Abstract
Background:
We present outcomes of Ahmed Glaucoma Valve (AGV) implantation in treating refractory glaucoma in a tertiary hospital in Oman. Refractory glaucoma was defined as previously failed conventional glaucoma surgery and an uncontrolled intraocular pressure (IOP) of more than 21 mm Hg despite treatment with three topical and/or oral therapy.
Materials and Methods:
This historical cohort study was conducted in 2010. Details of medical and surgical treatment were recorded. Ophthalmologists examined eyes and performed glaucoma surgeries using AGV. The best corrected distant vision, IOP, and glaucoma medications were prospectively reviewed on 1st day, 1st, 6th, 12th week postoperatively, and at the last follow up.
Result:
Glaucoma specialists examined and treated 40 eyes with refractory glaucoma of 39 patients (20 males + 19 females). Neo-vascular glaucoma was present in 23 eyes. Vision before surgery was <3/60 in 21 eyes. At 12 weeks, one eye had vision better than 6/12, seven eyes had vision 6/18 to 6/60, and eight eyes had vision 6/60 to 3/60. Mean IOP was reduced from 42.9 (SD 16) to 14.2 (SD 8) and 19.1 (SD 7.8) mmHg at one and 12 weeks after surgery, respectively. At 12 weeks, five (12.5%) eyes had IOP controlled without medication. In 33 (77.5%) eyes, pressure was controlled by using one or two eye drops. The mean number of preoperative anti-glaucoma medications (2.38; SD 1.1) was reduced compared to the mean number of postoperative medications (1.92; SD 0.9) at 12 weeks.
Conclusion:
We succeeded in reducing visual disabilities and the number of anti-glaucoma medications used to treat refractory glaucoma by AGV surgery.
Keywords: Ahmed glaucoma valve, blindness, complications, glaucoma surgery, intraocular pressure, refractory glaucoma
Introduction
Glaucoma is a priority disease in the disease control strategy for ’VISION 2020’ initiative to eliminate avoidable blindness.[1] Therefore, an intervention to treat glaucoma and its impact in reducing visual disability will be important.
The prevalence of glaucoma was 4.75% in Oman in 2005. The proportion of open and closed angle glaucoma in the Omani population was 40% each.[2] A public health approach to address visual disabilities due to glaucoma was implemented in all governorates of Oman since 2005.[3] Each governorate hospital has facilities for diagnosis and management of glaucoma. The treatment for glaucoma in Oman is affordable and accessible. However, more people opt for medical management of glaucoma. Compliance for medical treatment was noted to be 56% in patients with glaucoma.[4] Therefore, it is likely that glaucoma patients presenting for surgery could be in advanced stages of visual disabilities and would need more complex types of management.
Glaucoma could be of different types: Congenital, juvenile, primary open angle, primary angle closure, secondary glaucoma, etc., In advanced stages, medications after performance of successful conventional surgeries often fail to control IOP at a level below 21 mm Hg.[5] At such a stage, the glaucoma is termed as refractory to conventional treatment. The conventional methods include trabeculactomy, trabaculotomy, cyclodiathermy of cilliary body by diode laser or cryotherapy. The successful use of AGV to treat refractory glaucoma is widely documented in medical literature.[6–13] However, to our knowledge, very few studies have been conducted in adult Arab population with refractory glaucoma.[14,15] This is the first study conducted in Omani patients with refractory glaucoma. The purpose of this study was to evaluate in of the short term efficacy in reducing intraocular pressure and medications three months following AGV implantation, in patients with refractory glaucoma.
Materials and Methods
This was a historical cohort study. Those identified with refractory glaucoma visiting and planned for AGV implant surgery in a tertiary hospital of Oman between January 2005 and December 2009 were recruited. Their past information was collected from case records. Their surgical and post-operative information were collected prospectively. Although the follow up information of patients operated in early part of study was of long duration, we had planned to collect information till 6 months. The study was approved by the Institutional Ethical Committee. Two glaucoma specialists (R.Z., R.M.) performed the assessment and all the surgeries.
During preoperative assessment, intra ocular pressure (IOP) was measured by applanation tonometer (Goldman - USA) that was attached to the slit lamp bio-microscope (Topcon- USA). The distant visual acuity of each eye was noted as presented and then the best corrected visual acuity (BCVA) was noted. A projection Snellen’s chart that was held at six meter distance was used to test the distant visual acuity. If the vision was less than 6/60, the acuity was retested at a three meter distance. Perception of light and projection of rays from all four directions were tested in such cases. Investigators inquired about history of laser and surgical treatments for glaucoma in the past and diagnosis of glaucoma by any ophthalmologist in the past. A detailed anterior segment and posterior segment examination was done using slit lamp bio-microscope to determine the lens status and presence of other ocular co-morbidities. The angle of anterior chamber was examined by using Gonio lens. Information on all glaucoma medications (topical and systemic) was collected through a review of health records, reference letters, and by cross-checking with medications available with the patient. A thorough assessment by a physician was conducted to evaluate systemic status.
AGV (New Wold Medical for adult -model FP7 and for children -model FP8) were implanted in all patients. To collect operative data, operation log books were reviewed. Information collected included type of anesthesia, type of graft used (scleral patch or bovine pericardium), and intra-operative complications.
Surgery was performed using different anesthesia techniques (sub-tenon injection, peri-bulbar injection, and general anesthesia). AGV were implanted in the upper fornix in all cases except in one eye (inferior fornix) due to poor status of the bulbar conjunctiva above the limbus. The plate of the valve was secured to the sclera with 9-0 prolene interrupted sutures 8 to 9 mm posterior to the surgical limbus. The tube was trimmed bevel up, inserted at 2 mm behind the limbus, and placed in the anterior chamber through a 23-gauge needle track. Care was taken to ensure that the tube’s bevel was facing away from iris. The tube was anchored to the sclera using 9-0 prolene suture covered with a rectangular piece of scleral/bovine peri-cardiuial patch graft.[16] The conjunctiva was closed with 8-0 vicryl sutures.
The follow up assessments were conducted on the first day, in the first week, sixth week, and 12 weeks after surgery. If IOP was more than 25 mm Hg, we added medication to reduce IOP. We ensured that medication prescribed on previous visit was instilled on the day of visit before labeling as high IOP due to failed medication. The last follow up visit after three months was noted, and a similar assessment methods were used at all follow up visits. The glaucoma specialists noted postoperative visual acuity, IOP, details of glaucoma medicines (topical and systemic) being used after surgery, and early or late complications.
After the first month, if patient visited within two weeks of the scheduled day of follow up, the information was included in the analysis. The results of the final follow-up examination (IOP, visual acuity, and medications) were included in the analysis to define success of valve implant surgery.
Surgical success was defined as IOP of greater than 5 mmHg and 21 mmHg or less, with or without the use of additional glaucoma medications or additional glaucoma surgery (cyclo-photocoagulation) or occurrence of devastating complications that require removal of the implant) and without loss of light perception following surgery.[17] The criteria for success were defined before the study had commenced. Hypotony was defined as IOP ≤5 mm Hg on two consecutive visits. The reduction in number of medications following glaucoma surgery compared to those prescribed and used to reduce ocular pressure preoperatively was also defined as a success in reducing medication.
A pretested form was used to collect information from all computerized case records. The data was computed using Microsoft XL® spreadsheet. The demographic information included gender, age, and area of residence. The preoperative diagnosis of refractory glaucoma was categorized based on underlying cause that included neovascular glaucoma, glaucoma with aphakia or pseudophakia, primary angle closure glaucoma, post-uveitis and Sturge, Weber syndrome. The data was transferred for analysis into Statistical Package for Social Studies (SPSS 12) (IMB, Chicago, USA). Univariate analysis was carried out using the parametric method to calculate frequencies and percentage proportions of qualitative variables. For quantitative variables, we calculated the means and standard deviations. For statistical validation, we calculated 95% confidence intervals and the two-tailed P values. As the number of observations was few, the IOP was not showing a uniform distribution. Hence, we calculated log values which on plotting were found to have an even distribution. Therefore, we calculated the mean and standard deviation of log values of IOP and then estimated the 95% confidence interval by using Open EP calculator.[18]
The identities of patients were kept confidential by delinking the identity from other variables at the time of analysis. All patients were given treatment free of cost. The outcome of this study was discussed with other ophthalmologists in a national symposium, and suggestions to improve eye care of refractory glaucoma was proposed accordingly. If rubeosis iridis was noted in an eye, pan retinal photocoagulation (PRP) was applied.
Result
Our study comprised of 40 eyes with refractory glaucoma of 39 persons (27 male and 17 females). The mean age of study participants was 53.3 years (SD 15.3 Years). The age difference in male and female participants was not significant (Difference of mean - 3.1 years (95% Confidence Interval -7.6–13.7). All of them were Omani nationals. The characteristics and ocular profile of eyes with refractory glaucoma in our cohort are given in Table 1.
Table 1.
Ocular profile of patients with refractory glaucoma prior to the Ahmed valve surgery
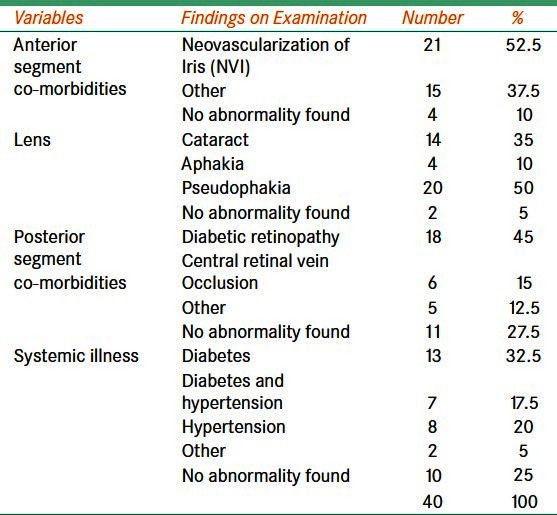
Neo-vascular and aphakic/pseudophakic glaucoma were present in 23 (57.5%) and nine (22.5%) eyes, respectively. One person had bilateral refractory glaucoma of congenital etiology. No eye in our cohort had primary open angle glaucoma. In 30 eyes, AGV surgery was not performed in the past. Diabetes mellitus was present in 19 (48.7%) participants.
Glaucoma treatment was given for less than one year in 24 (60%) eyes, one to five years in 15 (37.5%) eyes, and more than five years in one eye only. Trabeculectomy and trabeculotomy had been performed in seven (17.5%) and three (7.5%) eyes, respectively. In 13 eyes, peripheral iridectomy (PI) was performed in the past. Five eyes had cataract surgery with PI. In seven eyes, cryo therapy was applied in the past. In rest five eyes, surgery was performed, but details were not available. Systemic and topical medications were used prior to AGV implant surgery in 34 (95%) eyes. Three and four types of topical medications were used to control IOP in 10 (25%) and eight (20%) eyes, respectively.
The mean IOP before surgery was 42.9 mmHg (SD 16.0). It was 20.8 mmHg (SD 8.0) on six week follow up of 36 eyes of 36 patients. The mean IOP was 19.1 mmHg (SD 7.1) at 12 weeks follow up of 34 eyes of 34 patients. Ocular pressures measured before and after surgery were plotted for comparison [Figure 1]. There was a significant drop in ocular pressure following surgery. At 6 weeks, the mean decline was 22.1 mmHg and at 12 weeks, it was 23.8 mmHg. We were able to assess the optic nerve head of 37 eyes. The mean Cup to Disc ratio was 0.63 (SD ± 0.33).
Figure 1.
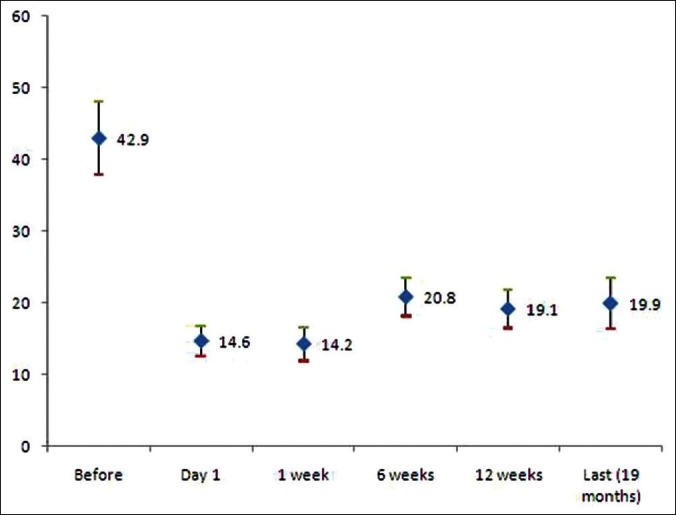
The intraocular pressure of eyes with refractory glaucoma is given before and after surgery using Ahemd valve. The decline in ocular pressure is significant after surgery but there is significant rise of IOP at 6 week follow up
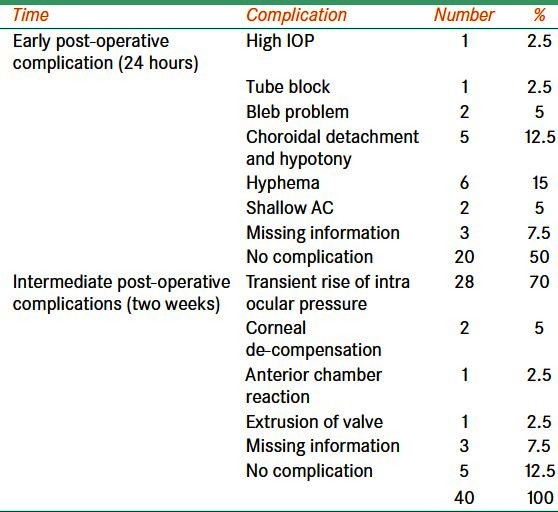
General anesthesia was used in nine patients. Nineteen eyes were operated using peri-bulbar anesthesia, whereas sub-tenon local anesthesia was applied for 12 eyes. We experienced intra-operative complication in only one eye. The details of early (within 1st week) and late postoperative complications (one week to six months) are given in Table 2. In all four eyes, the necrosis of conjunctiva resulted in graft exposure. It appears that the thickness and probable sharp edges of the pericardium were responsible for it. Subsequently, half thickness pericardium with trimmed edges was used to cover the implanted AGV tube. In this eye, necrosis of conjunctiva was not noted after surgery.
Table 2.
Post-operative complications following Ahmed valve surgery to treat refractory glaucoma
The number of medications used to control refractory glaucoma before and after surgery is shown in Table 3. There was decline in the number of eye drops and medications to control ocular pressure after surgery.
Table 3.
Distant vision before and after Ahmed valve surgery for treating refractory glaucoma
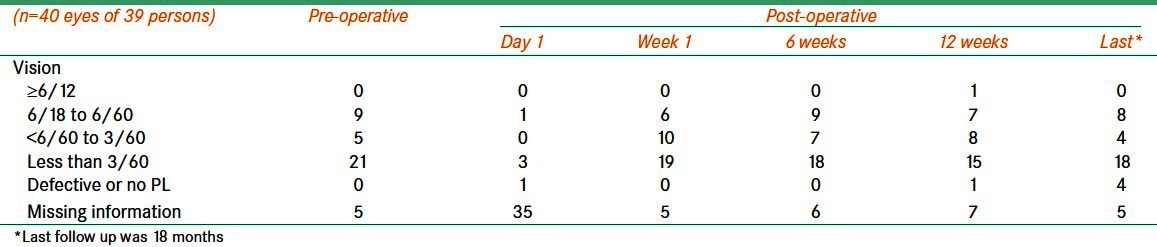
We grouped our participants based on the BCVA prior to the surgery. In five eyes, we were not able to assess the vision. Moderate visual impairment (6/18 to 6/60) was noted in nine eyes. Severe visual impairment (SVI) was noted in five eyes. In 21 (52.2%) eyes, BCVA was less than 3/60. The postoperative visual acuity is compared in Table 4 and Figure 2. Visual testing on the first day after surgery was not possible in a large number of participants. Moderate visual impairment (MVI) at six weeks following surgery was noted in 16 (40%) eyes.
Table 4.
Postoperative reduction in the number of oral/local medications following Ahmed valve surgery for treating refractory glaucoma
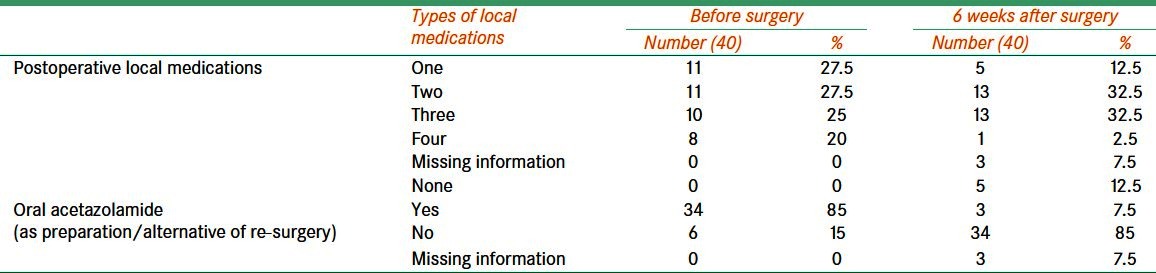
Figure 2.
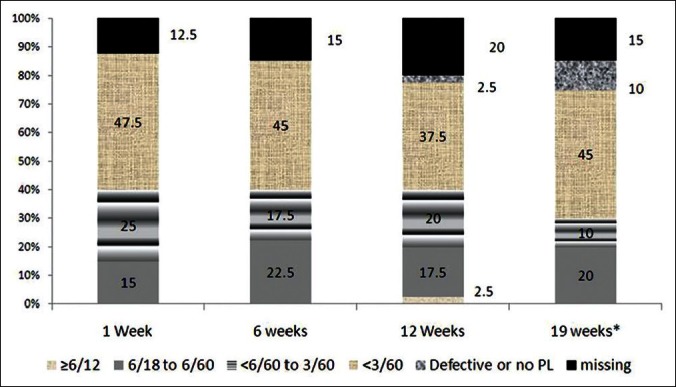
The percentage proportion of eyes with different grades of visual status is compared at different times of follow P after AGV surgery. The vision improved with time after surgery, but missing cases were a sizable proportion
Discussion
Refractory glaucoma is a major concern for care providers as they are complex cases to deal with. Results of conventional surgeries are not promising, and advanced interventions are needed to alleviate symptoms and signs in patients. Evidence suggests that the use of valves have improved the short term, intermediate, and long term surgical outcomes.[7,10,18] Our results also confirm that AGV implant surgery provides an acceptable level of success in the Omani population.
The study results demonstrated that IOP declined by 22.1 mmHg at six weeks and 23.8 mmHg at 12 weeks following AGV implant surgery among Omani patients with refractory glaucoma. The anti-glaucoma medication application to control IOP reduced from mean 2.38 (SD 1.1) before surgery to mean 1.92 (SD 0.9) at 12 weeks. In one eye, we had to remove valve due to complication. The follow up period in our study was three and six months, and it could be termed as short term follow up of AGV implant to treat glaucoma.
Decline in IOP was the main achievement in our study. Parihar JK et al. had noted decline of 16.7 mmHg at 12 months.[4] At three months, AGV implant in uvetitic glaucoma was not different from preoperative IOP.[19] Perhaps type of glaucoma is responsible for failure. In our study, none of the cases had uveitic etiology. This could be the reason for better outcomes. The decline of IOP was as high as 21.7 mmHg in a study in Tunis. However, the mean follow up period was 36 months.[9]
In our study, a transient rise of IOP was noted in 70% of operated eyes mainly noted at one week follow up. However, the IOP was stable in most of the eyes by adding glaucoma medications at six weeks following surgery. This matched with the results of other studies, in which researchers noted 88% and 89% of operated eyes with reduced intraocular pressure in the intermediate follow up periods.[7,20] In contrast, Wu et al. had reported that 59% of eyes operated for implanting AGV having ocular hypertension after six months, which were controlled by adding topical medication.[21]
The decline in number of glaucoma medications is an important achievement from the patient’s perspective. In our study, we had succeeded in reducing the number of eye medications in most of the cases. The decline in medication with an average follow up period of 29 months was 3.3 to 1.2 in a UK study.[22] Souza C et al. had noted a decline in the number of medications from 3.2 to 2.1, 60 months after surgery.[10] In Tunis, two-third of patients had reduced number of eye medications for controlling IOP.[9] The decline of medications was from 3.2 ± 1.0 to 1.6 ± 0.4 at 12 months in a study in USA.[10] The decline was noted 1.2 medications at two years by Wylega.[23] Our being short term study with follow up of 12 weeks, the results are likely to be better.
The proper management of intra and postoperative complications following AGV implant surgeries was crucial for high success rates.[24] In our study, hypotony was noted in seven eyes (17%) during the immediate postoperative period. In Tunis, El Afrit et al. also noted transient hypotony after surgery in 15% of eyes, which underwent AGV implantation.[9] Soon after surgery, hyphema was noted in 15% of eyes in our study, especially in eyes with neovascular changes in iris. This complication rate was higher than that reported by Chen H, et al.[20] The gradual decompression of anterior chamber/preoperative preparation of eyes with neovascular glaucoma with intra-vitreal anti-vascular endothelial growth factor (anti-VEGF) injection +/- PRP could reduce leakage from neo-vascular vessels.
Visual acuity was best at six weeks after surgery. Those eyes with residual vision (<3/60) before surgery did not improve. However, those with vision better than 3/60 showed improvement. Reduction of macular edema in cases with low IOP could be the main reason for improved vision at six weeks. Monteanez et al. had suggested that the visual assessment is not of value as it could be influenced by other ocular co-morbidities, which are common in the age groups having refractory glaucoma.[25] In a few other studies, researchers had considered ’not loosing perception of light’ following surgery as criteria for success.[10,26] Four out of 40 eyes (10%) in our study had no perception of light at the last follow up. This rate in our study is high compared to other studies. However, it should be noted that each of these four eyes had underlying co-morbidity, which could be held responsible for very poor eyesight.
Our study confirmed the previous researcher’s findings suggesting that AGV implant in Omani patients with refractory glaucoma enabled us to combat visual disabilities and improve glaucoma care by reducing the number of medications.[8,22] This study also revealed that refractory glaucoma was common among eyes with secondary glaucoma especially neovascular glaucoma and aphakic/pseudophakic glaucoma.[25,13]
A large number of cases were with neovascularization of iris. The presence of diabetes in half of the cohort explains this co-morbidity. Diabetes is noted in 11.6% of Oman’s adult population, and 14.5% of persons with diabetes were found to have diabetic retinopathy.[26,27] The prevalence of glaucoma among persons with diabetes in Oman was 9%.[28] The poor quality of primary prevention noted among cases of Sight Threatening stage of diabetic retinopathy (STDR) suggests that there are a large number of cases with refractory glaucoma in Oman.[29] Thus, refractory glaucoma due to neovascularization following advanced stages of diabetes is expected to be on the rise in the coming years.
This study had few limitations. In most of the studies, researchers have used parameters like the maintenance of reduced intraocular pressure over a short and long term, reduction in number of medications after surgery, and improvement in visual acuity in order to measure success. However, they used different cut off values and hence, the outcomes should be compared with caution. As our study was a historical cohort, some information was collected from the past, and other data was collected from case records and interview. With our study being historical cohort, the information was collected from case records and interview. Hence, the study could have been affected by recall bias and ’loss of data’ bias. The review of outcomes was planned for the entire series. However, the success would vary with underlying cause of refractory glaucoma. As the numbers in subgroups were few, we could not perform such analysis and hence, the result should be interpreted with caution.
Refractory glaucoma is a major concern for care providers as they are complex cases to deal with. As results of conventional surgeries are not promising, alternative interventions are needed to alleviate the pain and agony of patients. Evidence suggests that the use of valves have improved the short term, intermediate, and long term surgical outcomes.[7,10,23] Our results also confirm that AGV implant surgery provided a level of success in the Omani population that is comparable to many other studies.
Acknowledgment
We thank the staff of the eye unit of Al Nahdha hospital for assisting us in retrieving data and communicating with patients and their relatives. The co-operation of patients was a key for the success of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Pizzarello L, Abiose A, Ffytche T, Duerksen R, Thulasiraj R, Taylor H, et al. VISION 2020: The Right to Sight: A global initiative to eliminate avoidable blindness. Arch Ophthalmol. 2004;122:615–20. doi: 10.1001/archopht.122.4.615. [DOI] [PubMed] [Google Scholar]
- 2.Khandekar R, Jaffer MA, Al Raisi A, Zutshi R, Mahabaleshwar M, Shah R, et al. Oman Eye Study 2005: Prevalence and determinants of glaucoma. East Mediterr Health J. 2008;14:1349–59. [PubMed] [Google Scholar]
- 3.Khandekarl R, Zutshi R. Glaucoma in oman: A review. J Glaucoma. 2006;15:271–3. doi: 10.1097/01.ijg.0000212206.79899.cc. [DOI] [PubMed] [Google Scholar]
- 4.Khandekar R, Shama Mel-S, Mohammed AJ. Noncompliance with medical treatment among glaucoma patients in Oman--a cross-sectional descriptive study. Ophthalmic Epidemiol. 2005;12:303–9. doi: 10.1080/09286580500224602. [DOI] [PubMed] [Google Scholar]
- 5.Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: A systematic literature review and current controversies. Surv Ophthalmol. 2005;50:48–60. doi: 10.1016/j.survophthal.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Nassiri N, Kamali G, Rahnavardi M, Mohammadi B, Nassiri S, Rahmani L, et al. Ahmed glaucoma valve and single-plate Molteno implants in treatment of refractory glaucoma: A comparative study. Am J Ophthalmol. 2010;149:893–902. doi: 10.1016/j.ajo.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Parihar JK, Vats DP, Maggon R, Mathur V, Singh A, Mishra SK. The efficacy of Ahmed glaucoma valve drainage devices in cases of adult refractory glaucoma in Indian eyes. Indian J Ophthalmol. 2009;57:345–50. doi: 10.4103/0301-4738.55068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkisian SR., Jr Tube shunt complications and their prevention. Curr Opin Ophthalmol. 2009;20:126–30. doi: 10.1097/ICU.0b013e328323d519. [DOI] [PubMed] [Google Scholar]
- 9.El Afrit MA, Trojet S, Mazlout H, Hamdouni M, Kraiem A. Efficacy of the Ahmed glaucoma valve implant in eyes with refractory glaucoma. Tunis Med. 2007;85:941–4. [PubMed] [Google Scholar]
- 10.Souza C, Tran DH, Loman J, Law SK, Coleman AL, Caprioli J. Long-term outcomes of Ahmed glaucoma valve implantation in refractory glaucomas. Am J Ophthalmol. 2007;144:893–900. doi: 10.1016/j.ajo.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Chen TC, Bhatia LS, Walton DS. Ahmed valve surgery for refractory pediatric glaucoma: A report of 52 eyes. J Pediatr Ophthalmol Strabismus. 2005;42:274–83. doi: 10.3928/0191-3913-20050901-09. [DOI] [PubMed] [Google Scholar]
- 12.Netland PA. The Ahmed glaucoma valve in neovascular glaucoma (An AOS Thesis) Trans Am Ophthalmol Soc. 2009;107:325–42. [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida K, Netland PA. Ahmed glaucoma valve implantation in African American and white patients. Arch Ophthalmol. 2006;124:800–6. doi: 10.1001/archopht.124.6.800. [DOI] [PubMed] [Google Scholar]
- 14.Das JC, Chaudhuri Z, Sharma P, Bhomaj S. The Ahmed Glaucoma Valve in refractory glaucoma: Experiences in Indian eyes. Eye (Lond) 2005;19:183–90. doi: 10.1038/sj.eye.6701447. [DOI] [PubMed] [Google Scholar]
- 15.Al-Shahwan S. Transcorneal tube erosion of an ahmed valve implant in an adult. Middle East Afr J Ophthalmol. 2010;17:377–8. doi: 10.4103/0974-9233.71593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang JM, Kee C. The effect of surface area expansion with pericardial membrane (preclude) in Ahmed glaucoma valve implant surgery. J Glaucoma. 2004;13:335–9. doi: 10.1097/00061198-200408000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Eid TM, Radwan A, el-Manawy W, el-Hawary I. Intravitreal bevacizumab and aqueous shunting surgery for neovascular glaucoma: Safety and efficacy. Can J Ophthalmol. 2009;44:451–6. doi: 10.3129/i09-108. [DOI] [PubMed] [Google Scholar]
- 18.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 2.3.1. [Last updated on 2010 19 Sep, accessed on 2012 Aug 08]. Available from: http://www.OpenEpi.com .
- 19.Rachmiel R, Trope GE, Buys YM, Flanagan JG, Chipman ML. Ahmed glaucoma valve implantation in uveitic glaucoma versus open-angle glaucoma patients. Can J Ophthalmol. 2008;43:462–7. doi: 10.3129/i08-082. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Zhang SX, Liu L, Lin D, Tang X, Sun L, et al. Intermediate-term and long-term clinical evaluation of the Ahmed glaucoma valve implantation. Zhonghua Yan Ke Za Zhi. 2005;41:796–802. [PubMed] [Google Scholar]
- 21.Wu SC, Huang SC, Lin KK. Clinical experience with the Ahmed glaucoma valve implant in complicated glaucoma. Chang Gung Med J. 2003;26:904–10. [PubMed] [Google Scholar]
- 22.Wishart PK, Choudhary A, Wong D. Ahmed glaucoma valves in refractory glaucoma: A 7-year audit. Br J Ophthalmol. 2010;94:1174–9. doi: 10.1136/bjo.2009.165357. [DOI] [PubMed] [Google Scholar]
- 23.Wylega³a E, Tarnawska D, Lyssek-Boroñ A, Biliñska B, Jurewicz A, Petela A. A clinical study of the Ahmed glaucoma valve implant in refractory glaucoma. Klin Oczna. 2005;107:221–5. [PubMed] [Google Scholar]
- 24.Hille K, Moustafa B, Hille A, Ruprecht KW. Drainage devices in glaucoma surgery. Klin Oczna. 2004;106:670–81. [PubMed] [Google Scholar]
- 25.Montañez FJ, Laso E, Suñer M, Amaya C. Ahmed drainage device implant. Our experience between 1995 and 2003. Arch Soc Esp Oftalmol. 2005;80:239–44. doi: 10.4321/s0365-66912005000400007. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: A critical comparison of types. Curr Opin Ophthalmol. 2006;17:181–9. doi: 10.1097/01.icu.0000193080.55240.7e. [DOI] [PubMed] [Google Scholar]
- 27.Al-Riyami AA, Afifi M. Smoking in Oman: Prevalence and characteristics of smokers. East Mediterr Health J. 2004;10:600–9. [PubMed] [Google Scholar]
- 28.Khandekar R, Al Lawatii J, Mohammed AJ, Al Raisi A. Diabetic retinopathy in Oman: A hospital based study. Br J Ophthalmol. 2003;87:1061–4. doi: 10.1136/bjo.87.9.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khandekar R, Zutshi R. Glaucoma among Omani diabetic patients: A cross-sectional descriptive study: (Oman diabetic eye study 2002) Eur J Ophthalmol. 2004;14:19–25. doi: 10.1177/112067210401400104. [DOI] [PubMed] [Google Scholar]


