Abstract
Background:
Alzheimer's disease (AD) is a progressive neurodegenerative disease and nowadays the role of endothelial cell (EC) injury has been proposed in pathological process in AD. Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist has anti-inflammatory properties through activation in glial cells and improves vascular function and prevent atherosclerotic disease progression. The aim of this study is evaluation of pioglitazone effects as a drug of PPAR-γ agonist on endothelial apoptosis induced by sera from AD patients.
Methods:
Human umbilical vein endothelial cells (HUVECs) were treated with sera from AD patients (n = 10) and sera from controls (n = 10). Apoptosis was identified by annexin V-propidium iodide staining and cell death detection kit. Apoptosis was evaluated after and before adding of 10 μM pioglitazone on EC. Nitrite (NO2-) levels were determined in the culture supernatants.
Results:
Induced apoptosis by the serum of patients was inhibited markedly when pioglitazone used before treating HUVECs with the sera of AD. Also, the measurement of nitrite concentration showed significantly greater levels of dissolved NO2/NO3 metabolite in the culture media of HUVECs treated by sera of AD patients (P < 0.05), while the rate of nitric oxide significantly decreased when pioglitazone exists in culture media.
Conclusion:
Further studies are justified to investigate the novel role of the PPARs in the prevention of the neuronal and endothelial damage in neurological disorder and present a new therapeutic approach for Alzheimer's patients.
Keywords: Alzheimer's disease, apoptosis, endothelial cell
INTRODUCTION
Alzheimer's disease (AD) is a vascular disorder and neurodegenerative disease in the elderly.[1] Hypertension, diabetes, hypercholesterolemia, hyperhomocysteinemia, the apolipoprotein-4 genotype, and endothelial cell (EC) and microvascular injury are also important risk factors for AD.[2] Collaboration of endothelial dysfunction has been indicated in many acute and chronic neuroinflammatory diseases.[3] Apoptosis could be induced by many stimuli in vitro endothelial cells, suggesting that endothelial apoptosis is one of the important mechanism in CNS (central nervous system) vascular injury and proceeding inflammation.[4]
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a ligand-activated nuclear transcription factor[5] that is mainly expressed in endothelial cell, the immune system,[6] and also neuronal cultures.[7] It is a target of the class of drugs known as thiazolidinediones (TZDs) and commonly used to treat type II diabetes,[8] due to inhibitory action on microglial activation and neuronal damage.[9]
Recently, TZDs have received a great interest as potential therapeutic drugs for neurodegenerative diseases, traumatic injuries, and demyelinating diseases.[9] It has been presented that TZDs can attenuate neurodegeneration of experimental autoimmune encephalomyelitis (EAE).[10] PPAR-g agonists have anti-inflammatory properties through activation in glial cells,[11–13] also improve vascular function, and prevent atherosclerotic disease progression.[1–3]
On the contrary, induction of apoptosis was observed when sera from AD was exposed to endothelial cells suggesting that EC cultures represent an important model to study inflammatory mediators and to evaluate the therapeutic effect of anti- inflammatory molecules in AD and other neurodegenerative disorders.[14,15]
In the current study, we aimed to investigate whether pioglitazone as a drug of TZDs class could prevent endothelial apoptosis which induced by sera from AD patients.
METHODS
The study was performed in Departments of Physiology, Applied Physiology Research Center, and Neurology Outpatient Department of Al-Zahra Hospital, Isfahan University of Medical Sciences, between July 2010 and June 2011. A complete explanation of the study was given to each patient; written informed consent was received from all patients. The study protocol was reviewed and approved by the ethics in Research Committee, Isfahan University of Medical Sciences.
Patients and sample collection
Ten patients with AD and 10 healthy controls age- and sex-matched healthy subjects were eligible to participate in the study. Diagnosis of AD was based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and AD and Related Disorders Association.[16] Patients with a history of drug abuse, chronic systemic diseases, such as diabetes mellitus, hypertension, coronary heart disease, cigarette smoking, alcohol abuse, or acute illness, severe head injury, or seizure disorders, and who were treated with electroconvulsive therapy, major depression, cerebrovascular disease, intoxication and metabolic abnormalities and finally dementia that caused by other diseases than AD were ineligible for this study. Peripheral venous blood from 10 ADs and 10 healthy controls was sampled into serum tubes. To minimize the source of platelets, serum was centrifuged within 30 min after sampling and stored at –80°C until further analysis.
Cell culture
Human umbilical vein endothelial cells (HUVECs) (National Cell Bank of Iran affiliated with the Pasteur Institute, Tehran, Iran) were cultured in endothelial basal medium supplemented with, gentamicin, amphotericin B, and 10% fetal calf serum until the third passage before experiments was performed.
For evaluation effects of pioglitazone on HUVECs treated with sera of AD, we arranged different groups; in the first group, HUVECs were only treated by sera from AD for 24 h, in the second group HUVECs were treated by 10 μM pioglitazone (dissolved in dimethyl sulfoxide (DMSO)[17,18] for 24 h and then sera from AD was added to these cells for another 24 h. In the third group, HUVECs were exposed in the sera of AD for 24 h and then 10 μM pioglitazone was added to these cells for another 24 h. In the fourth group, HUVECs were treated by sera from healthy individuals for 24 h.
Apoptosis analysis
Flow cytometry and cell-death detection kit were used for apoptosis assessment in HUVECs. A total number of 105 cells were washed with ice-cold PBS once and were stained with annexin-PI as follows: Cells (105 /mL) were incubated with 1 μL annexin V-fluorescein isothiocyanate and 0.5 μL propidium iodide (PI, 10 mg/mL) in binding buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2). Subsequently, the cells were analyzed by fluorescence-activated cell sorting (FACScan, Becton-Dickinson). Apoptotic cells were designated as annexin-V+/PI– (povidone Iodine) cells. Data were analyzed by Cell Quest software. As an additional measure of apoptotic cell death, we assessed the formation of histone-associated DNA fragments by the Cell Death Detection ELISA kit from Roche (Basel, Switzerland) 21 as previously mentioned or according to the manufacturer instruction.
NO metabolite (NO2) measurement
NO metabolite in culture supernatants was determined using the Griess reaction (Parameter TM, total NO Assay kit, R and D Systems, and USA) according to manufacturer's instructions. Briefly, in this assay, nitrite is detected colorimetrically as an azo dye product of the Griess reaction. The Griess reaction is based on the two-step diazotization reaction in which acidified NO2 produces a nitrosating agent, which reacts with sulfanilic acid to produce the diazonium ion. This ion is then coupled to N-(1-naphthyl) ethylenediamine to form the chromophore azoderivative which absorbs light at 560 nm wavelength. Values were calculated using a standard curve using a standard curve produced with sodium nitrite.
Statistical analysis
The data were expressed as mean ± SE (standard error of mean). One-way analysis of variance followed by the Tukey's post hoc test was utilized for data analysis. All experiments were performed in three independent replicates. The data were analyzed using SPSS 16.0 statistical package (SPSS Inc.). P value more than 0.05 was regarded as significant.
RESULTS
Twenty-four hour treatment of HUVECs with the sera of untreated AD resulted in significantly greater apoptosis than healthy controls [P < 0.05, Figure 1]. There were no significant differences in the apoptosis rates of HUVECs between patients with AD (P > 0.05).
Figure 1.
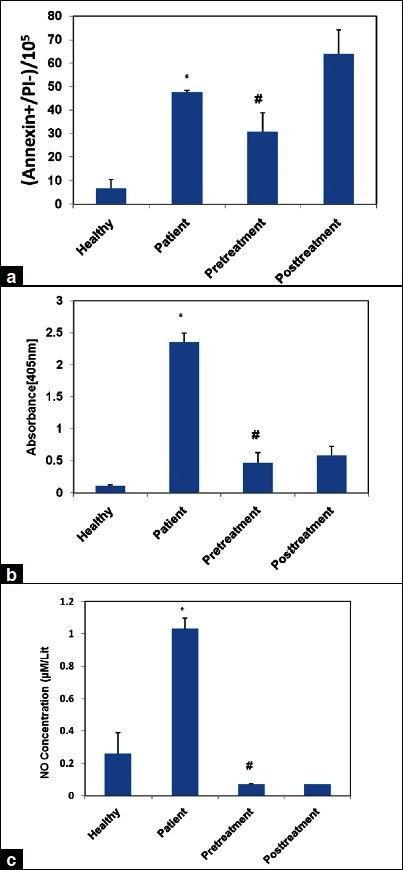
Induction of apoptosis in human umbilical vein endothelial cells (HUVECs) by sera from patients with Alzheimer's disease (AD) patients versus control group. Apoptosis rates of HUVECs were measured after exposure to media containing 10% serum of patients with AD or control group for 24 h. Assessment of suppression of AD serum-induced apoptosis by pre and post-treatment of pioglitazone (10 μM) for 24 h with flow cytometry (a) and cell death detection Kit (b). The concentration of nitrite in the culture medium was determined (c). %P< 0.001 versus healthy and # P< 0.05 versus patients
We examined the effects of pioglitazone on cultured EC apoptosis in two groups: 24 h before (pretreatment) and 24 h after (post treatment) adding patients’ serum. The addition of pioglitazone inhibited clearly the induction of apoptosis by the serum of patients only when used before treating HUVECs with the sera of AD [Figure 1a] [(mean ± SD) in (groups) control; 6.58 ± 3.79, in patients; 47.78 ± 0.65, in pretreatment; 30.65 ± 8.05; and in post treatment; 63.82 ± 10.39 (P ≤ 0.05)). Also apoptosis in different groups was evaluated by cell death detection kit determining internucleosomal degradation of genomic DNA during apoptosis. Again, we identified rising apoptosis rate in AD group in comparison with control group. Data showed pioglitazone pretreatment can distinctly overcome apoptosis HUVECs induced by the sera of patients [Figure 1b] [(mean ± SD) in (groups) control; 0.11 ± 0.01, in patients; 2.35 ± 0.14, in pretreatment; 0.47 ± 0.16; and in post treatment; 0.58 ± 0.14 (P ≤ 0.05)].
The measurement of nitrite concentration showed significantly greater levels of dissolved NO2/NO3 metabolite in the culture media of HUVECs untreated by 10 μM of pioglitazone (P < 0.05), while there was no significant differences between the two other treatments [Figure 1c] [(mean ± SD) in (groups) control; 0.26 ± 0.13, in patients; 1.03 ± 0.06, in pretreatment; 0.07 ± 0.001; and in post treatment; 0.07 ± 0.002 (P ≤ 0.05)].
DISCUSSION
Nitrite concentration and the number of apoptotic cells were significantly higher in the HUVEC media treated by AD serum compared with control and increased levels of dissolved NO2/NO3 metabolite considerably reduced by coincubation of TZDs such as pioglitazone both in pretreatment and post treatment group. But for apoptotic markers, this decline occurred only when we added pioglitazone before treatment of HUVECs with AD's serum.
The possible role of oxidative stress in the pathogenesis of AD via releasing cytotoxic molecules including cytokines and proinflammatory factors has been purposed.[19,20] In our study, the rate of apoptosis markedly raised when treated with sera of AD patients that presented apoptosis pathway might contribute of AD pathogenesis. It has been confirmed that in the brain of AD patients, P53 protein plays a critical role in neuronal apoptosis.[21–23] Following activation of microglia in AD, enormous amounts of oxidizing radicals such as superoxide, hydrogen peroxide, and nitric oxide were produced.[24] Indeed NO can interact with O2 to form peroxynitrite.
Elevated nitrite concentration in our study could explain that NO can interact with O2 to form peroxynitrite,[25] happens in neurodegenerative disorders including AD. In previous study, it has been indicated an increasing level of NO levels in sea of AD patients compared with controls.[26] We believe that following these event, we could detect nitrite level in treated HUVECs by AD's serum.
On the contrary, pioglitazone can attenuate vascular inflammation in nondiabetic patients[27] and EAE.[28] It was reported PPAR–γ agonists reduce NO production induced by proinflammatory mediators in cultured microglial cells.[29] Heneka et al., presented that troglitazone and pioglitazone can suppress cerebellar iNOS expression and protect cerebellar granule cells against lipopolysaccharide/ interferon-γ insult.[18] In previous literatures, they have recognized several protective mediators of pioglitazone including activation of the antiapoptotic protein Akt, upregulation of the bcl-2/ bax ratio, and inhibition of the proapoptotic protein p53.[30] In our study, pretreatment with pioglitazone could decline both apoptotic measurement and nitrite concentration. It supposes that pioglitazone scavenged-free radicals and peroxynitrate that are induced in the sera of AD patients.[31] In accordance with our study, pioglitazone acts as axonoprotective agent in vitro.[30]
CONCLUSIONS
Our study revealed that pioglitazone can possibly decrease NO levels and apoptotic measurement in culture treated with sera of AD patients. There are evidences confirming beneficial effects of PPAR-γ agonist for neurodegenerative disorders.[32,33]
However, the role of the elevation of NO level of serum on AD pathogenesis is unclear, and it requires further investigation. In the current study, we also provide evidence supporting a direct stabilizing effects of pioglitazone on the endothelium, which suggest that the PPAR-γ agonist pioglitazone protects EC and provide anti-inflammatory properties of TZDs. Further studies are justified to investigate the novel role of the PPARs in the prevention of the neuronal and endothelial damage in neurological disorder.
ACKNOWLEDGMENTS
This work was provided by Grant No. 289279 from the Deputy for Neurosciences Research, Isfahan University of Medical Sciences, Iran.
Footnotes
Source of Support: This work was provided by Grant No. 289279 from the Deputy for Neurosciences Research, Isfahan University of Medical Sciences, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011;300:H1566–82. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casado A, Encarnacion Lopez-Fernandez M, Concepcion Casado M, de La Torre R. Lipid peroxidation and antioxidant enzyme activities in vascular and Alzheimer dementias. Neurochem Res. 2008;33:450–8. doi: 10.1007/s11064-007-9453-3. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 4.Alexander JS, Zivadinov R, Maghzi AH, Ganta VC, Harris MK, Minagar A. Multiple sclerosis and cerebral endothelial dysfunction: Mechanisms. Pathophysiology. 2011;18:3–12. doi: 10.1016/j.pathophys.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993;268:26817–20. [PubMed] [Google Scholar]
- 6.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952–60. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimini A, Benedetti E, Cristiano L, Sebastiani P, D’Amico MA, D’Angelo B, et al. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130:325–37. doi: 10.1016/j.neuroscience.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Stumvoll M, Stefan N, Fritsche A, Madaus A, Tschritter O, Koch M, et al. Interaction effect between common polymorphisms in PPARgamma2 (Pro12Ala) and insulin receptor substrate 1 (Gly972Arg) on insulin sensitivity. J Mol Med (Berl) 2002;80:33–8. doi: 10.1007/s001090100282. [DOI] [PubMed] [Google Scholar]
- 9.Heneka MT, Landreth GE, Hull M. Drug insight: Effects mediated by peroxisome proliferator-activated receptor-gamma in CNS disorders. Nat Clin Pract Neurol. 2007;3:496–504. doi: 10.1038/ncpneuro0586. [DOI] [PubMed] [Google Scholar]
- 10.Raikwar HP, Muthian G, Rajasingh J, Johnson CN, Bright JJ. PPARgamma antagonists reverse the inhibition of neural antigen-specific Th1 response and experimental allergic encephalomyelitis by Ciglitazone and 15-deoxy-Delta12,14-prostaglandin J2. J Neuroimmunol. 2006;178:76–86. doi: 10.1016/j.jneuroim.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Benzie IF, Tomlinson B. Antioxidant power of angiotensin-converting enzyme inhibitors in vitro. Br J Clin Pharmacol. 1998;45:168–9. doi: 10.1046/j.1365-2125.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Cavanagh EM, Inserra F, Ferder L, Fraga CG. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol. 2000;278:R572–7. doi: 10.1152/ajpregu.2000.278.3.R572. [DOI] [PubMed] [Google Scholar]
- 13.Ceconi C, Francolini G, Bastianon D, Gitti GL, Comini L, Ferrari R. Differences in the effect of angiotensin-converting enzyme inhibitors on the rate of endothelial cell apoptosis: In vitro and in vivo studies. Cardiovasc Drugs Ther. 2007;21:423–9. doi: 10.1007/s10557-007-6068-5. [DOI] [PubMed] [Google Scholar]
- 14.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 15.Hossmann KA. Variability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–65. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Kakiuchi-Kiyota S, Arnold LL, Yokohira M, Suzuki S, Pennington KL, Cohen SM. Evaluation of PPAR gamma agonists on rodent endothelial cell proliferation. Toxicology. 2011;287:91–8. doi: 10.1016/j.tox.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–53. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 19.Calissano P, Matrone C, Amadoro G. Apoptosis and in vitro Alzheimer disease neuronal models. Commun Integr Biol. 2009;2:163–9. doi: 10.4161/cib.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH. Activation of caspase-8 in the Alzheimer's disease brain. Neurobiol Dis. 2001;8:1006–16. doi: 10.1006/nbdi.2001.0449. [DOI] [PubMed] [Google Scholar]
- 21.De la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J Alzheimers Dis. 2006;9:167–81. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 22.Dorszewska J, Florczak J, Rozycka A, Jaroszewska-Kolecka J, Trzeciak WH, Kozubski W. Polymorphisms of the CHRNA4 gene encoding the alpha4 subunit of nicotinic acetylcholine receptor are related to the oxidative DNA damage and the level of apoptotic proteins in lymphocytes of the patients with Alzheimer's disease. DNA Cell Biol. 2005;24:786–94. doi: 10.1089/dna.2005.24.786. [DOI] [PubMed] [Google Scholar]
- 23.Dezor M, Dorszewska J, Florczak J, Kempisty B, Jaroszewska-Kolecka J, Rozycka A, et al. Expression of 8-oxoguanine DNA glycosylase 1 (OGG1) and the level of p53 and TNF-alpha proteins in peripheral lymphocytes in patients with Alzheimer's disease. Folia Neuropathol. 2011;49:123–31. [PubMed] [Google Scholar]
- 24.Colton CA, Gilbert DL. Microglia, an in vivo source of reactive oxygen species in the brain. Adv Neurol. 1993;59:321–6. [PubMed] [Google Scholar]
- 25.Murphy S. Production of nitric oxide by glial cells:Regulation and potential roles in the CNS. Glia. 2000;29:1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Vural H, Sirin B, Yilmaz N, et al. The role of arginine-nitric oxide pathway in patients with Alzheimer disease. Biol Traceelem Res. 2009;129:58–64. doi: 10.1007/s12011-008-8291-8. [DOI] [PubMed] [Google Scholar]
- 27.Werner C, Kamani CH, Gensch C, Böhm M, Laufs U. The peroxisome proliferator activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56:2609–15. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 28.Raikwar HP, Muthian G, Rajasingh J, Johnson CN, Bright JJ. PPARgamma antagonists reverse the inhibition of neural antigen-specific Th1 response and experimental allergic encephalomyelitis by Ciglitazone and 15-deoxy-Delta12,14-prostaglandin J2. J Neuroimmunol. 2006;178:76–86. doi: 10.1016/j.jneuroim.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Ji H, Wang H, Zhang F, Li X, Xiang L, Aiguo S. PPARgamma agonist pioglitazone inhibits microglia inflammation by blocking p38 mitogenactivated protein kinase signaling pathways. Inflamm Res. 2010;59:921–9. doi: 10.1007/s00011-010-0203-7. [DOI] [PubMed] [Google Scholar]
- 30.Gray E, Ginty M, Kemp K, Scolding N, Wilkins A. The PPAR-gamma agonist pioglitazone protects cortical neurons from inflammatory mediators via improvement in peroxisomal function. J Neuroinflammation. 2012;9:63. doi: 10.1186/1742-2094-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceconi C, Francolini G, Bastianon D, Gitti GL, Comini L, Ferrari R. Differences in the effect of angiotensin-converting enzyme inhibitors on the rate of endothelial cell apoptosis: In vitro and in vivo studies. Cardiovasc Drugs Ther. 2007;21:423–9. doi: 10.1007/s10557-007-6068-5. [DOI] [PubMed] [Google Scholar]
- 32.Heneka MT, Landreth GE, Hull M. Drug insight: Effects mediated by peroxisome proliferator-activated receptor-gamma in CNS disorders. Nat Clin Pract Neurol. 2007;3:496–504. doi: 10.1038/ncpneuro0586. [DOI] [PubMed] [Google Scholar]
- 33.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]


