Abstract
Small gauge vitrectomy, also known as minimally invasive vitreous surgery (MIVS), is a classic example of progress in biomedical engineering. Disparity in conjunctival and scleral wound location and reduction in wound diameter are its core principles. Fluidic changes include increased pressure head loss with consequent reduction in infusional flow rate and use of higher aspiration vacuum at the cutter port. Increase An increase in port open/port closed time maintains an adequate rate of vitreous removal. High Intensity Discharge (HID) lamps maintain adequate illumination in spite of a decrease in the number of fiberoptic fibers. The advantages of MIVS are, a shorter surgical time, minimal conjunctival damage, and early postoperative recovery. Most complications are centered on wound stability and risk of postoperative hypotony, endophthalmitis, and port site retinal break formation. MIVS is suited in most cases, however, it can cause dehiscence of recent cataract wounds. Retraction of the infusion cannula in the suprachoroidal space may occur in eyes with scleral thinning. As a lot has been published and discussed about sutureless vitrectomy a review of this subject is necessary. A PubMed search was performed in December 2011 with terms small gauge vitrectomy, 23-gauge vitrectomy, 25-gauge vitrectomy, and 27 gauge vitrectomy, which were revised in August 2012. There were no restrictions on the date of publication but it was restricted to articles in English or other languages, if there abstracts were available in English.
Keywords: Pars plana vitrectomy, sutureless vitrectomy, vitreoretinal surgery
Introduction
Development of small gauge vitrectomy or minimally invasive vitreous surgery (MIVS) is a reflection of the aims of surgeons, who strive continuously at minimizing invasiveness without compromising the outcome. Vitreous surgery, once limited to manual anterior vitrectomy during cataract extraction, has undergone major advances and refinements, and evolved as a highly controlled closed globe intervention. Small gauge vitrectomy, once highly criticized, has now become an indispensible part of our practice. As a result of this, almost all vitreoretinal instruments available in 20 gauge (G) are now available in sizes of 23-G and 25-G. The aim of the review is to discuss the science behind the feasibility of sutureless vitreoretinal intervention and analyze the conclusions of scientific studies done in the recent past, to help the reader adopt the technique in light of the current contemporary scientific literature. A PubMed search was performed in December 2011, using the terms small gauge vitrectomy, 23-gauge vitrectomy, 25-gauge vitrectomy, 27-gauge vitrectomy, which was revised in August 2012. There were no restrictions on the date of publication, but was restricted to articles in English or other languages, if there abstracts were available in English.
History of sutureless vitreoretinal intervention
The development of vitreoretinal surgical technology is a leading example of the application of biomedical engineering in Ophthalmology. The history of pars plana vitreoretinal intervention dates to Robert Machemer, who developed closed pars plana vitrectomy to operate in a closed system with controlled intraocular pressure, thereby eliminating the need for an open-sky approach.[1] This served as the start of a distinct and separate vitreoretinal specialty. Even as Machemer developed the vitreous infusion suction cutter (VISC) that needed a 17-G sclerotomy port, other major advancements were development of a three-port vitrectomy with a 20-G (0.89 mm) system by Conor O’Malley and Ralph Heinz and a lightweight, reusable, bellows-driven, pneumatic, axial cutter driven by the Ocutome 800 console (Berkley Bioengineering, 1972).[2] At the same time Gholam Peyman developed the electric solenoid-driven axial (guillotine) cutter and R. Kloti in Europe developed a three-port system with an electric cutter.[3]
Novel attempts to shorten surgical time and trauma led to the development of a 20-G transconjunctival approach in 1996, by Chen et al., who described a scleral tunnel technique after limited peritomy and insertion of the microvitreoretinal (MVR) blade in the vitreous cavity at the base of the tunnel.[4] Kwok et al. described a variation of this method with an initial radial incision, still placed 3–4 mm behind the corneoscleral limbus.[5] They used a 20-G round body hypodermic needle rather than a MVR blade. Gotzaridis in his technique created adhesion between the conjunctiva and the sclera using a diathermy.[6] A 20-G MVR was used to create a combined conjunctival scleral incision in the inferotemporal area. A 4 mm self-retaining infusion cannula was inserted through this incision. Numerous other modifications have also been described, which are out of scope of this review.[7–9] The 20-G sutureless technique did not gain much popularity due to high rates of wound leakage and choroidal detachment, with a need for suture placement in many of the cases.
De Juan developed a 25-G instrument set for pediatric use, in 1990, since the ’conventional’ 20-G vitreous cutters had proven to be big, lacking in precision, and unsuitable for pediatric use.[10] De Juan and Hickingbotham stated that due to the reduced aspiration rate 25-G vitrectomy was to be used only in selected, delicate cases requiring particularly precise and careful intervention. It was 12 years later, when eventually a complete 25-G transconjunctival vitrectomy system, was introduced by Fuji et al.,[11] which consisted of microtrocar cannulas and afforded the ease and safety of instrument introduction and withdrawal, as well as an array of integrated 25-G instruments. The development of 23-G systems materialized due to a need to combine the advantage of sutureless intervention and rigidity of the 20-G system. Singh et al. introduced the first electronic 23-Gvitrectome as early as in 1995.[12] It was used only for carrying out vitreous biopsies and office procedures. Almost 10 years passed before a fully integrated 23-Gvitrectomy system for routine clinical use was designed, when in 2005, Eckardt in cooperation with DORC (The Netherlands) eventually introduced complete 23-G instrumentation and demonstrated its safety and efficiency in a first evaluation study.[13]
Wound construction in sutureless vitrectomy
The principles of sutureless pars plana intervention are, small wound size, multiplanar incision, and construction and disparity in the site of the internal and external wounds, which is similar to a self-sealing limbal incision used for phacoemulsification.
Two-step technique
In the initially described two-step technique an angled MVR was used to create a slit opening in the sclera followed by placement of the cannula [Figure 1]. Oliveira et al.,[14] reported the use of a 0.7 mm sapphire knife as a variation on the stiletto blade initially used by Eckardt.[13] The technique suffered a disadvantage – it was difficult to locate the initial point of the trocar insertion.
Figure 1.
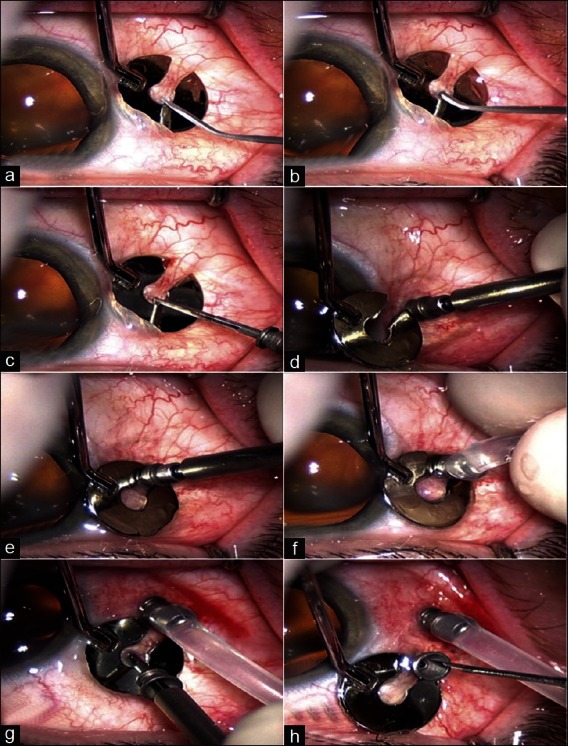
Two-step entry system, as described by Eckardt, using an angled microvitreoretinal blade (MVR) followed by placement of cannula (a,b) placement of fixation plate at appropriate distance from limbus and initiation of creation of sclerotomy using angled MVR. (c) Insertion of trocar mounted cannula. (d) Complete insertion of trocar mounted cannula. (e) Removal of trocar, to leave the cannula in place. (f) Attachment of infusion line on the cannula (g) Insertion of second trocar cannula assembly (h) Removal of trocar leaving the cannula in place
Single-step technique
In the modern single-step technique, the Entry Site Alignment (ESA) system is the primary component of instrumentation. The ESA system components include: The trocar-mounted microcannulas [Figures 2 and 3], cannula plugs, and an infusion line [Figure 4]. The microcannulas are preloaded on the needle trocars and the combined components of the trocar needle, microcannula, and trocar handle are referred to as the trocar/cannula assembly. As the name suggests, the ESA system serves to maintain alignment between the entry holes in the conjunctiva and sclera as well as provide unobstructed instrument access. The techniques of the insertion are as follows [Figure 5].
Figure 2.

Components of the entry alignment system (ESA): Cannule mounted trocars
Figure 3.

23-G cannulae and trocars separately
Figure 4.
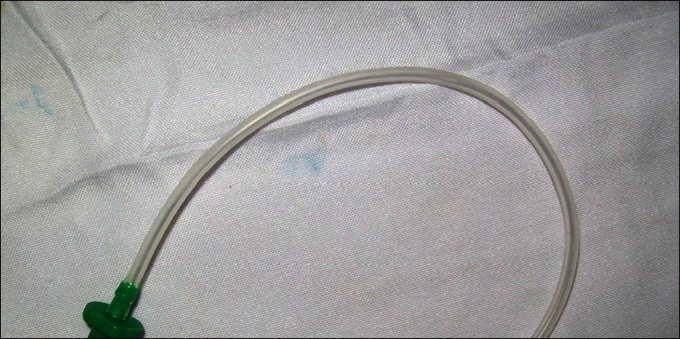
23-G self-retaining infusion cannula
Figure 5.
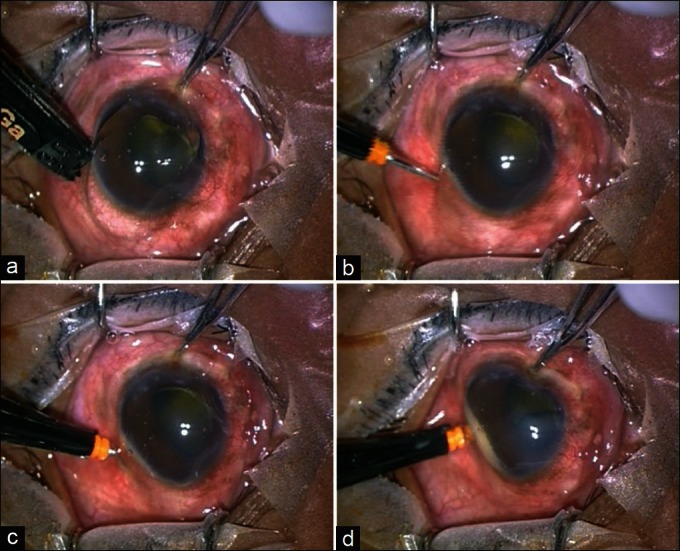
Single-step entry technique using the entry site alignment system (ESA), using angled incision. (a) Measurement of distance from pars plana. (b) Scleral entry at 45°. (c) Change in the direction, from oblique to vertical. (d) Entry into the vitreous cavity perpendicularly. Note the buckling of the sclera due to preoperative hypotony
Perpendicular entry
The direction of the trocar was kept perpendicular to the globe, as in 20-G vitrectomy. This incision was used for 25-G port construction, but it was abandoned due to the high complication rate.[15–17]
Angled (Beveled) incision
The conjunctiva is displaced laterally. The trocar is then inserted intrasclerally at 45° to the surface, changing the direction tangential to the ocular surface once the sclera has penetrated fully [Figure 6]. This causes a valve-like effect to occur and helps in wound apposition.[18]
Figure 6.
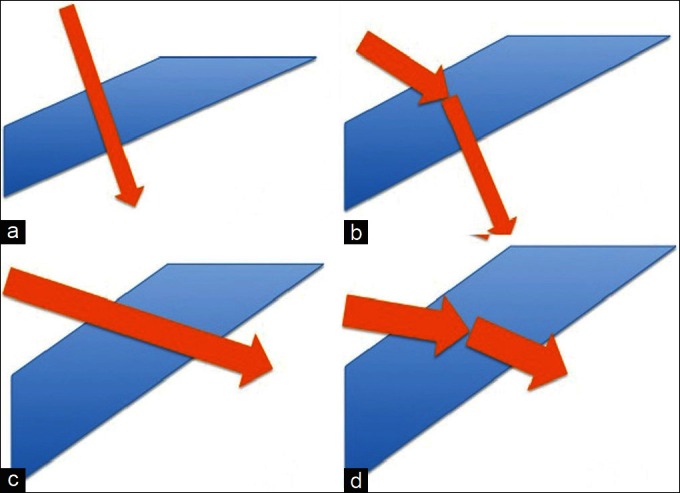
Graphic representation of various single-step entry techniques. (a) Perpendicular entry, (b) Angled entry, (c) Zorro’s incision, 6d: Pollack’s incision
Zorro’s incision
Inserting the system obliquely without straightening modifies the incision direction. The blade is inserted 4.0 mm from the limbus, at an oblique angle of 10–15°, and enters the vitreous without straightening.[19]
Pollack’s incision
Pollack has improved Zorro’s direct and oblique technique by suggesting a biplanar insertion modality. The technique is as follows: The trocar bevel is held up with the tip at approximately 5° to the sclera. The trocar is then inserted to 50% of the scleral depth, until just past the end of the beveled tip. The handle of the trocar shaft is raised till it is at about a 30° angle to the sclera, and then insertion is completed.[20] The physics of incision construction is that the longer tunnel provides better sealing action. Trocar entry at 45° results in a tunnel length of 1.154 mm compared to 30°, which has a tunnel length of 1.414 mm – a 30% increase.[20]
Physics of sutureless vitrectomy
The physics of sutureless vitrectomy encompasses the mechanics of wound construction, fluidic changes at the inner end of the infusion cannula, fluidic changes at the tip of the aspiration port of the vitrectomy cutter, and the mechanism for sufficient illumination, in spite of the reduction in diameter of the endoilluminator.
Mechanics of entry site alignment system
The performance of the trocar/cannula insertion is influenced by the trocar needle puncture, sliding friction, cannula insertion, and the overall size of the wound. When the initial scleral puncture is initiated the force applied causes the tissues to either give away or displace. The total force applied to the trocar handle to initiate the puncture is affected by the trocar point size and the state of the patient’s tissue. The pressure necessary for the puncture is dictated by the composition of the tissue, while the total force needed to reach this pressure is governed by the sharpness of the trocar point.
For the trocar to reach the critical puncture pressure, the tissue must oppose the applied force. The tissue at, or near the puncture site will usually deflect prior to fully opposing the applied trocar force. The tissue characteristics and the intraocular pressure govern the amount of deflection or buckling. As the tissue buckles there is a rise in the intraocular pressure leading to a rise in the point pressure till the ocular coat punctures. The amount of tissue buckling is directly proportional to the trocar point area and inversely proportional to the intraocular pressure.
Once the trocar point punctures, the trocar side bevels enlarge the wound. As the trocar enters, the wound tissue exerts frictional force on the trocar surface, which is determined by the frictional coefficient and surface area of the shaft. The next critical event that follows is the encircling cannula in the wound that has been created by the trocar. The most important parameter that has an impact on the cannula insertion and its performance is the ratio of the internal diameter of cannula to the external diameter of the trocar [Trocar//Cannula (T/C)]. The ratio provides a measure of how easily the cannula is able to slide relative to the trocar shaft. If the T/C Delta is too small, the cannula insertion will be smooth, but it may be difficult for the surgeon to remove the trocar from the cannula. A large trocar handle force and a large cannula counterforce may be necessary to remove the trocar. Second, the T/C Delta governs the extent to which the wound stretches to accept the cannula, and thus, the normal force applied to the outer surface of the cannula. This normal force affects both the amount of friction that is holding the cannula in place during the case, and the amount of force required for cannula removal at the conclusion.
A T/C Delta that is too small may allow an easy insertion, but may not provide enough wound holding force to prevent accidental cannula removal during the surgical procedure. Too large a T/C Delta may lead to large insertion forces, cannula buckling, and large cannula removal forces.
Fluidics of small gauge vitrectomy
Reduction in the internal diameter of the ports brings about critical changes in the fluidics at two levels, the infusion line and the fluid aspiration tip.
First, at the level of the infusion cannula, reduction in the diameter results in increased frictional forces and loss of pressure head and decrease of volume flow at the intraocular end of the infusion cannula. This can be explained on the basis of Poiseuille’s law that describes the volume flow rate of an incompressible viscous fluid through a tube of constant circular cross-section. It states that Δ P = 8 μLQ/πr4
where:
Δ P is the pressure drop
L is the length of pipe
μ is the dynamic viscosity
Q is the volumetric flow rate
r is the radius
π is the mathematical constant Pi
If the inner radius reduces by half, the volume flow rate decreases by a factor of sixteen. To simplify, the volume of fluid flowing inside the eye is compromised by a narrower tube diameter, increased length of the infusion cannula, and lowered bottle height. In addition, the volume flow rate is directly proportional to the pressure differential, while it is inversely proportional to the fluid viscosity and length of the tube. Hence, the infusion pressure has to be raised from 30-40 mmHg, as used in 20-G vitrectomy, to 40-50 mm Hg for 25-G vitrectomy. Vitrectomy with 23-G instrumentation can still be performed at low infusion pressures, without encountering hypotony.
The second change that occurs as a consequence of reduced internal diameter is at the distal end of the vitrectomy probe. Unlike the infusion cannula the vitrectomy cutter is exposed to balanced salt solution, vitreous and other intraocular solids. In spite of the Poiseuille’s law not being fully applicable to the vitreous, there are similarities in concepts between the fluidics at aspiration and the infusion lines. Applying the Poiseuille’s law to create a flow rate equal to a 20-G system, the aspiration vacuum would have to be higher in a 23-G or a 25-G system to counter higher pressure head loss, with reduction in the caliber of the tubing. The 20-G machines normally work at a suction pressure of 150 mmHg. In the 23- and 25-G systems these suction pressures are kept at 250 mmHg and 500 mmHg to be able to achieve comparative volume aspiration rates; (these are the authors preferences and may vary between surgeons) [Figure 7]. The second factor affecting the outflow through the vitrectomy cutter is the duty cycle of the cutter. The duty cycle is the percentage of time the cutter port is open relative to each cutting cycle. When the port is open, the aspiration pressure draws some vitreous into the cutter. During cutting, a chunk of vitreous is removed from the main ’block’ of the vitreous. The opening of the cutter tip is dependant upon the spring in the cutter. As the mechanical properties of the spring remain constant the time taken by the spring to open the port does not change with the varying cut rates. At higher cut rates the proportion of time for which the port remains open is compromised due to the jet of air closing the cutter before it opens fully. T. Magalhaes et al.,[21] have compared the infusion and extrusion volumes of three different 25-G systems. The average infusion rates are 167.23 μL/s with the Bausch and Lomb (MilleniumTM), 190.53 μL/s with Alcon (AccurusTM), and 250.09 μL/s with DORC (AssociateTM), respectively. With the cutter off, the Bausch and Lomb and Alcon systems have lower aspiration flow rates than the DORC system. They also have a variation in the aspiration flow rate of <10% between a cut rate of 0 cuts/minute and 1100 cuts/minute compared to >50% in the DORC system, demonstrating a lower power, but greater flow stability. To maintain adequate outflow rates at high cut rates, newer vitrectomy systems employ the dual pneumatic cutter technology to sustain an adequate flow rate. Dual pneumatic cutters employ air jets to open and close the ports obviating the use of springs, achieving a port open/port close ratio of 50% even at 5000 cuts/minute.
Figure 7.
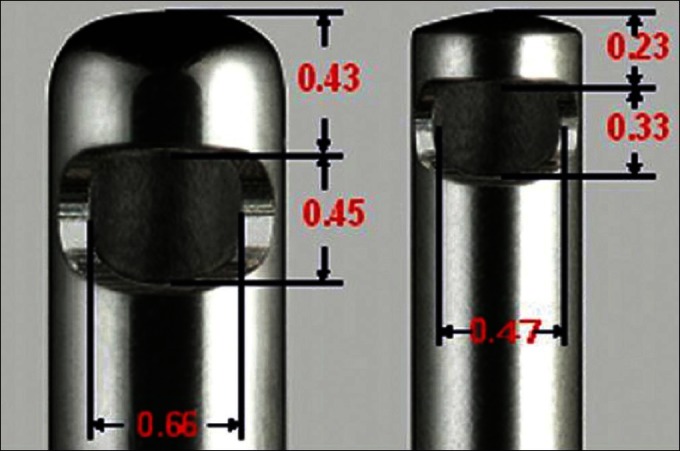
Comparison of the port diameter of 20-G and 23-G vitrectomy cutter
Illumination systems
The smaller diameter of the 23- and 25-G fiber optic light pipes also decreases illumination from the conventional vitrectomy light sources. To obviate this, high intensity discharge lamps (HID) are utilized. They have short arcs that are generated between two electrodes under high pressure. Although short, these arcs generate high temperature, efficiency, and have a long life (20,000+ hours) in an efficient package. There are four categories of HID lamps: Mercury vapor, metal halide, high-pressure sodium, and low-pressure sodium. Only two HID lamps: Metal halide and high-pressure sodium are used in Ophthalmology as primary light sources.
Metal halide lamps utilize mercury and argon gases inside the arc tube to improve efficacy and allow variable wattage of the lamp. Unfortunately, metal halides suffer from spectrum shifting with the change of voltage, and lower life compared to other HID lamps. Currently, metal halide lamps are used in the Millennium system from Bausch and Lomb Inc. (St. Louis, MO, USA). High-pressure sodium lamps consist of a high-voltage starter ballast with an arc tube that is filled with gases such as xenon, sodium, and mercury. These lamps can be used at high wattages, and have higher good efficacy, with minimal spectrum shifting. The disadvantage is that they require an additional cooling system. Currently, xenon lamps are used in the Accurus system from Alcon Laboratories, Inc (Fort Worth, TX, USA) and the Photon light source from Synergetics Inc. (O’Fallon, MO, USA).
Advantage of small gauge vitrectomy
The chief advantage of small gauge vitrectomy is reduced operative time, although few studies found no difference in the total surgical time. The time gained by faster port construction was balanced against slower vitreous removal.[22,23]
Many of the studies with small gauge vitrectomy have also found postoperative decrease in pain and inflammation,[24] and improved patient comfort.[25] A recent study evaluated the feasibility of No-patch 23-G vitrectomy under topical anesthesia. In a study involving five patients, four patients had grade 0 discomfort during trocar removal.[26]
Sutured sclerotomies induce astigmatism in many eyes following 20-G vitrectomy,[27] which is uncommon following 23- and 25-G vitrectomy,[28,29] helping rapid visual recovery.
Complications of small gauge vitrectomy
Intraoperative
Intraocular pressure rise
The complications of small gauge vitrectomy are enumerated in Table 1. The force applied during insertion of the trocar and cannula assembly is known to cause dehiscence of recently operated cataract wounds, and iris prolapse, due to the rise of intraocular pressure (IOP). In in vivo and experimental settings it has been seen that the intraocular pressure rises to above 60 mmHg.[30] The limited sharpness of the trocar and the difference between the higher diameter of the cannula and the trocar creates frictional resistance to its entry through the scleral canal, causing more force to be applied during insertion. A theoretical risk of increased IOP during port construction has been proposed in patients with compromised intraocular blood flow. It is advisable to close any recent corneal or scleral wounds before the trocar/cannulas are inserted.[31] Wu PC et al. have proposed a simple modification of the twisting maneuver for sutureless vitrectomy trocar insertion to reduce IOP, wherein the stab motion is replaced with a twisting movement during wound placement, resulting in the highest IOP of 30 mmHg, in sharp contrast to 63.9 mm when employing the conventional method, for entry.[32]
Table 1.
Complications of small gauge vitrectomy
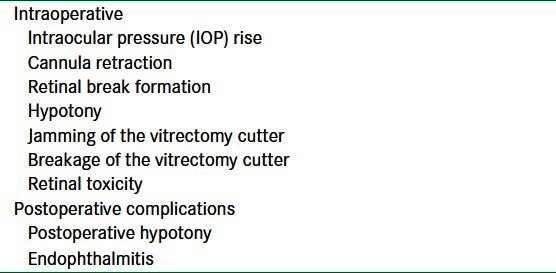
Cannula retraction
Intraoperative retraction of the infusion cannula is known to occur leading to choroidal detachment. In a retrospective series the complication has been seen in 3.5% of the cases, with serous and hemorrhagic detachments occurring at equal frequency.[33] We in our own experience have seen this phenomenon in surgery being done for failed scleral buckling. The intravitreal length of the infusion cannula is compromised due to choroidal edema. We have had a similar case of intraoperative displacement during a redetachment surgery. Repeated surgeries lead to scleral thinning and reduced friction between the cannula sleeve and globe wall, which predisposes to cannula displacement. The cannula attached to the infusion line may also spontaneously dislocate during scleral depression, causing severe hypotony and choroidal detachment. Sometimes the cannula sleeve is pulled out of the sclera when instruments are withdrawn from the eye, as a result of the friction between the instrument and inner wall of the cannula sleeve. The cannula sleeve can usually be reinserted through the same scleral incision by placing the trocar blade back through the sleeve and reinserting it into the same scleral tunnel. This generally happens when disposable instruments are resterilized to be used in multiple cases (a common practice in developing nations).
Retinal break formation
The rate of retinal tears discovered during sutureless vitreous surgery has been reported to be between 0 and 24%, with most series reporting an incidence of less than 5%.[34,35] In the largest retrospective series of 177 consecutive 25-G pars plana vitrectomy cases, the incidence of intraoperative retinal breaks was 15.8%, with roughly two-thirds of these occurring away from the superior sclerotomies.[36] In one comparative series of 25-G and 20-G vitrectomies, no statistically significant difference in the incidence of intraoperative retinal breaks was found (3.1% of the 25-gauge cases compared with 6.4% of the 20-G cases).[37]
Other complications
In situations where 20-G instrumentation is used for aspiration and 23- or 25-G ports are used for infusion, hypotony can occur. This situation generally arises when performing phacofragmentation or while inducing posterior vitreous detachment in difficult situations.[38] Jamming[39] and breakage of the vitrectomy cutter may occur with 25-G probes.[40]
Retinal toxicity has been reported in an eye with 23- and 25-G vitrectomy, where subconjunctival gentamicin was administered at the conclusion of the case.[41] Aminoglycosides or any other retinotoxic agents such as 5-fluorouracil should not be given subconjunctivally if there are any sutureless sclerotomies.
Postoperative complications
Postoperative hypotony
(defined as IOP < 6 mmHg) has been reported in 0 to 25% of the cases of sutureless vitrectomy.[42,43] This hypotony is usually transient and in most cases resolves with conservative measures. However, in some cases, the hypotony may be severe leading to large choroidal mounds, with accompanying hypotonic maculopathy and optic choroidopathy. Wound leak is likely to occur in the following situations, reoperation done on a vitrectomized eye,[44] multiple exchanges of instruments, younger patient age, extensive vitreous base dissection, and variations in wound construction. The two-step technique, although cumbersome, has been shown to have lesser leakage rates than the one-step technique.[45] Angled (oblique) entry or biplanar (oblique-parallel) entry may reduce the wound leak in contrast to direct insertion of the cannula perpendicular to the scleral surface by effectively lengthening the wound tract and maximizing scleral reapposition. Intentional conjunctival displacement during trocar insertion to misalign the conjunctival and scleral wounds may also decrease hypotony and improve wound reapposition.[46] In the one-step technique itself, wound leakage is lowest for the extreme oblique entry followed by the oblique entry and the straight entry. There is no difference in the incidence of wound leakage between 23-G and 25-G incisions.[47] This has further been supported by imaging of the wound healing by anterior segment optical coherence tomography; after 25-G vitrectomy, the scleral wounds evaluated by optical coherence tomography closed in 60.5% at one month and 63.9% at three months. After 23-G vitrectomy 57.4% of the scleral wounds closed at one month and 61.1% at three months postoperatively.[48] The sclerotomy flap apposition is not the only mechanism of wound closure. Endoscopic evaluations of the sclerotomies show vitreous plugging the wounds in both the 23- and 25-G cases.[49] It has also been shown that vitrectomy procedures done for macular indication have lesser wound leaks, which may be due to less extensive vitrectomy in these indications.[50]
Endophthalmitis
Small gauge vitrectomy received criticism in the initial phases, when reports of increased incidence of endophthalmitis started pouring in. The first reported case of endophthalmitis following 25-G surgery was published in 2005.[51] It was cited that contamination with conjunctival flora during insertion of cannulas and exchange of instruments,[52] ingress associated with postoperative hypotony, vitreous wick effect through unsutured conjunctival wounds, decreased bacterial clearance because of diminished infusional flow, and sequestration of bacteria in residual peripheral vitreous were responsible for increased incidence of endophthalmitis.[53] Singh et al. showed the passage of India ink into the eye, in over two-thirds of the eyes with unsutured sclerotomies, while no eyes had entry of India ink if the 20-G or 25-G sclerotomies were sutured.[54] A very large series of cases reported from the Wills Eye Institute showed an endophthalmitis rate of 0.23% (7/3,103 eyes) for 25 g vitrectomy compared to only 0.018% (1/5,498 eyes) for 20 g vitrectomy, representing a 12-fold increased risk for 25 g vitrectomy.[55] Another large retrospective series of a total of 7682 cases reported an even higher incidence of endophthalmitis in 25 g cases of 1/119 patients (0.84%) compared to 1/3188 (0.03%) for 20G cases.[56]
In a recent retrospective multicentric analysis from the Latino nations the five-year post-pars plana vitrectomy, endophthalmitis incidence rates were 0.020, 0.028, and 0.021% for 20-G, 23-G, and 25-G, respectively, the difference of which did not reach statistical significance.[57] Shimada H et al. recommended irrigation of the ocular surface with 1.25% povidone iodine, as it decreased the risk of bacterial contamination of the vitreous cavity.[58] Some surgeons advocated aggressive removal of the vitreous around the cannulas to prevent vitreous prolapse into the wounds. In one study by Shimada and colleagues, this method decreased the rate of vitreous prolapse from 20% to zero.[59]
Outcomes
Most studies examining 25-G and 23-G outcomes have found no significant difference in efficacy and safety when compared with the same in 20-G instrumentation. Most published series are retrospective and include a variety of surgical indications: Epiretinal membrane (ERM), macular hole, diabetic vitreous hemorrhage, tractional macular edema, and rhegmatogenous retinal detachment.[60–63] However, this might not be the case is retinal detachment.[64] Most surgeons agree that the removal of peripheral vitreous is important in the management of retinal detachment by vitrectomy, and indentation of the periphery is helpful in locating retinal breaks. Both these steps are more difficult in 25-G surgery and there were early reports of high re-attachment rates following 25-G vitrectomy. In a series of 53 eyes managed by 25-G vitrectomy, the primary success rate was only 74%,[65] however, more recent studies have reported reasonable success rates (primary success 92.9%) for 25 g vitrectomy and gas, without the use of a supplementary buckle.[66] 23-G and 25-G vitreoretinal surgery has also been successfully combined with phacoemulsification for management of vitreoretinal pathologies with concomitant cataract. The advantages of combined surgery are: Reduced surgical cost, obviating repeated trauma and anesthesia, lower risk of lens touch, easy postoperative examination, and vitreous base shaving during vitrectomy. It has been shown that the presence of an additional wound does not increase the risk of postoperative hypotony. In addition, surgically induced astigmatism with combined surgery is not higher than either of the procedures (phacoemulsification, vitrectomy) done alone.[67] Park et al. demonstrated that concomitant 23-G vitrectomy did not hamper the stability of the toric intraocular lens inserted in the bag with 66.7% of the cases undergoing r < 5 degree rotation,[68] while Lee et al. showed that 87.8% of the patients were within one diopter of emmetropia.[69] The indication of surgery in a majority of the patients in these studies were macular conditions such as ERM, macular hole, retinal vein occlusions or uncomplicated retinal detachments, making it is difficult to comment on the feasibility of this intervention in situations where IOP fluctuations are marked, such as, silicon oil-perfluorocarbon liquid exchange in vitreoretinal surgery. 23-G and 25-G ports have also been employed for silicon oil removal. Silicon oil is allowed to flow out passively through the ports, with the infusional flow creating a pressure gradient.[70,71] Patwardhan et al., in a prospective randomized study comparing 20-G and 23-G technique for silicon oil removal, found the two techniques to be equally safe with no additional risk of hypotony with 23-G transconjunctival oil removal. Eyes that had 23-G port creation had significantly less ocular surface inflammation compared to the 20-G group.[72]
Future directions and conclusions
The success of 23-G and 25-G instrumentation is driving the vision engineers to further smaller instrumentation. 27-G instrumentation has been introduced recently and has been applied in selected situations. This may lead to true ultra minimally invasive vitreous surgery.
In spite of the popularity of sutureless intervention 20-G surgery still has a significant role, especially in difficult situations, such as, retinal detachments with advanced proliferative vitreoretinopathy. In second place 20-G has importance in complex diabetic vitrectomies, which have compromised optic disc perfusion and are sensitive to high intraocular pressure, usually encountered in 23- or 25-G vitrectomies.
Finally, the issue of cost benefit cannot be ignored. In one large teaching center, the cost of sutureless microincision vitrectomy surgery was calculated to be 3.4 times higher than that of sutured 20-G vitrectomy surgery. Giving the patient the benefit of early postoperative recovery and earlier rehabilitation at thrice the expense, especially in a developing nation, is an issue to think upon.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: A pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:813–20. [PubMed] [Google Scholar]
- 2.Charles S, Calzada J, Wood B. Text Book of Vitreous Microsurgery. 2nd ed. London: Williams and Wilkins; 1999. p. 45. [Google Scholar]
- 3.Klöti R. [Vitrectomy. I. A new instrument for posterior vitrectomy] Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;187:161–70. doi: 10.1007/BF00411214. [DOI] [PubMed] [Google Scholar]
- 4.Chen JC. Sutureless pars plana vitrectomy through self-sealing sclerotomies. Arch Ophthalmol. 1996;114:1273–5. doi: 10.1001/archopht.1996.01100140473024. [DOI] [PubMed] [Google Scholar]
- 5.Kwok AK, Tham CC, Lam DS, Li M, Chen JC. Modified sutureless sclerotomies in pars plana vitrectomy. Am J Ophthalmol. 1999;127:731–3. doi: 10.1016/s0002-9394(98)00427-9. [DOI] [PubMed] [Google Scholar]
- 6.Gotzaridis EV. Sutureless Transconjunctival 20 Gauge pars plana Vitrectomy. Semin Ophthalmol. 2007;22:179–83. doi: 10.1080/08820530701501170. [DOI] [PubMed] [Google Scholar]
- 7.Patil BB, Mowatt L, Ho S, Scott R. Reverse self-sealing sclerostomies. Eye (Lond) 2005;19:1235–7. doi: 10.1038/sj.eye.6701748. [DOI] [PubMed] [Google Scholar]
- 8.Saad A, Assi A. Modified 20 gauge single-step sutureless sclerotomies for pars plana vitrectomy. Retina. 2009;29:848–53. doi: 10.1097/IAE.0b013e3181a3b71d. [DOI] [PubMed] [Google Scholar]
- 9.Lafetá AP, Claes C. Twenty-gauge transconjunctival sutureless vitrectomy trocar system. Retina. 2007;27:1136–41. doi: 10.1097/IAE.0b013e318150d846. [DOI] [PubMed] [Google Scholar]
- 10.de Juan E, Jr, Machemer R, Charles ST, Hirose T, Tasman WS, Trese MT. Surgery for stage 5 retinopathy of prematurity. Arch Ophthalmol. 1987;105:21. doi: 10.1001/archopht.1987.01060010023005. [DOI] [PubMed] [Google Scholar]
- 11.Fujii GY, De Juan E, Jr, Humayun MS, Pieramici DJ, Chang TS, Awh C, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109:1807–12. doi: 10.1016/s0161-6420(02)01179-x. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Josephberg RJ, Zaidman GW. Office based diagnostic pars plana vitrectomy. Invest Ophthalmol Vis Sci. 1996;37:402. [Google Scholar]
- 13.Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25:208–11. doi: 10.1097/00006982-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira LB, Reis PA. Silicone oil tamponade in 23-gauge transconjunctival sutureless vitrectomy. Retina. 2007;27:1054–8. doi: 10.1097/IAE.0b013e318113235e. [DOI] [PubMed] [Google Scholar]
- 15.Acar N, Kapran Z, Unver YB, Altan T, Ozdogan S. Early postoperative hypotony after 25-gauge sutureless vitrectomy with straight incisions. Retina. 2008;28:545–52. doi: 10.1097/IAE.0b013e318162b008. [DOI] [PubMed] [Google Scholar]
- 16.Byeon SH, Lew YJ, Kim M, Kwon OW. Wound leakage and hypotony after 25-gauge sutureless vitrectomy: Factors affecting postoperative intraocular pressure. Ophthalmic Surg Lasers Imaging. 2008;39:94–9. doi: 10.3928/15428877-20080301-04. [DOI] [PubMed] [Google Scholar]
- 17.Byeon SH, Chu YK, Lee SC, Koh HJ, Kim SS, Kwon OW. Problems associated with the 25-gauge transconjunctival sutureless vitrectomy system during and after surgery. Ophthalmologica. 2006;220:259–65. doi: 10.1159/000093081. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Shinoda K, Shinoda H, Kawamura R, Suzuki K, Ishida S. Two-step oblique incision during 25-gauge vitrectomy reduces incidence of postoperative hypotony. Clin Experiment Ophthalmol. 2007;35:693–6. doi: 10.1111/j.1442-9071.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- 19.Rizzo S, Genovesi-Ebert F. Angled incision techniques for 25-G and 23-G surgery. Euretina, Montecarlo. 2007 [Google Scholar]
- 20.Pollack J. Microincision vitrectomy surgery transitional course. American Academy of Ophthalmology meeting; 8 November; New Orleans. 2007. [Google Scholar]
- 21.Magalhaes O, Jr, Maia M, Maia A, Penha F, Dib E, Farah ME, et al. Fluid dynamics in three 25-gauge vitrectomy systems: Principles for use in vitreoretinal surgery. Acta Ophthalmol (Oxf) 2008;86:156–9. doi: 10.1111/j.1600-0420.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang CJ, Chang YH, Chiang SY, Lin LT. Comparison of clear corneal phacoemulsification combined with 25-gauge transconjunctival sutureless vitrectomy and standard 20-gauge vitrectomy for patients with cataract and vitreoretinal diseases. J Cataract Refract Surg. 2005;31:1198–207. doi: 10.1016/j.jcrs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 23.Kellner L, Wimpissinger B, Stolba U, Brannath W, Binder S. 25-gauge vs 20-gauge system for pars plana vitrectomy: A prospective randomised clinical trial. Br J Ophthalmol. 2007;91:945–8. doi: 10.1136/bjo.2006.106799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadonosono K, Yamakawa T, Uchio E, Yanagi Y, Tamaki Y, Araie M. Comparison of visual function after epiretinal membrane removal by 20-gauge and 25-gauge vitrectomy. Am J Ophthalmol. 2006;142:513–5. doi: 10.1016/j.ajo.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 25.Mentens R, Stalmans P. Comparison of postoperative comfort in 20 gauge versus 23 gauge pars plana vitrectomy. Bull Soc Belge Ophtalmol. 2009;311:5–10. [PubMed] [Google Scholar]
- 26.Deka S, Bhattacharjee H, Barman MJ, Kalita K, Singh SK. No-patch 23-gauge vitrectomy under topical anesthesia: A pilot study. Indian J Ophthalmol. 2011;59:143–5. doi: 10.4103/0301-4738.77038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avitabile T, Castiglione F, Bonfiglio V, Castiglione F. Transconjunctival sutureless 25-gauge versus 20-gauge standard vitrectomy: Correlation between corneal topography and ultrasound biomicroscopy measurements of sclerotomy sites. Cornea. 2010;29:19–25. doi: 10.1097/ICO.0b013e3181ab98ae. [DOI] [PubMed] [Google Scholar]
- 28.Park DH, Shin JP, Kim SY. Surgically induced astigmatism in combined phacoemulsification and vitrectomy; 23-gauge transconjunctival sutureless vitrectomy versus 20-gauge standard vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1331–7. doi: 10.1007/s00417-009-1109-3. [DOI] [PubMed] [Google Scholar]
- 29.Jampel HD, Thompson JT, Nunez M, Michels RG. Corneal astigmatic changes after pars plana vitrectomy. Retina. 1987;7:223–6. doi: 10.1097/00006982-198707040-00004. [DOI] [PubMed] [Google Scholar]
- 30.Dalma-Weiszhausz J, Gordon-Angelozzi M, Ustariz-Gonzalez O. Intraocular pressure rise during 25-gauge vitrectomy trocar placement. Graefes Arch Clin Exp Ophthalmol. 2008;246:187–9. doi: 10.1007/s00417-007-0713-3. [DOI] [PubMed] [Google Scholar]
- 31.Wong RW, Kokame GT, Mahmoud TH, Mieler WF, Tornambe PE, McDonald HR. Complications associated with clear corneal cataract wounds during vitrectomy. Retina. 2010;30:850–5. doi: 10.1097/IAE.0b013e3181c70111. [DOI] [PubMed] [Google Scholar]
- 32.Wu PC, Tiong IS, Chuang YC, Kuo HK. Twisting maneuver for sutureless vitrectomy trocar insertion to reduce intraoperative intraocular pressure rise. Retina. 2011;31:887–92. doi: 10.1097/IAE.0b013e3181f57d8a. [DOI] [PubMed] [Google Scholar]
- 33.Tarantola RM, Folk JC, Shah SS, Boldt HC, Abràmoff MD, Russell SR, et al. Intraoperative choroidal detachment during 23-gauge vitrectomy. Retina. 2011;31:893–901. doi: 10.1097/IAE.0b013e3181f4429b. [DOI] [PubMed] [Google Scholar]
- 34.Gupta OP, Weichel ED, Regillo CD, Fineman MS, Kaiser RS, Ho AC, et al. Postoperative complications associated with 25-gauge pars plana vitrectomy. Ophthalmic Surg Lasers Imaging. 2007;38:270–5. doi: 10.3928/15428877-20070701-01. [DOI] [PubMed] [Google Scholar]
- 35.Scartozzi R, Bessa AS, Gupta OP. Intraoperative sclerotomy-related retinal breaks for macular surgery, 20- vs 25-gauge vitrectomy systems. Am J Ophthalmol. 2007;143:155–6. doi: 10.1016/j.ajo.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 36.Hikichi T, Matsumoto N, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, et al. Comparison of one-year outcomes between 23- and 20-gauge vitrectomy for preretinal membrane. Am J Ophthalmol. 2009;147:639–43. doi: 10.1016/j.ajo.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Tewari A, Shah GK, Fang A. Visual outcomes with 23-gauge transconjunctival sutureless vitrectomy. Retina. 2008;28:258–62. doi: 10.1097/IAE.0b013e318159ec5a. [DOI] [PubMed] [Google Scholar]
- 38.Sallam A, Zambarakji HJ. Infusion aspiration mismatch during 25-gauge vitrectomy with conversion to 20-gauge vitrector. Ann Ophthalmol (Skokie) 2008;40:51–2. [PubMed] [Google Scholar]
- 39.Shinoda H, Nakajima T, Shinoda K, Suzuki K, Ishida S, Inoue M. Jamming of 25-gauge instruments in the cannula during vitrectomy for vitreous haemorrhage. Acta Ophthalmol. 2008;86:160–4. doi: 10.1111/j.1600-0420.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 40.Inoue M, Noda K, Ishida S, Nagai N, Imamura Y, Oguchi Y. Intraoperative breakage of a 25-gauge vitreous cutter. Am J Ophthalmol. 2004;138:867–9. doi: 10.1016/j.ajo.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 41.Cardascia N, Boscia F, Furino C. Gentamicin-induced macular infarction in transconjunctival sutureless 25-gauge vitrectomy. Int Ophthalmol. 2008;28:383–5. doi: 10.1007/s10792-007-9148-4. [DOI] [PubMed] [Google Scholar]
- 42.O'Reilly P, Beatty S. Transconjunctival sutureless vitrectomy: Initial experience and surgical tips. Eye (Lond) 2007;21:518–21. doi: 10.1038/sj.eye.6702255. [DOI] [PubMed] [Google Scholar]
- 43.Kusuhara S, Ooto S, Kimura D, Itoi K, Mukuno H, Miyamoto N, et al. Outcomes of 23- and 25-gauge transconjunctival sutureless vitrectomies for idiopathic macular holes. Br J Ophthalmol. 2008;92:1261–4. doi: 10.1136/bjo.2008.140533. [DOI] [PubMed] [Google Scholar]
- 44.Amato JE, Akduman L. Incidence of complications in 25-gauge transconjunctival sutureless vitrectomy based on the surgical indications. Ophthalmic Surg Lasers Imaging. 2007;38:100–2. doi: 10.3928/15428877-20070301-02. [DOI] [PubMed] [Google Scholar]
- 45.Teixeira A, Allemann N, Yamada AC, Uno F, Maia A, Bonomo PP. Ultrasound biomicroscopy in recently postoperative 23-gauge transconjunctival vitrectomy sutureless self-sealing sclerotomy. Retina. 2009;29:1305–9. doi: 10.1097/IAE.0b013e3181b09487. [DOI] [PubMed] [Google Scholar]
- 46.López-Guajardo L, Vleming-Pinilla E, Pareja-Esteban J, Teus-Guezala MA. Ultrasound biomicroscopy study of direct and oblique 25-gauge vitrectomy sclerotomies. Am J Ophthalmol. 2007;143:881–3. doi: 10.1016/j.ajo.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 47.Singh RP, Bando H, Brasil OF, Williams DR, Kaiser PK. Evaluation of wound closure using different incision techniques with 23-gauge and 25-gauge microincision vitrectomy systems. Retina. 2008;28:242–8. doi: 10.1097/IAE.0b013e318156dea3. [DOI] [PubMed] [Google Scholar]
- 48.Sawada T, Kakinoki M, Sawada O, Kawamura H, Ohji M. Closure of sclerotomies after 25- and 23-gauge transconjunctival sutureless pars plana vitrectomy evaluated by optical coherence tomography. Ophthalmic Res. 2011;45:122–8. doi: 10.1159/000318875. [DOI] [PubMed] [Google Scholar]
- 49.Nagpal M, Wartikar S, Nagpal K. Comparison of clinical outcomes and wound dynamics of sclerotomy ports of 20, 25, and 23 gauge vitrectomy. Retina. 2009;29:225–31. doi: 10.1097/IAE.0b013e3181934908. [DOI] [PubMed] [Google Scholar]
- 50.Nakano T, Uemura A, Sakamoto T. Incidence of Iatrogenic retinal breaks in 23 gauge vitrectomy for macular diseases. Retina. 2011;31:1997–2001. doi: 10.1097/IAE.0b013e31820f49ea. [DOI] [PubMed] [Google Scholar]
- 51.Taylor SR, Aylward GW. Endophthalmitis following 25-gauge vitrectomy. Eye (Lond) 2005;19:1228–9. doi: 10.1038/sj.eye.6701737. [DOI] [PubMed] [Google Scholar]
- 52.Gupta OP, Maguire JI, Eagle RC, Jr, Garg SJ, Gonye GE. The competency of pars plana vitrectomy incisions: A comparative histologic and spectrophotometric analysis. Am J Ophthalmol. 2009;147:243–50. doi: 10.1016/j.ajo.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Scott IU, Flynn HW, Jr, Dev S, Shaikh S, Mittra RA, Arevalo JF, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: Incidence and outcomes. Retina. 2008;28:138–42. doi: 10.1097/IAE.0b013e31815e9313. [DOI] [PubMed] [Google Scholar]
- 54.Kunimoto DY, Kaiser RS Wills Eye Retina Service. Incidence of endophthalmitis after 20- and 25-Gauge vitrectomy. Ophthalmology. 2007;114:2133–7. doi: 10.1016/j.ophtha.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Taban M, Ufret-Vincenty RL, Sears JE. Endophthalmitis after 25-gauge transconjunctival sutureless vitrectomy. Retina. 2006;26:830–1. doi: 10.1097/01.iae.0000244272.13890.cc. [DOI] [PubMed] [Google Scholar]
- 56.Wu L, Berrocal MH, Arévalo JF, Carpentier C, Rodriguez FJ, Alezzandrini A, et al. Endophthalmitis after pars plana vitrectomy: Results of the Pan American Collaborative Retina Study Group. Retina. 2011;31:673–8. doi: 10.1097/IAE.0b013e318203c183. [DOI] [PubMed] [Google Scholar]
- 57.Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Effect of operative field irrigation on intraoperative bacterial contamination and postoperative endophthalmitis rates in 25-gauge vitrectomy. Retina. 2010;30:1242–9. doi: 10.1097/IAE.0b013e3181cea6ab. [DOI] [PubMed] [Google Scholar]
- 58.Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Vitreous prolapse through the scleral wound in 25-gauge transconjunctival vitrectomy. Eur J Ophthalmol. 2008;18:659–62. doi: 10.1177/112067210801800431. [DOI] [PubMed] [Google Scholar]
- 59.Fine HF, Iranmanesh R, Iturralde D, Spaide RF. Outcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmology. 2007;114:1197–200. doi: 10.1016/j.ophtha.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 60.Ibarra MS, Hermel M, Prenner JL, Hassan TS. Long term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol. 2005;139:831–6. doi: 10.1016/j.ajo.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Lakhanpal RR, Humayun MS, de Juan E, Jr, Lim JI, Chong LP, Chang TS, et al. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112:817–24. doi: 10.1016/j.ophtha.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 62.Oshima Y, Ohji M, Tano Y. Surgical outcomes of 25-gauge transconjunctival vitrectomy combined with cataract surgery for vitreoretinal diseases. Ann Acad Med Singapore. 2006;35:175–80. [PubMed] [Google Scholar]
- 63.Heimann H. Primary 25 and 23 gauge vitrectomy in the treatment of rhegmatogenous retinal detachment: Advancement of surgical technique or erroneous trend? Klin Monbl Augenheild. 2008;225:947–56. doi: 10.1055/s-2008-1027917. [DOI] [PubMed] [Google Scholar]
- 64.Lai MM, Ruby AJ, Sarrafizadeh R, Urban KE, Hassan TS, Drenser KA, et al. Repair of primary rhegmatogenous retinal detachment using 25-gauge transconjunctival sutureless vitrectomy. Retina. 2008;28:729–34. doi: 10.1097/IAE.0b013e318162b01c. [DOI] [PubMed] [Google Scholar]
- 65.Miller DM, Riemann CD, Foster RE, Petersen MR. Primary repair of retinal detachment with 25-gauge pars plana vitrectomy. Retina. 2008;28:931–6. doi: 10.1097/IAE.0b013e31816b313a. [DOI] [PubMed] [Google Scholar]
- 66.Yuen CY, Cheung BT, Tsang CW, Lam RF, Baig NB, Lam DS. Surgically induced astigmatism in phacoemulsification, pars plana vitrectomy, and combinedphacoemulsification and vitrectomy: A comparative study. Eye (Lond) 2009;23:576–80. doi: 10.1038/eye.2008.57. [DOI] [PubMed] [Google Scholar]
- 67.Park DH, Shin JP, Kim SY. Combined 23-gauge microincisonal vitrectomy surgery and phacoemulsification with AcrySof toric intraocular lens implantation: A comparative study. Eye (Lond) 2011;25:1327–32. doi: 10.1038/eye.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee DK, Lee SJ, You YS. Prediction of refractive error in combined vitrectomy and cataract surgery with one-piece acrylic intraocular lens. Korean J Ophthalmol. 2008;22:214–9. doi: 10.3341/kjo.2008.22.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapran Z, Acar N. Removal of silicone oil with 25-gauge transconjunctival sutureless vitrectomy system. Retina. 2007;27:1059–64. doi: 10.1097/IAE.0b013e3180592c13. [DOI] [PubMed] [Google Scholar]
- 70.Cekiç O, Cakir M, Yilmaz OF. Passive silicone oil removal in 23-gauge transconjunctival vitrectomy. Ophthalmic Surg Lasers Imaging. 2011;42:514–5. doi: 10.3928/15428877-20111017-01. [DOI] [PubMed] [Google Scholar]
- 71.Kapran Z, Acar N, Unver YB, Altan T, Ocak B. Passive removal of silicone oil with a 25-gauge sutureless system. Jpn J Ophthalmol. 2008;52:63–6. doi: 10.1007/s10384-007-0490-x. [DOI] [PubMed] [Google Scholar]
- 72.Patwardhan SD, Azad R, Shah V, Sharma Y. The safety and efficacy of passive removal of silicone oil with 23-gauge transconjunctival sutureless system. Retina. 2010;30:1237–41. doi: 10.1097/IAE.0b013e3181dde612. [DOI] [PubMed] [Google Scholar]


