Abstract
Background:
The aim of this study was to compare the effects of olanzapine versus haloperidol to control the signs and symptoms of stuttering.
Methods:
Ninety-three patients were recruited in a 12-week single-blind randomized clinical trial, which was held between October 2009 and October 2010. Forty-three patients received olanzapine (5 mg/day) and 50 patients, haloperidol (2.5 mg/day). Before and after the study, they were evaluated by a speech pathologist by Van Riper's questionnaire. The data were analyzed using the SPSS version 16. T-test was used to compare the data between the two groups.
Results:
Mean of stuttering score (SD) before treatment was 4.67 (0.81) and 4.40 (1.14) in haloperidol and olanzapine groups, respectively (P > 0.05). After treatment, the mean (SD) score was 2.87 (1.32) and 1.56 (0.71) in haloperidol and olanzapine groups, respectively (P = 0.000).
Conclusions:
It seems that olanzapine does have better impact in controlling stuttering, and it may be recommended to prescribe olanzapine for stutters as the first choice to control the stuttering under a careful follow-up.
Keywords: Haloperidol, olanzapine, stuttering
INTRODUCTION
Stuttering is a developmental speech disorder that occurs in 5% of children with spontaneous remission in approximately 70% of cases.[1] The treatment of stuttering has been described as a controversial and puzzling issue for speech language pathologists,[2] and recent concerns have been expressed about the absence of strict documentation regarding the efficacy of particular interventions.
It is generally agreed that stuttering is a speech disorder that has an adverse effect on communication. Communication difficulties are a central component of stuttering.[3]
Several data indicate that it may be related to a dysfunction in dopaminergic neurotransmission; moreover, it can be relieved by dopaminergic receptor blockers,[4] and positron emission tomography (PET) studies have shown substantial increase in dopamine uptake activity in cortical and subcortical areas.[3] The management of stuttering is difficult and most of the times frustrating. Positive clinical results with biperiden, clomipramine, haloperidol,[4] cocaine,[5] and fluoxetine[6] have been reported. The aim of the present study was to compare efficacy of olanzapine with haloperidol to improve stuttering.
METHODS
The present 12-week, single-blind, randomized clinical trialwas conducted in the Isfahan University of medical sciences between October 2009 and October 2010. The patients were selected among those who were willing to take part in this research at the neurological disorder, psychiatric disorder, and speech disorder clinics of Isfahan University of medical sciences. The patients included those who were diagnosed as having developmental stuttering and needed to take medications. The study was approved by the human ethics committee of Isfahan University of Medical Sciences. All participants and their families received a full explanation of the nature of the study and were required to sign an agreement form. After providing written informed consent, each subject underwent a diagnostic evaluation by aneurologist using the Structured Clinical Interview for DSM-IV.
Patients were included in the study if they met the following criteria: Agreeing to participate in the study and signing the informed consent form and having the age between 10 and 50 years.
Subjects were excluded if they were unable to provide informed consent, had been treated with antipsychotics before, had current substance abuse or significant medical illness (including cerebral stroke, multiple sclerosis, brain tumors, diabetes mellitus, and hypertension), had pregnancy, had a history of intolerance of antipsychotics, were receiving treatment with agents that interfere with olanzapine or haloperidol, and receiving medications that induced stuttering such as risperidone.
The patients’ profile, including family history, intelligence quality (IQ), was recorded by a neurologist. He also performed a neurological physical examination. After completing baseline assessments, the subjects were randomly assigned to haloperidoland olanzapine. Each subject of olanzapine group was given 5 mg/day olanzapine. Similarly, patients of haloperidol group were given 2.5 mg/day haloperidol. Before the study, all subjects were examined by a speech therapist and the severity of stuttering was evaluated with Van Riper's questionnaire.[7] The stuttering scores were between 1 and 7. At the end of the study, the subjects were again evaluated for the severity of stuttering. The speech pathologist who examined the patients was not aware of the kind of drugs the patients received. All subjects took part in speech pathologist sessions weekly. Speech therapy sessions included a mixed treatment sessions including “air flow technique” and “break Valsalva maneuver” as well as “desensitization” from Van Riper's protocol. Duration of each session was 30 minutes and subjects in sessions were active. Safety laboratory tests, including complete blood count, creatinine, fasting blood glucose, level of serum prolactin, and liver function tests, based on standard hematological and clinical chemistry values, were performed at baseline and week 12.
The data were analyzed using the SPSS version 16. Significance was determined as P> 0.05. T-test was used to compare the data between the two groups.
RESULTS
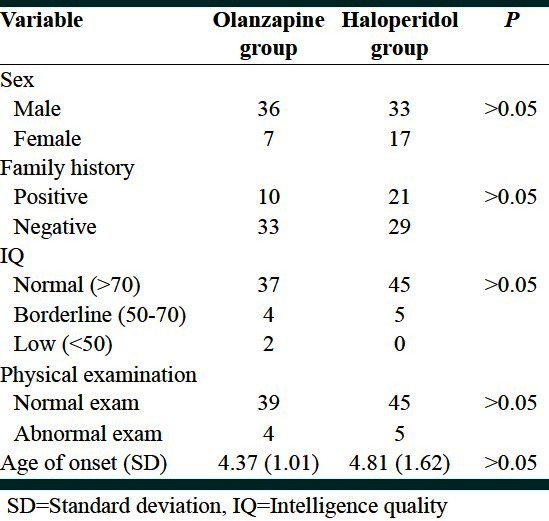
A total of 43 subjects were included in olanzapine group, and 50 were enrolled in haloperidol group. Side effects, such as mild drowsiness, dry mouth, and lethargy, occurred in over 20% of the patients treated with haloperidol, and mild to moderate drowsiness in olanzapine group, but without any missing patients in both groups at the end of the study. The mean age (standard deviation [SD]) inolanzapine group was 18.98 (9.85) and in haloperidol group it was 16.96 (6.58) (P > 0.05). There were no significant differences between sexes, family history of stuttering, IQ, physical examination, and age of onset in the two groups [Table 1]. Mean of stuttering score (SD) before treatment was 4.67 (0.81) and 4.40 (1.14) in haloperidol and olanzapine groups, respectively (P > 0.05). After treatment the mean (SD) was 2.87 (1.32) and 1.56 (0.71) in haloperidol and olanzapine groups, respectively (P = 0.000). The mean difference of stuttering score (SD) defined as stuttering score after treatment minus stuttering score before treatment was calculated to be –1.82 (1.58) and –2.84 (0.94), respectively (P = 0.000).
Table 1.
Homogeneous of demographic variables between olanzapine and haloperidol groups
In addition, after 12 weeks, no significant change in complete blood count, creatinine, fasting blood glucose, level of serum prolactin, and liver function tests in both groups was detected. Besides, no extrapyramidal symptoms appeared.
DISCUSSION
Olanzapine reduced the severity of stuttering more than haloperidol. This finding may be due to blockade of inhibitory neurotransmitter, dopamine. It has been shown through PET that stuttering is associated with hypometabolism of the striatum possibly mediated by a hyperdopaminergic state.[8] PET studies have also shown that medications act by blocking dopamine and increasing striatal metabolism.[3] It goes without saying that both haloperidol and olanzapine exert their effects by dopamine blockade; indeed, the difference in effectiveness might be associated to other neurotransmitters such as serotonin. Antagonism at the serotonin receptor 2A by the atypical antipsychotics such as olanzapine has been explained as the cause of the adverse effects of the drugs on glucose-insulin homeostasis[9] and likewise, such differences may incite some beneficial effects of atypical antipsychotic drugs over typical drugs.
A few reports have shown the possible effects of olanzapine to treat stuttering. Lavid et al.,[10] in 1999 described three cases whose symptoms were successfully controlled with olanzapine. He suggested that olanzapine may be a pharmacological option in the management of stuttering. Maguire et al.,[8] in 2004 performed a double-blind placebo-controlled trial to evaluate the effects of olanzapine in controlling the symptoms of stuttering. That was the first report which systematically applied for assessment of therapeutic effects of olanzapine on stuttering. They suggested that olanzapine is a good medication for the treatment of stuttering.[8] But in that study olanzapine was not compared with haloperidol, which is used for the treatment of stuttering routinely.
Although olanzapine has shown to reduce the severity of stuttering, it could induce stuttering, especially in patients having pre-existing brain pathology or concomitant use of antidepressants.[11,12] Olanzapine also induces obesity and insulin resistant states, which haloperidol does not.[13,14] Thus, following up patients seems to be necessary when they are receiving olanzapine treatment and controlling weight. In contrast, olanzapine makes patients less sedate than haloperidol,[15] so they feel more comfortable when using olanzapine.
CONCLUSION
Finally, it is recommended to use olanzapine as the first choice to control the stuttering under a careful follow-up. More studies are warranted with larger case populations in children and adolescents and adults separately.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39:1333–44. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingham JC, Riley G. Guidelines for documentation of treatment efficacy for young children who stutter. J Speech Lang Hear Res. 1998;41:753–70. doi: 10.1044/jslhr.4104.753. [DOI] [PubMed] [Google Scholar]
- 3.Wu JT, Huang GF, Huang CS, Noordhoff MS. Nasopharyngoscopic evaluation and cephalometric analysis of velopharynx in normal and cleft palate patients. Ann Plast Surg. 1996;36:117–22. doi: 10.1097/00000637-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Murray MG, Newman RM. Paroxetine for treatment of obsessive-compulsive disorder and comorbid stuttering. Am J Psychiatry. 1997;154:1037. doi: 10.1176/ajp.154.7.1037a. [DOI] [PubMed] [Google Scholar]
- 5.Linazasoro G, Van Blercom N. Severe stuttering and motor tics responsive to cocaine. Parkinsonism Relat Disord. 2007;13:57–8. doi: 10.1016/j.parkreldis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Balan S. Fluoxetine for persistent developmental stuttering. Clin Neuropharmacol. 2007;30:58–9. doi: 10.1097/01.wnf.0000240950.18821.19. [DOI] [PubMed] [Google Scholar]
- 7.Van Riper C. The nature of stuttering. Englewood Cliffs: Prentice Hall; 1982. [Google Scholar]
- 8.Maguire GA, Riley GD, Franklin DL, Maguire ME, Nguyen CT, Brojeni PH. Olanzapine in the treatment of developmental stuttering: A double-blind, placebo-controlled trial. Ann Clin Psychiatry. 2004;16:63–7. doi: 10.1080/10401230490452834. [DOI] [PubMed] [Google Scholar]
- 9.Melkersson KI, Gunes A, Dahl ML. Impact of serotonin receptor 2A gene haplotypes on C-peptide levels in clozapine- and olanzapine-treated patients. Hum Psychopharmacol. 2010;25:347–52. doi: 10.1002/hup.1114. [DOI] [PubMed] [Google Scholar]
- 10.Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: Three case reports. Ann Clin Psychiatry. 1999;11:233–6. doi: 10.1023/a:1022365513865. [DOI] [PubMed] [Google Scholar]
- 11.Nurnberg HG, Greenwald B. Stuttering: An unusual side effect of phenothiazines. Am J Psychiatry. 1981;138:386–7. doi: 10.1176/ajp.138.3.386. [DOI] [PubMed] [Google Scholar]
- 12.Bär KJ, Häger F, Sauer H. Olanzapine- and clozapine- induced stuttering. A case series. Pharmacopsychiatry. 2004;37:131–4. doi: 10.1055/s-2004-818992. [DOI] [PubMed] [Google Scholar]
- 13.Baptista T, Zárate J, Joober R, Colasante C, Beaulieu S, Páez X, et al. Drug induced weight gain, an impediment to successful pharmacotherapy: Focus on antipsychotics. Curr Drug Targets. 2004;5:279–99. doi: 10.2174/1389450043490514. [DOI] [PubMed] [Google Scholar]
- 14.Mathews M, Muzina DJ. Atypical antipsychotics: New drugs, new challenges. Cleve Clin J Med. 2007;74:597–606. doi: 10.3949/ccjm.74.8.597. [DOI] [PubMed] [Google Scholar]
- 15.Kumra S, Oberstar JV, Sikich L, Findling RL, McClellan JM, Vinogradov S, et al. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:60–71. doi: 10.1093/schbul/sbm109. [DOI] [PMC free article] [PubMed] [Google Scholar]


