Abstract
Background:
Diabetes is one of the most common chronic diseases in which antioxidant capacity changes. Omega-3 fatty acids have extensive biological effects including their advantage on lipoprotein metabolism, platelet function, cytokine production, clotting, fibrinolysis, and inflammatory factors. This study aimed to investigate the effect of omega-3 fatty acid supplements on antioxidant capacity in patients with type 2 diabetes.
Methods:
This clinical trial enrolled 71 women with type 2 diabetes in two case (treated with omega-3 capsules) and control (treated with placebo) groups. In the first stage, participants filled out a demographics questionnaire including age, height, weight, waist circumference, and hip circumference.Their blood sample was taken to evaluate glycosylated hemoglobin and antioxidant capacity. Then the case group received intervention for 8 weeks and weight, waist circumference, and hip circumference were measured and a blood sample was taken again. The data were analyzed using SPSS 18 software.
Results:
The mean difference of antioxidant capacity before and after intervention was significant (P < 0.001). Antioxidant capacity increased in the case group and reduced in the control group.
Conclusions:
With regard to the results of the present study, patients with type 2 diabetes increase their antioxidant capacity, enhance their antioxidant defense system, and probably prevent diabetes complications and related disease progress by taking omega-3 supplements.
Keywords: Antioxidant capacity, omega-3 supplement, type 2 diabetes
INTRODUCTION
Diabetes is a relatively prevalent disease that occurs as a result of lack or dysfunction of insulin.[1] Approximately 6% American have different levels of glucose metabolism disorder, which indicate diabetes or the tendency to have diabetes.[2] According to WHO, the prevalence of type 2 diabetes was estimated to be 5.5, 5.7, and 6.8% in 1995, 2000, and 2005, respectively.[3]
Stephens et al. studied the biological relationship and measured plasma markers of oxidative stress in diabetic and cardiovascular patients and found a close relationship between diabetes mellitus and increased oxidative stress.[4]
Adiposity and a longer duration of obesity are important risks factors for type 2 diabetes, and even little weight losses are associated with a maodulataion of hyperglycemia in persons with prediabetes.[5]
Review studies have shown that the level of free radicals increases and the level of antioxidants decreases during diabetes.[6] Linoleic acid (omega-6) and Linolenic acid (omega-3) have important roles in body functions, but they cannot be produced in the absence or lack of an enzyme.[7] Mori et al.[7] studied the effect of EPA, DHA, and olive oil on oxidative stress and biochemical parameters of patients with type 2 diabetes and found that EPA and DHA reduce oxidative stress without changing inflammatory markers in patients with type 2 diabetes.[7] Thorsdottir et al.,[8] from Iceland concluded that omega-3 fatty acids in milk reduces type 2 diabetes in men.[8] Lean et al.[9] concluded that omega-3 fatty acids significantly reduce serum lipids and control serum sugar in patients with type 2 diabetes.[9] With regard to high prevalence and irreparable complications of diabetes, there is probable role of free radicals in the developmentand progress of the disease as well as the fact that omega-3 fatty acids have extensive biological effects on lipoprotein metabolism, platelet function, cytokine production, clotting, fibrinolysis, and inflammatory factors.[7] Hajianfar et al. found that omega-3 reduced weight in the case group that used omega-3 vs. control group.[10] This study aimed to investigate the effect of omega-3 fatty acid supplement on antioxidant capacity of patients with type 2 diabetes.
METHODS
This study was a double-blind randomized placebo controlled clinical trial. The study population consisted of 45-65 year-old women with type 2 diabetes admitted to Shariaty Clinic in Isfahan. Considering the extra 15%, sample size in each group was estimated 39 people.
Thirty-seven volunteers were recruited in the case group and 34 entered the placebo group after signing the informed written consent form. Inclusion criteria were type 2 diabetes for at least 5 years, no insulin shot, no secondary complications of diabetes such as ophthalmologic complications, renal complications, amputation, renal diseases, blindness, cardiovascular diseases, and no high CRP.
At the beginning of study, demoghraphic characteristics were gathered by questionnaire. Blood sampling was done after 10-12 hrs fasting. Biochemical indices including serum antioxidant capacity and HbA1c were measured. Then, the subjects received supplement or placebo according to the groups for 8 weeks. At the end of study, we measured anthropometric and biochemical indices again.
Anthropometrical measurements
A digital scale (seca) was used to measure weight with an accuracy of +100g. Subjects were weighed without shoes and light clothing. Height was measured without shoes to the nearest 0.5 cm with the use of a commercial stadiometer with the shoulders in relaxed position and arms hanging freely. Body mass index (BMI) was calculated by dividing weight (kg) by height (m2).
Both the lab technician and the patients were unaware of the intervention. Data were analyzed using SPSS 18 software, and tables and charts were drawn. Fisher's exact test was used for qualitative information, t test was used for quantitative test, Chi square test was used for weight changes, and Pearson's correlation test was used for data correlation. Changes between two groups were compared using independent t test. Pearson's regression was used to find relationship between variables.
Findings
The results showed that the omega-3 group and placebo group were not significantly different in terms of age, education, and occupation. Furthermore, other variables like comorbid diseases, medication, and diet were not significantly different between the two groups. Therefore, they cannot be considered confounding factor is this study.
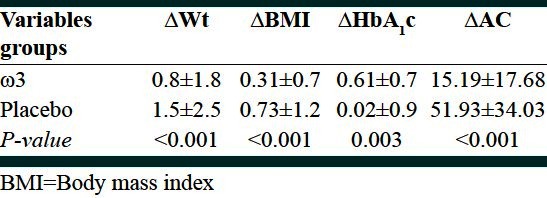
Mean age of patients was 53.6 ± 4.3 and 53.9 ± 5.4 in the case and control groups, respectively. According to t test, no significant difference was found between the two groups in this regard (P = 0.79). Mean of serum age, BMI, weight, HbA1c concentration and antioxidant capacity and comparison of the changes before and after intervention at two groups is showen in Table 1 and 2.
Table 1.
Mean of age, BMI, weight, concentration of serum HbA1c and antioxidant capacity before and after intervention at two groups
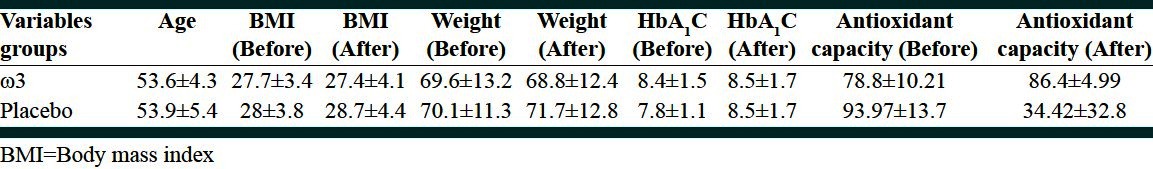
Table 2.
Comparison of changes in anthropometric and biochemical indices between two groups (ω3 and placebo)
DISCUSSION
The main objective of this study was to determine the effect of omega-3 supplement on antioxidant capacity of patients with diabetes type 2. The results show that taking omega-3 supplement increases antioxidant capacity and reduces HbA1c in patients with type 2 diabetes. This finding shows the role of omega-3 supplement in controlling HbA1c, which is a criterion to evaluate the control level of serum glucose in diabetes. This finding is in agreement with that of other studies.[11] Omega-3 consumption reduced HbA1c in diabetic patients,[12,13] but this change was not significant in Kesuvalu's study.[14] Several studies confirm the results of this study about increase in antioxidant capacity after taking omega-3 supplement. Suresh et al.,[15] concluded that omega-3 can improve oxidative stress in diabetes, which corresponds with the result of the present study. Mori et al.,[7] found that EPA and DHA reduced oxidative stress in type 2 diabetes. This finding is against that of some studies by Fokine et al.,[16] Samaoui et al.,[17] studied the relationship between fat in diet and antioxidants in diabetic patients and found that antioxidant capacity in diabetic patients who did not control diabetes well was reduced. Blaszzak et al.[18] reported an increase in antioxidant capacity of diabetic patients, which is in line with our findings. Pasaogala et al., (2004) found that the level of oxidative stress in patients with type 2 diabetes was higher than that in the control group. The amount of free radicals in patients with type 2 diabetes increases and antioxidant resistance reduces, therefore oxidative stress increases in such people.[19] Kasuvalu showed that omega-3 reduced free radicals and increased antioxidant enzymes in this group,[14] which is in line with our finding. Antioxidants reduce in these patients because increased free radicals cause different diseases including diabetes, and weaken antioxidant system. Tayyebi-Khosroshahi[20] concluded that omega-3 in hemodialysis patients increased antioxidant factors like glutathione peroxide and superoxide mutase Hiratsuka[21] found that DHA rich diet has antioxidant activity in rat's brain. Omega-3 reduces triglyceride and oxidative stress.[22]
This study resulted in the following, aiming to investigate the effect of omega-3 supplement on antioxidant capacity of patients with type 2 diabetes. HbA1c and antioxidant capacity in these people were significant, indicating that the intervention was useful and increased antioxidant capacity in these people. Considering the probable role of antioxidant system in causing type 2 diabetes, we can prevent type 2 diabetes in healthy people and reduce diabetes complications and its progress by boosting their antioxidant defense system.
CONCLUSIONS
Some studies found a significant relationship between the level of serum visfatin and BMI of the participants.[23–26] This finding is against the findings of Pagano et al.,[27] on fat non-diabetic patients and that of Samara et al.,[28] on non-diabetic people with different weights; they found an inverse relationship between BMI and Visfatin. Furthermore, Chen et al.,[29] found a significant relationship between serum visfatin and BMI in patients with type 2 diabetes.
Recommendation
Since weakening of antioxidant system can cause diabetes and because findings of the present study showed that omega-3 supplement can increase antioxidant capacity, consumption of omega-3 supplement is recommended as primary prevention and secondary prevention of diabetes complications.
ACKNOWLEDGMENT
This study was conducted by a grant from school of public health, Tehran University of Medical Sciences. The authors have no conflict of interest. All finance payment by first author.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mokini Z, Marcovecchio M, Chiarelli F. Moleculare pathology of oxidative stress in diabetic angiopathy: Role of mitochondrial and cellular pathway: A review. Diabetes Res Clin Pract. 2009;75:435–45. doi: 10.1016/j.diabres.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Afzali Z, Pilevariyan A. Maleki Compare stress oxidative in patient diabetics type 2 with healthy. J Mens health. 2008;2,4:129–34. [Google Scholar]
- 3.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 4.Stephens J, Khanolkar M, Bain S. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;20:321–9. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Franz MJ. Medical nutrition therapy for diabetes mellitus and hypoglycemia of nondiabetic origion. In: Mahan K, Escott Stump S, Raymond J, editors. Krause's Food and Nutrition Care Process. 13th ed. USA: Saunders Elsevier; pp. 678–9. [Google Scholar]
- 6.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 7.Mori T, Woodman R, Burke V, Puddy I, Croft K, Beilin L. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–81. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 8.Thorsdottir I, Hill J, Ramel A. Omega 3 fatty acid supply from milk associates with lower type 2 diabetes in men and coronary heart disease in women. Prev Med. 2004;39:630–4. doi: 10.1016/j.ypmed.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 9.MacLean CH, Mojica WA, Morton SC, Pencharz J, Hasenfeld Garland R, et al. Effects of omega-3 fatty acids on lipids and glycemic control in type II diabetes and the metabolic syndrome and on inflammatory bowel disease, rheumatoid arthritis, renal disease, systemic lupus erythematosus, and osteoporosis. Evid Rep Technol Assess (Summ) 2004;89:1–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Hajianfar H, Hosseinzadeh MJ, Bahonar A, Mohammad K, Askari GR, Entezari MH, et al. The effect of omega-3 on the serum visfatin concentration on the patients with type II diabetes versus placebo. J Res Med Sci. 2011;16:490–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Doughman SD, Krupanidhi S, Sanjeevi CB. Omega-3 fatty acid for nutrition and medicine: Considering microalgae oil as a vegetarian source of EPA and DHA. Curr Diabetes Rev. 2007;3:198–203. doi: 10.2174/157339907781368968. [DOI] [PubMed] [Google Scholar]
- 12.Wolfarm S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, et al. Pigallocatechingallate supplementation alleviates diabetes in rodent. J Nutr. 2006;136:2512–8. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 13.McManus RM, Jumpson J, Finegood DT, Clandinin MT, Tyan EA. A comparison of the effects of n-3 fatty acids from linseed oil and fish oil in well controlled type II diabetes. Diabetes Care. 1996;19:463–7. doi: 10.2337/diacare.19.5.463. [DOI] [PubMed] [Google Scholar]
- 14.Kesavulu MM, Kameswararao B, Apparao Ch, Kumar EG, Harinarayan CV. Effect of omega3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetes patients. Diabetes Metab. 2002;28:20–6. [PubMed] [Google Scholar]
- 15.Suresh Y, Das U. Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus. Nutrition. 2003;19:213–28. doi: 10.1016/s0899-9007(02)00855-9. [DOI] [PubMed] [Google Scholar]
- 16.Fukino Y, Shimbo M, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J Nutr Sci Vitaminol (Tokyo) 2005;51:335–42. doi: 10.3177/jnsv.51.335. [DOI] [PubMed] [Google Scholar]
- 17.Smaoui M, Koubaa N, Hammami S, Abid N, Feki M, Chaaba R, et al. Association between dietary fat and antioxidant status of Tunisian type 2 diabetic patients. Prostaglandins Leukot Essent Fatty Acids. 2006;5:323–9. doi: 10.1016/j.plefa.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Blaszzak R, Kujawski K, Kedziora-Kornatowska K, Kornatowski T, Kedziora J, Szadurski L, et al. The total antioxidant capacity and low-molecular antioxidants concentration in plasma in diabetes type 2 patient in different stage of metabolic compensation and concomitant diabetic nephropathy. Pol Merkur Lekarski. 2005;18:29–30. [PubMed] [Google Scholar]
- 19.Pasaoglu H, Sancak B, Bukan N. Lipid peroxidation and resistance to oxidation in patients with type 2 diabetes mellitus. Tohoku J Exp Med. 2004;203:211–8. doi: 10.1620/tjem.203.211. [DOI] [PubMed] [Google Scholar]
- 20.Tayyebi-Khosroshahi H, Houshyar J, Tabrizi A, Vatankhah AM, Razzagi Zonouz N, Dehghan-Hesari R. Effect of omega-3 fatty acid on oxidative stress in patients on hemodialysis. Iran J Kidney Dis. 2010;4:322–6. [PubMed] [Google Scholar]
- 21.Hiratsuka S, Ishihara K, Kitagawa T, Wada S, Yokogoshi H. Effect of dietary docosahexaenoic acid connecting phospholipids on the lipid peroxidation of the brain in mice. J Nutr Sci Vitaminol (Tokyo) 2008;54:501–6. doi: 10.3177/jnsv.54.501. [DOI] [PubMed] [Google Scholar]
- 22.Zak A, Zeman M, Turzicka E, Stolba P. The effect of fish oil on metabolic parameters in patient with type 2 diabetes associated with dyslipidemia. Cas Lak Cesk. 1996;135:354–9. [PubMed] [Google Scholar]
- 23.Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–81. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 24.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 25.Bemdt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–6. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 26.Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:565–70. doi: 10.1016/j.metabol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, et al. Reduced plasma visfatin/pre B-cell colony enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006;91:3165–70. doi: 10.1210/jc.2006-0361. [DOI] [PubMed] [Google Scholar]
- 28.Samara A, Ptister M, Marie B, Visvikis-Siest S. Visfatin, low-grade inflammation and body mass index (BMI) Clin Endocrinol (Oxf) 2008;69:568–74. doi: 10.1111/j.1365-2265.2008.03205.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–9. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]


