Abstract
Background:
Multiple sclerosis (MS) is an autoimmune disease of central nerves system, in which neurological disabilities occur in young adults. Despite increasing number of studies on MS, some aspects of this disorder are still unclear. In the previous studies, it has been proven that there is direct relation between MS incidence and vitamin D deficiency. Thereby, strong evidence in MS pathogenesis suggests that endothelial cells (EC) could be harmed in MS. In addition, functional changes in EC and macrovascular injuries lead blood-brain barrier disruption in MS. Current study is the first investigation to elucidate positive influences of vitamin D against EC apoptosis in MS.
Methods:
Human umbilical vein endothelial cells (HUVECs) were cultured and then treated with sera from patients with active MS (in relapse) and sera from healthy volunteer participants as control group (each group n=15). 3-(4,5-dimethylthiazol-2-yl)-5- (3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay for cell surveillance and cell-death detection kit for evaluating apoptosis were used in this study.
Results:
There was a significant decrease in apoptosis rate by the serum of patients, just when 1,25(OH)2D3 applied before treating HUVECs with sera from active MS (in relapse). Furthermore, the cells surveillance increased markedly with the presence of 1,25(OH)(2)D(3) in culture, too.
Conclusion:
Withregard to increment in EC apoptosis rate, which treated by the sera from MS patients and decrement in apoptosis rate by the presence of vitamin D in culture media, it could be proposed that vitamin D pre-treatment can be used for MS patients, due to its beneficial effects on protecting EC apoptosis.
Keywords: Vitamin D, Apoptosis, Endothelial Cells, Multiple Sclerosis
INTRODUCTION
Multiple sclerosis (MS) is an autoimmune disease of central nerves system (CNS), in which, neurological disabilities occur in young adults.[1–3] The all aspects of etiology and pathogenesis of MS have not been fully recognized.[4,5] However, a combination of environmental factors and genetics are responsible to MS prevalence world-wide.[6–8] Vitamin D deficiency as environmental factor is prevalent among MS patients. Conducted researches have been revealed that vitamin D deficiency is associated with increased risk of MS. More precisely, MS incidence is linked to vitamin D levels. Based on researches, in most industrialized countries, vitamin D insufficiency has been seen as an epidemic issue.[9–12]
In the past, the immune system was believed to be a chief actress in MS pathogenesis, but in recent studies MS has been considered as a both auto-inflammatory and vascular disease. The relation between vascular system and MS risk is strengthened by blood-brain barrier (BBB) disruption caused by functional changes in cerebral endothelial cells (CECs) and consequently, further formation of demyelinating lesions.[13,14] In vitro studies show that, at least in the earlier stages of MS, there are strong interactions between the CECs, CD8+ T-lympho-cytes, macrophages, chemokines, activated CD4+ T-lympho-cytes, and cytokines. The impairment in CECs function allows inflammatory cells trafficking across the BBB, which stimulate persistent of cascade of Th1 cytokine and chemokine inside the CNS environment. In some studies have been shown that 1,25-dihydroxyvitamin D3[1,25(OH)2D3] can be caused endothelial cells(EC) proliferation. Based on research, CEC abnormality is related to many neurological disorders and considered as basic mechanism for “vascular hypothesis of MS.” CEC apoptosis may occur due to many stimuli's inductions.[15–23] Regarding the fact that, endothelial apoptosis is associated to many inflammatory and immune-mediated disorders, current study is the first investigation to elucidate positive influences of vitamin D against EC apoptosis in MS.
METHODS
This research was performed in cytogenetic lab and neurology out-patient department of Al-Zahra hospital, Isfahan University of Medical Sciences between July 2010 and June 2011. In this study, all of the patients were agreed about the research and the Ethics Committee of the Isfahan University of Medical Sciences approved the study protocols. Also written informed consent was obtained from all study participants before inclusion in the study.
Patients
Fifteen patients with MS were recruited from Isfahan MS society patients[24] were defined as being in a clinical relapse with gadolinium-enhancing brain lesions on Magnetic Resonance Imaging (MRI) and in remission as being in a clinical remission and having no contrast-enhancing lesions on their brain. Fifteen healthy age and sexwith no history of any neurological symptoms appliedas the control group. After drawing of venous blood and centrifugation, serum aliquots were frozen at –80°C until analysis.
Cell culture
Human umbilical vein endothelial cells (HUVECs) (National Cell Bank of Iran affiliated with the Pasteur Institute, Tehran, Iran) were cultured in endothelial basal medium supplemented with, gentamicin, amphotericin B, and 10% fetal calf serum until the thirdpassage before experiments was performed.
For evaluation effects of 1,25(OH)2D3 on HUVECs treated with sera of MS, we arranged different groups; in the first group, HUVECs were only treated by sera from MS for 24 h, in the second group HUVECs were treated by 10-7 M 1,25(OH)2D3[23,25] (dissolved in culture medium) for 24 h and then sera from MS was added to these cells for another 24 h. In the third group, HUVECs were exposed in the sera of MS for 24 h and then 10–7 M of 1,25(OH)2D3 was added to these cells for another 24 h. In the fourth group, HUVECs were treated by sera from healthy individuals for 24 h.
Apoptosis analysis
The rate of apoptosis in HUVECs was evaluated by MTS assay for cell viability and cell-death detection kit for apoptosis rate.In order to evaluate whether 1,25(OH)2D3 (Alborz Daru, Tehran, Iran) would overcome sera from MS patient-induced apoptosis in these cells, 10-7 M 1,25(OH)2D3[23,25] was administrated to HUVECs in treated groups and assessed by MTS assay. As an additional measure of apoptotic cell-death, we assessed the formation of histone-associated DNA fragments by the cell-death detection ELISA kit from Roche (Basel, Switzerland) 21 as previously mentioned or according to the manufacturer instruction.
Statistical analysis
Data were analyzed by the SPSS (Version 18) and were expressed as mean ± SE (standard error).One way ANOVA followed by the Tukey's post-hoc test was used for data analysis. All experiments were repeated in three independent replicates. Differences between groups were considered as statistically significant at P < 0.05.
RESULTS
In order to evaluate whether 1,25(OH)2D3 would overcome apoptosis, which resulted from MS patient serum, 10-7 M 1,25(OH)2D3 was added to treated and trypsinized HUVECs 24 h before, and 10-7 M 1,25(OH)2D3 was added to trypsinized ECs before and after that treated by sera from MS patients. The MTS results showed that pre-administration of 10-7 M 1,25(OH)2D3 significantly increased more ECs surveillances ((mean ± SD) in (groups) control; 100 ± 00, in patients; 49.43 ± 9.20, in pre-treatment; 104.23 ± 12.83; and in post-treatment; 78.56 ± 6.77 (P ≤ 0.05)). Furthermore, the rate of apoptosis in different groups was assessed by cell-death detection kit that detects inter-nucleosomal degradation of genomic DNA during apoptosis ((mean ± SD) in (groups) control; 6.78 ± 2.69, in patients; 67.78 ± 1.06, in pre-treatment; 30.65 ± 6.05; and in post-treatment; 43.82 ± 10.39 (P ≤ 0.05). In this experiment, we observed increasing apoptosis rate in MS group in comparison with control group. Data showed 1,25(OH)2D3 pre-treatment and post-treatment prevented the induction of apoptosis by the serum of MS in HUVECs [Figure 1a and b].
Figure 1.
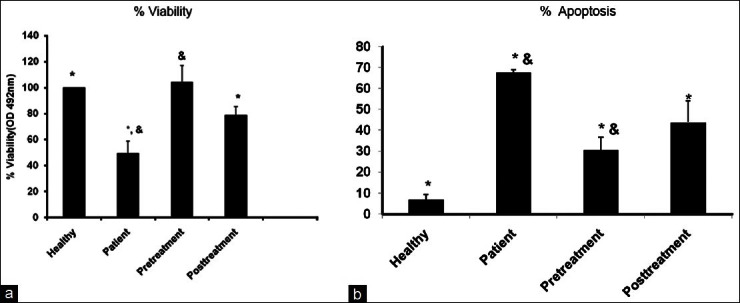
Vitamin D increased cell viability in endothelial cells in Multiple sclerosis (MS) patients. Treatmentsinclude: (i) Healthy, (ii) patients (24 h), (iii) vitamin D (24 h) and sera from MS patient, (iv) sera from MS patient (24 h) and vitamin D (24 h). (a) By cell viability by MTS assay and (b) Apoptosis percent by cell-death detection kit. Superscript letter indicates significant difference (P≤0.05) from means labeled with a different letter as determined by one-way ANOVA analysis of three independent replicates
DISCUSSIONS
Studies have been shown that Insufficient amounts of vitamin D in Human body is related toboth development and higher prevalence of wide ranges of immunity disorders including, MS, type 1 diabetes mellitus, Rheumatoid arthritis, Bechcet's disease, and inflammatory bowel diseases.[26] Regards to previous literature, which stated the rate of ECs apoptosis raise when these cells exposed to MS serum.[27] In current study, we found that the surveillance of ECs was noticeably increased when these cells were incubated with 1,25(OH)2D3 before treating them by sera from MS patients. Above all, this occurrence is observed in the absence of immune cells, expressing that releasing stable circulating factors during different stages of MS, may affect the cerebral vasculature independently of immune cell mediated effects. This protective effect of 1,25(OH)2D3 was associated with increased EC proliferation.[23]
Disruption of the BBB, caused by CEC abnormality is a critical early stage, which is tolerant for the subsequent formation of demyelinating lesions, characterizing MS. Dysfunctional CECs then authorize the in-trafficking of inflammatory cells, which provokea persistent cascade of Th1 cytokines and chemokines within the CNS.[16] On the basis of in vitro studies many stimuli can induceapoptosis in EC, suggestingthat CEC apoptosis is an important mechanism underlying CNS vascular injury that leads to weaken barrier, immune cell penetration of the CNS, inflammation, and coagulation.[16] Furthermore, Vitamin D deficiency is associated with increased risk of MS.[9–14] Disruption of the BBB permitting transendothelial penetration of activated leukocytes into the CNS is among the earliest event seen in the brain in MS, which results from inflammatory interactions between activated immunocompetent cells with CECs, their associated astrocytes, neurons, and oligodendrocytes.[28] Several recent studies have underlined vitamin D caused decrement in the rate of relapses inMS patients prevent the occurrence of the disease.
In the other hand, differentiation are arrested by vitamin D and maturation of populations of dendritic cells, T-cells, and B-cells, with increased production of interleukin (IL)-10 and reduced IL-12, resulting in attenuation of the immune response by suppressing dendritic cell development and inducing apoptosis of inflammatoryimmunecells.Vitamin D also decreases the concentration of the pro-inflammatory cytokine IL-17.[26] Overall it could be concluded that not only vitamin D protect the ECs from apoptosis, also resulted in ECs surveillances.
ACKNOWLEDGMENTS
We are grateful to Isfahan Neurosciences Research Isfahan and all the patients those who helped progression of our project.
Footnotes
Source of Support: We are grateful from Isfahan Neurosciences research center
Conflict of Interest: None declared.
REFERENCES
- 1.Luessi F, Siffrin V, Zipp F. Neurodegeneration in multiple sclerosis: Novel treatment strategies. Expert Rev Neurother. 2012;12:1061–77. doi: 10.1586/ern.12.59. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Minagar A. A Review article: Current and future therapies for multiple sclerosis. Scientifica. 2013;2013:1–11. doi: 10.1155/2013/249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo MF, Ji N, Ma CG. Immunologic pathogenesis of multiple sclerosis. Neurosci Bull. 2008;24:381–6. doi: 10.1007/s12264-008-2429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mechelli R, Annibali V, Ristori G, Vittori D, Coarelli G, Salvetti M. Multiple sclerosis etiology: beyond genes and environment. Expert Rev Clin Immunol. 2010;6:481–90. doi: 10.1586/eci.10.11. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9:599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 7.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol. 2007;61:288–99. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 8.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–13. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 9.Etemadifar M, Abtahi SH, Razmjoo H, Abtahi MA, Dehghani A, Salari M, et al. 25-hydroxyvitamin D concentrations in patients with optic neuritis as a clinically isolated syndrome and healthy controls. Int J Prev Med. 2012;3:313–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. New Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gloth FM, 3rd, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–6. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 13.Minagar A, Maghzi AH, McGee JC, Alexander JS. Emerging roles of endothelial cells in multiple sclerosis pathophysiology and therapy. Neurol Res. 2012;34:738–45. doi: 10.1179/1743132812Y.0000000072. [DOI] [PubMed] [Google Scholar]
- 14.Minagar A, Jy W, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res. 2006;28:230–5. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- 15.Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–41. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- 16.Alexander JS, Zivadinov R, Maghzi AH, Ganta VC, Harris MK, Minagar A. Multiple sclerosis and cerebral endothelial dysfunction: Mechanisms. Pathophysiology. 2011;18:3–12. doi: 10.1016/j.pathophys.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Harvey L, Burne T, Cui X, Mackay-Sim A, Eyles D, McGrath J. Vitamin D and the Brain: A neuropsychiatric perspective. Clinic Rev Bone Miner Metab. 2009;7:199–205. [Google Scholar]
- 18.Gomez JM, Curiel MD. Vitamin D deficiency and consequences for the health of people in mediterranean countries. Vitamin D Nutrition Health. 2010:453–67. Chapter 23. [Google Scholar]
- 19.Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 20.Cantorna MT. Vitamin D and its role in immunology: Multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–4. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 22.van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254:581–90. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 23.Xiang W, He XJ, Ma YL, Yi ZW, Cao Y, Zhao SP, et al. 1,25(OH)(2)D(3) influences endothelial cell proliferation, apoptosis and endothelial nitric oxide synthase expression of aorta in apolipoprotein E-deficient mice. Zhonghua Er Ke Za Zhi. 2011;49:829–33. [PubMed] [Google Scholar]
- 24.Saadatnia M, Etemadifar M, Maghzi AH. Multiple sclerosis in Isfahan, Iran. Int Rev Neurobiol. 2007;79:357–75. doi: 10.1016/S0074-7742(07)79016-5. [DOI] [PubMed] [Google Scholar]
- 25.Yiu YF, Chan YH, Yiu KH, Siu CW, Li SW, Wong LY, et al. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:E830–5. doi: 10.1210/jc.2010-2212. [DOI] [PubMed] [Google Scholar]
- 26.Arnson Y, Amital H. Is vitamin D a new therapeutic agent in autoinflammatory and pain syndromes? Isr Med Assoc J. 2011;13:234–5. [PubMed] [Google Scholar]
- 27.Haghjooy Javanmard S, Saadatnia MM, Homayouni VV, Nikoogoftar MM, Maghzi AH, Etemadifar M, et al. Interferon-beta-1b protects against multiple sclerosis-induced endothelial cells apoptosis. Front Biosci (Elite Ed) 2012;4:1368–74. doi: 10.2741/e466. [DOI] [PubMed] [Google Scholar]
- 28.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]


