesive strength in the vertical meridian of human eye bank corneas. Invest Ophthalmol Vis Sci. 1993;34:2962–9. [PubMed] [Google Scholar]
- 23.Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948–56. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 24.Edmund C. Corneal topography and elasticity in normal and keratoconic eyes. A methodological study concerning the pathogenesis of keratoconus. Acta Ophthalmol Suppl. 1989;193:1–36. [PubMed] [Google Scholar]
- 25.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–62. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Kotecha A. What biomechanical properties of the cornea are relevant for the clinician? Surv Ophthalmol. 2007;52:S109–14. doi: 10.1016/j.survophthal.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Franco S, Lira M. Biomechanical properties of the cornea measured by the ocular response analyzer and their association with intraocular pressure and the central corneal curvature. Clin Exp Optom. 2009;92:469–75. doi: 10.1111/j.1444-0938.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 28.Kamiya K, Miyata K, Tokunaga T, Kiuchi T, Hiraoka T, Oshika T. Structural analysis of the cornea using scanning-slit corneal topography in eyes undergoing excimer laser refractive surgery. Cornea. 2004;23:S59–64. doi: 10.1097/01.ico.0000136673.35530.e3. [DOI] [PubMed] [Google Scholar]
- 29.Jaycock PD, Lobo L, Ibrahim J, Tyrer J, Marshall J. Interferometric technique to measure biomechanical changes in the cornea induced by refractive surgery. J Cataract Refract Surg. 2005;31:175–84. doi: 10.1016/j.jcrs.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 30.Touboul D, Roberts C, Kérautret J, Garra C, Maurice-Tison S, Saubusse E, et al. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg. 2008;34:616–22. doi: 10.1016/j.jcrs.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Kerautret J, Colin J, Touboul D, Roberts C. Biomechanical characteristics of the ectatic cornea. J Cataract Refract Surg. 2008;34:510–3. doi: 10.1016/j.jcrs.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Lam AK, Chen D, Tse J. The usefulness of waveform score from the ocular response analyzer. Optom Vis Sci. 2010;87:195–9. doi: 10.1097/OPX.0b013e3181d1d940. [DOI] [PubMed] [Google Scholar]
- 33.Wolffsohn JS, Safeen S, Shah S, Laiquzzaman M. Changes of corneal biomechanics with keratoconus. Cornea. 2012;31:849–54. doi: 10.1097/ICO.0b013e318243e42d. [DOI] [PubMed] [Google Scholar]
- 34.Zarei-Ghanavati S, Ramirez-Miranda A, Yu F, Hamilton DR. Corneal deformation signal waveform analysis in keratoconic versus post-femtosecond laser in situ keratomileusis eyes after statistical correction for potentially confounding factors. J Cataract Refract Surg. 2012;38:607–14. doi: 10.1016/j.jcrs.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Mandalos A, Anastasopoulos E, Makris L, Dervenis N, Kilintzis V, Topouzis F. Inter-examiner Reproducibility of Ocular Response Analyzer Using the Waveform Score Quality Index in Healthy Subjects. J Glaucoma. 2013;22:152–5. doi: 10.1097/IJG.0b013e318227e63e1. [DOI] [PubMed] [Google Scholar]
- 36.Ehrlich JR, Haseltine S, Shimmyo M, Radcliffe NM. Evaluation of agreement between intraocular pressure measurements using Goldmann applanation tonometry and Goldmann correlated intraocular pressure by Reichert’s ocular response analyser. Eye (Lond) 2010;24:1555–60. doi: 10.1038/eye.2010.83. [DOI] [PubMed] [Google Scholar]
- 37.Ayala M, Chen E. Measuring corneal hysteresis: Threshold estimation of the waveform score from the Ocular Response Analyzer. Graefes Arch Clin Exp Ophthalmol. 2012;250:1803–6. doi: 10.1007/s00417-012-2053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rad AS, Jabbarvand M, Saifi N. Progressive keratectasia after laser in situ keratomileusis. J Refract Surg. 2004;20:S718–22. doi: 10.3928/1081-597X-20040903-18. [DOI] [PubMed] [Google Scholar]
- 39.Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1796–802. doi: 10.1016/s0886-3350(01)01090-2. [DOI] [PubMed] [Google Scholar]
- 40.Dawson DG, Randleman JB, Grossniklaus HE, O’Brien TP, Dubovy SR, Schmack I, et al. Corneal ectasia after excimer laser keratorefractive surgery: Histopathology, ultrastructure, and pathophysiology. Ophthalmology. 2008;115:2181–2191. doi: 10.1016/j.ophtha.2008.06.008. e1. [DOI] [PubMed] [Google Scholar]
- 41.Winkler von Mohrenfels C, Salgado JP, Khoramnia R. Keratectasia after refractive surgery. Klin Monbl Augenheilkd. 2011;228:704–11. doi: 10.1055/s-0029-1245754. [DOI] [PubMed] [Google Scholar]
- 42.Randleman JB. Post-laser in-situ keratomileusis ectasia: Current understanding and future directions. Curr Opin Ophthalmol. 2006;17:406–12. doi: 10.1097/01.icu.0000233963.26628.f0. [DOI] [PubMed] [Google Scholar]
- 43.O’Keefe M, Kirwan C. Laser epithelial keratomileusis in 2010 - a review. Clin Experiment Ophthalmol. 2010;38:183–91. doi: 10.1111/j.1442-9071.2010.02198.x. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–73. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 45.Schweitzer C, Roberts CJ, Mahmoud AM, Colin J, Maurice-Tison S, Kerautret J. Screening of forme fruste keratoconus with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2010;51:2403–10. doi: 10.1167/iovs.09-3689. [DOI] [PubMed] [Google Scholar]
- 46.Binder PS. Analysis of ectasia after laser in situ keratomileusis: Risk factors. J Cataract Refract Surg. 2007;33:1530–8. doi: 10.1016/j.jcrs.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 47.Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. 1998;24:1007–9. doi: 10.1016/s0886-3350(98)80057-6. [DOI] [PubMed] [Google Scholar]
- 48.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 49.Kozobolis V, Sideroudi H, Giarmoukakis A, Gkika M, Labiris G. Corneal biomechanical properties and anterior segment parameters in forme fruste keratoconus. Eur J Ophthalmol. 2012;22:920–30. doi: 10.5301/ejo.5000184. [DOI] [PubMed] [Google Scholar]
- 50.Randleman JB, Trattler WB, Stulting RD. Validation of the ectasia risk score system for preoperative laser in situ keratomileusis screening. Am J Ophthalmol. 2008;145:813–8. doi: 10.1016/j.ajo.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan CC, Hodge C, Sutton G. External analysis of the Randleman Ectasia Risk Factor Score System: A review of 36 cases of post LASIK ectasia. Clin Experiment Ophthalmol. 2010;38:335–40. doi: 10.1111/j.1442-9071.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 52.Touboul D, Bénard A, Mahmoud AM, Gallois A, Colin J, Roberts CJ. Early biomechanical keratoconus pattern measured with an ocular response analyzer: Curve analysis. J Cataract Refract Surg. 2011;37:2144–50. doi: 10.1016/j.jcrs.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 53.Belin MW, Ambrósio R., Jr Corneal ectasia risk score: Statistical validity and clinical relevance. J Refract Surg. 2010;26:238–40. doi: 10.3928/1081597X-20100318-01. [DOI] [PubMed] [Google Scholar]
- 54.Randleman JB. Evaluating risk factors for ectasia: What is the goal of assessing risk? J Refract Surg. 2010;26:236–7. doi: 10.3928/1081597X-20100318-02. [DOI] [PubMed] [Google Scholar]
- 55.Dauwe C, Touboul D, Roberts CJ, Mahmoud AM, Kérautret J, Fournier P, et al. Biomechanical and morphological corneal response to placement of intrastromal corneal ring segments for keratoconus. J Cataract Refract Surg. 2009;35:1761–7. doi: 10.1016/j.jcrs.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 56.Bron AJ. Keratoconus. Cornea. 1988;7:163–9. [PubMed] [Google Scholar]
- 57.Zimmermann DR, Fischer RW, Winterhalter KH, Witmer R, Vaughan L. Comparative studies of collagens in normal and keratoconus corneas. Exp Eye Res. 1988;46:431–42. doi: 10.1016/s0014-4835(88)80031-9. [DOI] [PubMed] [Google Scholar]
- 58.McMonnies CW. The evidentiary significance of case reports: Eye rubbing and keratoconus. Optom Vis Sci. 2008;85:262–9. doi: 10.1097/OPX.0b013e318169287a. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiological study of keratoconus: Evidence for major gene determination. Am J Med Genet. 2000;93:403–9. [PubMed] [Google Scholar]
- 60.Ortiz D, Piñero D, Shabayek MH, Arnalich-Montiel F, Alió JL. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J Cataract Refract Surg. 2007;33:1371–5. doi: 10.1016/j.jcrs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 61.Shah S, Laiquzzaman M, Bhojwani R, Mantry S, Cunliffe I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci. 2007;48:3026–31. doi: 10.1167/iovs.04-0694. [DOI] [PubMed] [Google Scholar]
- 62.Fontes BM, Ambrósio R, Jr, Velarde GC, Nosé W. Corneal biomechanical evaluation in healthy thin corneas compared with matched keratoconus cases. Arq Bras Oftalmol. 2011;74:13–6. doi: 10.1590/s0004-27492011000100003. [DOI] [PubMed] [Google Scholar]
- 63.Saad A, Lteif Y, Azan E, Gatinel D. Biomechanical properties of keratoconus suspect eyes. Invest Ophthalmol Vis Sci. 2010;51:2912–6. doi: 10.1167/iovs.09-4304. [DOI] [PubMed] [Google Scholar]
- 64.Kirwan C, O’Malley D, O’Keefe M. Corneal hysteresis and corneal resistance factor in keratoectasia: Findings using the Reichert ocular response analyzer. Ophthalmologica. 2008;222:334–7. doi: 10.1159/000145333. [DOI] [PubMed] [Google Scholar]
- 65.Mollan SP, Wolffsohn JS, Nessim M, Laiquzzaman M, Sivakumar S, Hartley S, et al. Accuracy of Goldmann, ocular response analyser, Pascal and TonoPen XL tonometry in keratoconic and normal eyes. Br J Ophthalmol. 2008;92:1661–5. doi: 10.1136/bjo.2007.136473. [DOI] [PubMed] [Google Scholar]
- 66.Shah S, Laiquzzaman M. Comparison of corneal biomechanics in pre and post-refractive surgery and keratoconic eyes by ocular response analyser. Cont Lens Anterior Eye. 2009;32:129–32. doi: 10.1016/j.clae.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Fontes BM, Ambrósio R, Jr, Jardim D, Velarde GC, Nosé W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology. 2010;117:673–9. doi: 10.1016/j.ophtha.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 68.Galletti JG, Pförtner T, Bonthoux FF. Improved keratoconus detection by ocular response analyzer testing after consideration of corneal thickness as a confounding factor. J Refract Surg. 2012;28:202–8. doi: 10.3928/1081597X-20120103-03. [DOI] [PubMed] [Google Scholar]
- 69.Kara N, Altinkaynak H, Baz O, Goker Y. Biomechanical Evaluation of Cornea in Topographically Normal Relatives of Patients With Keratoconus. Cornea. 2013;32:262–6. doi: 10.1097/ICO.0b013e3182490924. [DOI] [PubMed] [Google Scholar]


