Abstract
Background:
Restless legs syndrome (RLS) is a common movement disorder. The occurrence of this syndrome is due to genetic factors and lifestyle. This study performed to determine restless legs syndrome (RLS) prevalence in Iranian multiple sclerosis (MS) patients and the possible risk factors.
Methods:
This cross-sectional study was conducted with MS patients, and the age- and sex-matched control group comprised healthy persons. Then, all subjects were asked about RLS symptoms. After the diagnosis of RLS, the patients were divided into two groups: With and without RLS. In both groups, the following variables were evaluated: Age, sex, other underlying disease, duration of MS, MS course, family history of RLS, history of anemia, and drug intakes. The severity of the disease in subjects diagnosed with RLS was also evaluated.
Results:
A total of 126 patients in the MS group and 126 healthy controls were included in the study, with no statistically significant differences between them in terms of age and gender. In MS group, 82 (65.1%) and, in control group, 16 (12.7%) had RLS. The frequency of RLS in the MS patients was significantly higher than that in the control group. Among MS patients, 60 male (73.2%) and 22 female (26.8%) had RLS. Mean age of MS patients with RLS was significantly higher than that in MS patients without RLS. MS patients and higher EDSS score had more RLS symptoms.
Conclusions:
We suggest that RLS always be considered during neurological examinations of MS patients.
Keywords: Case-control, Iran, multiple sclerosis, restless legs syndrome
INTRODUCTION
Restless legs syndrome (RLS) is a common movement disorder that affects up to 5-10% of the general population.[1–3] RLS is an important cause of sleep disruption and disability in several clinical and neurological disorders and can be identified on the basis of clinical criteria.[4] The four criteria for considering this diagnosis include, an urge to move the legs (usually accompanied or caused by uncomfortable leg sensations), temporary relief with movement, onset or worsening of symptoms during rest, and worsening or onset of symptoms at evening and/or at night.[4] The diagnosis is further supported by the presence of periodic limb movements in sleep (PLMS) and positive response to dopaminergic treatment.[5]
The majorities of RLS cases is commonly classified as idiopathic and include sporadic and inherited forms.[5] The terms “symptomatic” or “secondary” refer to RLS forms significantly related to physiological or pathological conditions, such as iron deficiency.[6] Among the neurological disorders, peripheral neuropathy, spinocerebellar ataxia, essential tremor, Parkinson disease, and myelopathies are reported to be associated with RLS.[7–9] Available studies suggest that RLS prevalence among MS patients is higher compared with people without MS.[10–12] However, MS has not been considered as a secondary cause for RLS yet.[12] We sought to determine RLS prevalence in Iranian MS patient and the possible risk factors for the same.
METHODS
Study design
A cross-sectional study was conducted at the University Hospital of Kashani, Isfahan, Iran, from September to December 2011. Patients diagnosed with MS according to McDonald Criteria[13] were recruited from MS outpatient clinic in Kashani Hospital; the control group comprised of healthy volunteers, healthcare personnel, or companions of the patients. Then all subjects were asked about RLS symptoms. The diagnosis of RLS in patients was based on the four diagnostic criteria of RLS according to the revised IRLSSG guidelines.[4] After the diagnosis of RLS, the patients were divided into two groups: With and without RLS. The present study was approved by the Ethical Committee of Isfahan University of Medical Sciences.
Inclusion criteria
Inclusion criteria for the study were patients with definite MS according to the standard diagnostic criteria for MS and with an age of 25-65 years. There was no specific limitations regarding chronic MS treatments such as interferon, cyclophosphamide, or azatioprine.
Exclusion criteria
Exclusion criteria for the study were the usage of dopaminergic and antidopaminergic drugs, renal failure, pregnancy, sideropenic anemia, recent MS diagnosis (< 6 months before the time of the interview), recent clinical MS relapse (within 3 months of the interview), and history of alcohol or drug abuse. Patients who received a recent (within 6 months from the time of the interview) acute high-dose steroid treatment were excluded.
Assessment procedures
The diagnosis of RLS in the patients was based on the four diagnostic criteria of RLS according to the revised IRLSSG guidelines. Only those patients fulfilling all four criteria were assigned to the RLS population. In both groups, the following variables were evaluated: Age, sex, other underlying disease, duration of MS, MS course, family history of RLS, history of anemia, and drug intakes (related to MS). The severity of the disease in subjects diagnosed with RLS was also evaluated. Neurological examination of all patients was carried out and their disability was defined according to Expanded Disability Status Scale score and by using points for functional systems.
Statistical analysis
Statistical analysis was done by utilizing SPSS 18.0. Continuous and categorical variables were articulated as mean and percentages, respectively. Parametric and nonparametric comparisons of categorical and continuous variables were performed using the Chi-square test, Student t-test, and correlation test, as suitable. P < 0.05 were the threshold of statistical significance.
RESULTS
A total of 126 patients in the MS group (96 females, 30 males, mean age 32.37 ± 8.7 years) and 126 healthy controls (96 females, 30 males, and mean age 33.34 ± 8.3 years) were included in the study. There were no statistically significant differences between the 2 groups in terms of age and gender (33.34 ± 8.3). Mean EDSS score in MS groups was 1.8 ± 1.74 (range 0-7). Mean EDSS score in women were 1.62 ± 1.67 and, in men, it was 2.41 ± 1.9. According to statistical analysis, there was significant difference in disability between two sex (P < 0.035) [Figure 1]. The demographic characters of patients are shown in Table 1.
Figure 1.
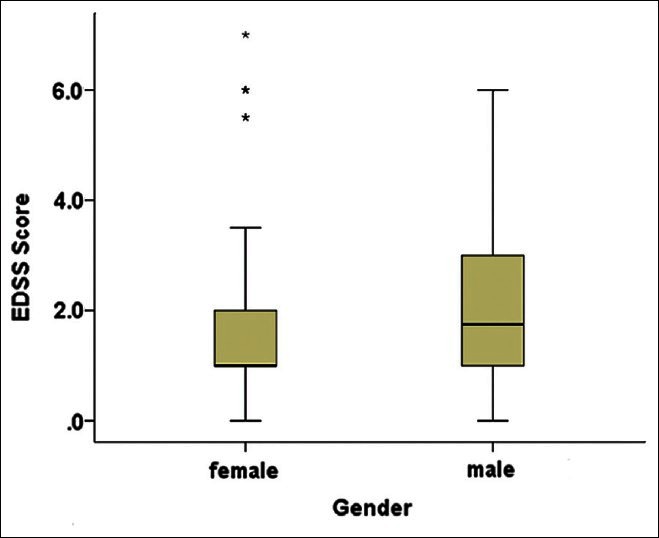
Mean range and 25%, 75% of EDSS score
Table 1.
Demographic characteristics of patients in the case and control population

MS duration was 43.2 ± 33.4 months (range 3-204 month). According to Pierson correlation test, there was significant correlation between MS duration and EDSS score (P < 0.001). According to this test, correlation between MS duration and EDSS was 0.39 [Figure 2].
Figure 2.
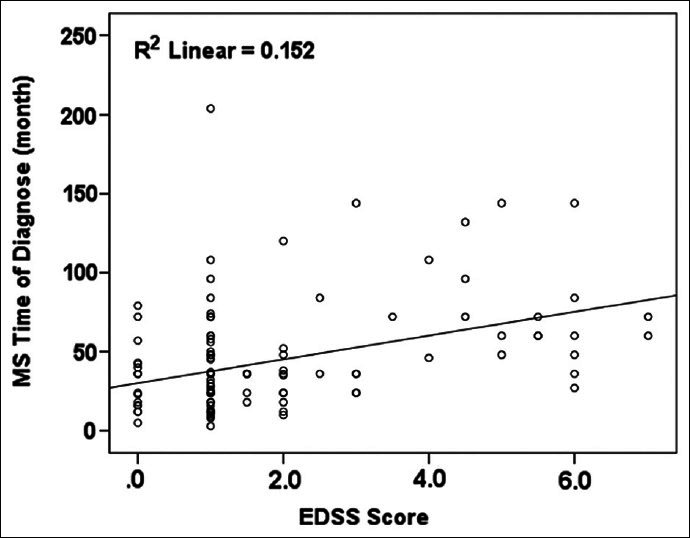
Correlation between MS duration and EDSS score
In this study, 82 (65.1%) in the MS group and 16 (12.7%) in the control group had RLS. The frequency of RLS in the MS patients was significantly higher than that in the control group (P < 0.001). Among MS patients, 60 male (73.2%) and 22 female (26.8%) had RLS. In this study, the mean age of MS patients with RLS was 33.8 ± 9 years. This was significantly higher than MS patients without RLS (P < 0.05).
Patients with MS and higher EDSS score had more RLS symptoms (P < 0.005).
Other significant factors include family history of RLS and MS predominant sign. Mean RLS score was 10.9 ± 10.2 in the MS group and 17.9 ± 6.6 in the control group. According to t test, RLS score was significantly higher in the control groups (P = 0.008) [Figure 3].
Figure 3.
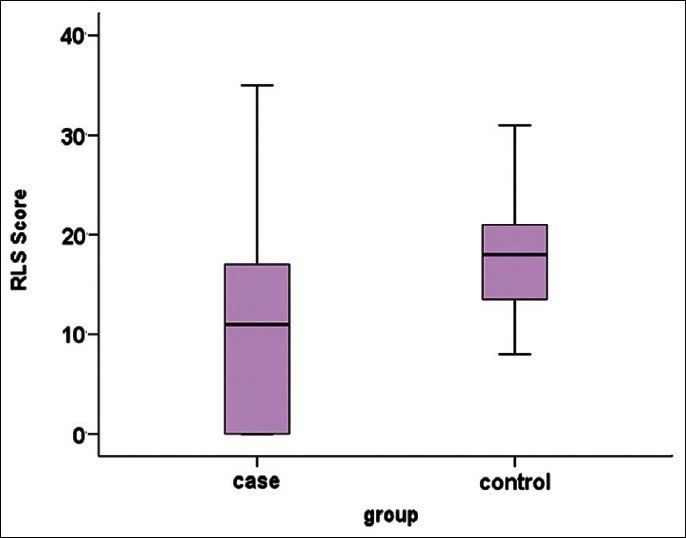
Mean range and 25%, 75% of RLS score
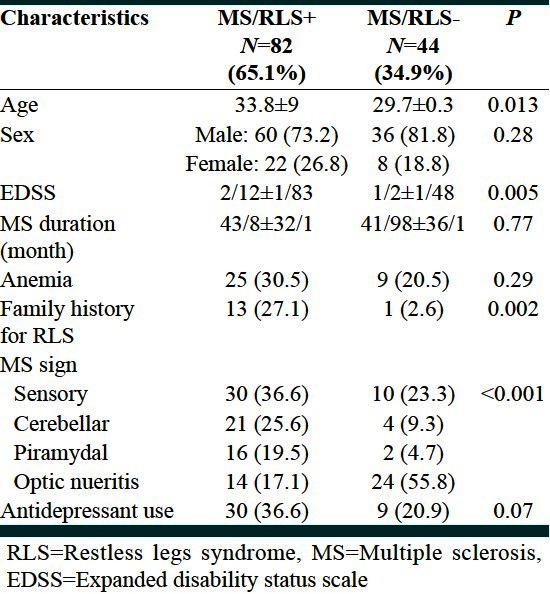
Table 2 showed characteristics of MS patients with and without RLS.
Table 2.
Characteristics of MS patients without and with RLS
DISCUSSION
RLS is common in MS patients, and this has been investigated in several studies. In our study, the prevalence of RLS was 65.1%. In other studies, the prevalence of RLS in MS patients has been reported to be ranging from 13.3 to 57.5%.[14–17] In our study, older MS patients had higher prevalence of RLS symptoms; Similar findings were obtained in other studies.[10,18]
Although the biological mechanism linking MS and RLS is unknown, one study comparing MRI parameters between MS patients with and without RLS found no difference in brain MRI scans, but did find more tissue damage in the cervical spinal cords of patients with MS with RLS as compared with patients with MS without RLS.[17] Low iron levels in the brain and iron deficiency have been associated with RLS.[19]
However, in this study, we did not find significant difference in the prevalence of iron deficiency anemia in MS patients with or without RLS (P < 0.29).
An important aspect that differentiated patients with and without RLS was clinical severity of MS according to the EDSS score. The number of patients with higher EDSS score were significantly superior in the MS/RLS positive group (P < 0.005). The same finding has been investigated in other studies.[10,18]
The MS/RLS positive phenotype may be the result of a particular lesion pattern, which eventually involves specific unknown nerve centers or their connection pathways important for the pathogenesis of RLS. Because inflammatory damages in MS are randomly disseminated in the central nervous system, the higher likelihood of severe MS patients developing secondary RLS might be simply due to a probabilistic reason. In patients with a higher number of lesions, there are more chances of impaired specific neurological regions involved in the pathogenesis of RLS than in patients with a milder MS course.[18]
We determined that the difference in severity of RLS between our MS group and controls was statistically significant (the severity was greater in control group). This is in contrast with a lot of studies that found that severity of RLS was greater than in the MS group.[10] Our hypothesis is that MS patients have other complaints like spasticity and weakness in their lower limbs, so may be their complaint of RLS severity is less than in the normal subjects. We found that MS patients and RLS had higher family history of RLS than MS patients without RLS (P ≤ 0.002) This finding is compatible with a few studies.[18] This finding show that RLS in MS patients may be affected by primary RLS.
In the present study, no differences were found between groups with and without RLS in their gender, MS duration, anemia, and antidepressant use. Another parameter that affects RLS prevalence in MS is the predominant sign of disease. Those who have sensory symptoms significantly have higher prevalence of RLS. Current concepts of RLS assume a dysfunction of the dopaminergic system; this may involve the striatum, the spinal cord, and the hypothalamus. They are involved in modulating spinal excitability and sensory processing of leg afferents. Damages including demyelization along this route may cause a dysfunction of the dopaminergic system manifesting as RLS. This concept is supported by reports of RLS in patients with spinal cord lesions.[20]
The high prevalence rate of RLS in MS patients, the association between RLS and more severe disability are the central aspects supporting the hypothesis of a new symptomatic form of RLS secondary to the central nervous system damage. As for other neurological diseases, such as Parkinson's disease, spinocerebellar atrophy, and peripheral neuropathies, MS should be considered as another risk factor for RLS.[15–18]
CONCLUSIONS
We suggest that RLS always be considered during neurological examinations of MS patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Etemadifar M, Abtahi SH. Multiple sclerosis in Isfahan, Iran: Past, Present and Future. Int J Prev Med. 2012;3:301–2. [PMC free article] [PubMed] [Google Scholar]
- 2.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Zucconi M, Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5:293–9. doi: 10.1016/j.sleep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–46. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Abele M, Burk K, Laccone F, Dichgans J, Klockgether T. Restless legs syndrome in spinocerebellar ataxia types 1, 2, and 3. J Neurol. 2001;248:311–4. doi: 10.1007/s004150170206. [DOI] [PubMed] [Google Scholar]
- 8.Ondo WG, Lai D. Association between restless legs syndrome and essential tremor. Mov Disord. 2006;21:515–8. doi: 10.1002/mds.20746. [DOI] [PubMed] [Google Scholar]
- 9.Rye DB. Parkinson's disease and RLS: The dopaminergic bridge. Sleep Med. 2004;5:317–28. doi: 10.1016/j.sleep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Aydar G, Kurt S, Karaer Unaldi H, Erkorkmaz U. Restless legs syndrome in multiple sclerosis. Eur Neurol. 2011;65:302–6. doi: 10.1159/000327315. [DOI] [PubMed] [Google Scholar]
- 11.Canturk I, Turkes M, Isik N, Aydin T, Arici Duz O, Candan F. Restless legs syndrome in multiple sclerosis. Mult Scler. 2010;16:S220. [Google Scholar]
- 12.Deriu M, Cossu G, Molari A, Murgia D, Mereu A, Ferrigno P, et al. Restless legs syndrome in multiple sclerosis: A case-control study. Mov Disord. 2009;24:697–701. doi: 10.1002/mds.22431. [DOI] [PubMed] [Google Scholar]
- 13.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 14.Manconi M, Ferini-Strambi L, Filippi M, Bonanni E, Iudice A, et al. Italian REMS Study Group. Multicenter case-control study on restless legs syndrome in multiple sclerosis: The REMS study. Sleep. 2008;31:944–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Fragoso YD, Finkelsztejn A, Gomes S, Kaimen-Maciel DR, Oliveira CL, Lopes J, et al. Restless legs syndrome and multiple sclerosis: A Brazilian multicenter study and meta-analysis of the literature. Arq Neuropsiquiatr. 2011;69:180–3. doi: 10.1590/s0004-282x2011000200007. [DOI] [PubMed] [Google Scholar]
- 16.Moreira NC, Damasceno RS, Medeiros CA, Bruin PF, Teixeira CA, Horta WG, et al. Restless leg syndrome, sleep quality and fatigue in multiple sclerosis patients. Braz J Med Biol Res. 2008;41:932–7. doi: 10.1590/s0100-879x2008001000017. [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Choco MJ, Iranzo A, Blanco Y, Graus F, Santamaria J, Saiz A. Prevalence of restless legs syndrome and REM sleep behavior disorder in multiple sclerosis. Mult Scler. 2007;13:805–8. doi: 10.1177/1352458506074644. [DOI] [PubMed] [Google Scholar]
- 18.Manconi M, Fabbrini M, Bonanni E, Filippi M, Rocca M, Murri L, et al. High prevalence of restless legs syndrome in multiple sclerosis. Eur J Neurol. 2007;14:534–9. doi: 10.1111/j.1468-1331.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 19.van Toorn R, Schoeman JF, Solomons R, Rensburg MA, van Rensburg SJ. Iron status in children with recurrent episodes of tumefactive cerebral demyelination. J Child Neurol. 2010;25:1401–7. doi: 10.1177/0883073810366179. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann M, Pfister R, Pfadenhauer K. Restless legs syndrome associated with spinal cord lesions. J Neurol Neurosurg Psychiatry. 1999;66:688–9. doi: 10.1136/jnnp.66.5.688a. [DOI] [PMC free article] [PubMed] [Google Scholar]


