Abstract
Background:
The challenge of antiepileptic drugs (AEDs) management is to attain the best compromise between the desire to maximize seizure control and the need to keep side-effects within tolerable limits for the individual patient. To reduce devastation in Iranian epileptic patients, the aim of this study was to explore the overall outcome following AEDs prescription.
Methods:
A cross-sectional study of 36 patients located at the epilepsy ward, conducted to Isfahan Neurosciences Research Centre was carried out during the year 2011. Female (n = 17) and male subjects (n = 19) with a mean age of 27 years (range; 7-74 years) were studied. Variables including, sex, age, age of seizure onset, type, and number of AEDs, biochemical and hematological data were recorded in d-Base and statistical analyses were performed using SPSS (version 18) for windows.
Results:
The main drug to control seizure attack was carbamazepine and valproic-acid. The following tests were the most frequently influenced; alkaline phosphatase (AP), lymphocyte (Lymph), white blood cell (WBC) counts and hemoglobin (Hgb). There was a significant increase in (AP) (mean; 534.6 u/l↑; [P = 0.02] in three patients and (Lymph) (55%↑; [43-84] %↑; [P = 0.04] in seven patients. WBC was lower than 4400 mm3↓ (P = 0.02) in six patients. Hgb was significantly lower in 70.6% of women (11.8↓; [10-14.2] g/dl↓; [P = 0.04] and 68.4% of men population (12.3↓; [9.7-13.8] g/dl↓; [P = 0.01]. Mean age of epilepsy onset was 15.6 years (range: Birth-74 years). Analysis of drug prescriptions showed that the incidence of monotherapy and polypharmacy (2 up to six AEDs simultaneously) was 19.4% plus 80.6% respectively.
Conclusions:
In Iranian epileptic population, effectiveness of treatment should be attributed by the close supervising of AEDs in relation to clinical circumstance, laboratory data, and therapeutic drug monitoring. Any significant change in patients’ biochemical and hematological data may require close verifying for the rapid detection of severe anemia, leukopenia, lymphocytosis, osteomalacia, or liver failure.
Keywords: Antiepileptic drugs, biochemical, hematological, side effects
INTRODUCTION
Epilepsy is a disorder of the brain that generates epileptic seizures by the neurobiological, cognitive, psychological, and social consequences of this condition. Antiepileptic drugs (AEDs) are frequently used for several conditions including, epilepsy, movement or mind disorders and neuropanic tenderness. Epileptic patients in 70% are seizure free with one drug based on cautious drug administration and dosage modification. Judgment to treatment of epilepsy is normally based on monotherapy. AEDs polypharmacy may contribute to cause side-effects in patients.[1,2] Weight gain, metabolic acidosis, nephrolithiasis, angle closure glaucoma, skin rash, hepatotoxicity, colitis, movement, and behavioral disorders are some reported side-effects.[3] Multiple organ toxicity including, hypochromic anemia following repeated dose oral administration in rats,[4] eosinophilic leukemoid reaction, exfoliative dermatitis associated to carbamazepine,[5–7] cross hypersensitivity syndrome between phenytoin and carbamazepine,[8] neutropenia in a patient treated with clozapine in combination with other psychotropic drugs,[9] pure red cell aplasia during carbamazepine monotherapy,[10–12] hypersensitivity syndrome induced by possible cross-reactivity between carbamazepine and lamotrigine[13] are a number of reported adverse effects in patients with AEDs. Common side-effects of AEDs noted as dizziness, drowsiness, mental slowing, and hematologic side-effects reported as purpura, anemia, thrombocytopenia, lymphadenopathy, increased white blood cell (WBC) count, lymphocytosis, non-Hodgkin's lymphoma, and increased bleeding time. Other side-effects may include: Agranulocytosis, aplastic anemia, leukopenia, and thrombocytopenia.[15–17] Phenobarbital, carbamazepine, phenytoin, and valproic acid have numerous deficits such as suboptimal reply rates, imperative adverse effects, numerous drug interactions, and a constricted therapeutic window. Gabapentin, lamotrigine, levetiracetam, oxcarbazepine, and zonisamide have improved acceptability outline, low interface potential, and significantly less enzyme inducing or inhibiting properties.[3] The simultaneous use of many drugs for treatment of epilepsy could cause adverse effects, therefore, monotherapy continues as the “gold standard.” Development in the understanding of AEDs mechanisms of action have discovered two major patterns increasing inhibition either through GABA or glycine, or decreasing excitation due to glutamate. However, some AEDs reduce membrane excitability by interrelating with neurotransmitter receptors or ion channels (carbamazepine and valproic acid) but the methods of action for most of them are not fully understood (levetiracetam and zonisamide). Monotherapy is preferable treatment for epilepsy as it keeps side-effects and drug interactions to a minimum level. Some patients will achieve seizure control based on monotherapy on low-doses and others can tolerate high-doses with superior outcome and no side-effects. Rational polypharmacy aims at multiple receptors or ion channels to increase inhibition and simultaneously reduce excitation. Interactions between AEDs based on kinetics and rate of elimination from the liver appear to be accountable for the greater efficiency or adverse effects.[2,18–24] As prescription of AEDs persists to augments, an efficient lesson connected to recommendation in provisions of management approach is of interest to be examined. This study is an effort to emphasize the changes in hematological and biochemical parameters and its’ correlation with AEDs monotherapy or polypharmacy in Iranian epileptic population.
METHODS
A cross-sectional study of 36 patients located at the Epilepsy Ward of the Kashani Hospital; conducted to Isfahan Neurosciences Research Centre was carried out during the year 2011. There was no induction in treatment procedure. The study was approved by the Institute Research Ethics Committee. The average age of the patients was 27 years (range; 7-74 years); 17 patients were female and 19 male. Approximately 15 varieties of AEDs have been used for the treatment of epilepsy. At least 1 year before the study, route of administration was oral. All data were recorded initially in d-Base and processed using Microsoft Excel and SPSS (version, 18.0) for windows. The variables of interest were sex, age, age of seizure onset, biochemical, hematological parameters, 15 types of AEDs and number of drugs used by each patient. Descriptive statistics included; calculation of means, range, frequency, and proportions for categorical ones. The correlation of AEDs with biochemical and hematological data was defined by multiple logistic regression analysis (step-wise). A P ≤ 0.05 was considered as significant.
RESULTS
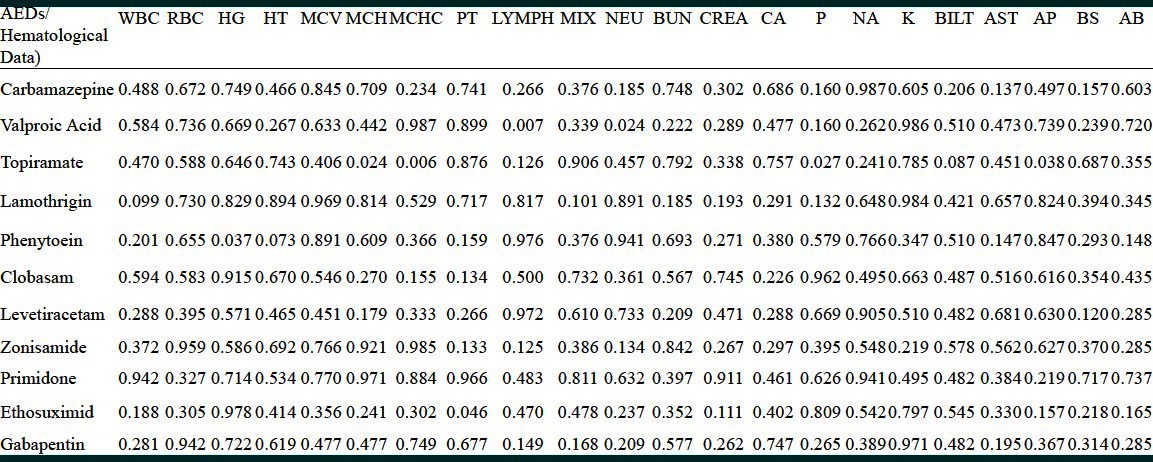
The mean age of epilepsy onset was 15.6 years (range: Birth-74 years). An epileptic attack was happened approximately around 6 (25%), 11 (50%) and 17 years of life (75%). The incidence of monotherapy and polypharmacy was 19.4% plus 80.6% respectively [Figure 1]. The number of anticonvulsant for each subject ranged from one to six with a mean of three AEDs. Prescriptions were concentrated on two compounds, carbamazepine, and valproic acid. Preliminary analysis of medical prescriptions implicated [Figure 2]: Carbamazepine (n = 22), valproic acid (n = 15), topiramate (n = 12), lamotrigine (n = 9), phenytoin (n = 7), clobasam (n = 6), levetiracetam (n = 5), clonazepam (n = 4), zonisamide (n = 3), primidone (n = 2), risperidone (n = 2), ethosuximide (n = 2), gabapentin (n = 2), phenobarbital (n = 2), oxcarbazepine (n = 1). Table 1 presents biochemistry and hematology parameters in the 36 patients with AEDs. Before starting the AEDs no attempt was made to achieve level of biochemistry and hematology parameters, therefore abnormal results reflect dysfunction prevailing around the time of study. Table 2 shows the most significant determinant in patients with defined code number and their AEDs regimen. The corresponding variables were alkaline phosphatase (AP), lymphocyte (Lymph), WBC counts and hemoglobin (Hgb). In three patients AP with a mean value of 506 u/l↑ was significantly higher (473, 492, 553) u/l↑] than normal range (60-360 u/l) (P = 0.02). One 29-year-old female (Code no; 568) who presented seizure attack from the age of 9 year showed a value of 553 u/l↑ for AP. She was on three AEDs: Carbamazepine (200 mg T.D.S)/valproic-acid (200 mg T.D.S)/zonisamide (50 mg T.D.S). In this lady variables such as Lymph (84%↑), platelet (314000/mm3↑) and Hgb (10.4 g/dl↓) were also in abnormal side. In another 14-year-old lady (Code no; 580) who presented seizure attack from the age of 7 year showed a value of 473 u/l↑for AP. She was on two AEDs primidone (50 mg T.D.S)/valproic-acid (500 mg T.D.S). In a 24-year-old male subject (Code no; 560) with two AEDs topiramate (50 mg T.D.S)/valproic-acid (200 mg T.D.S), the value for AP was 492 u/l↑.
Figure 1.
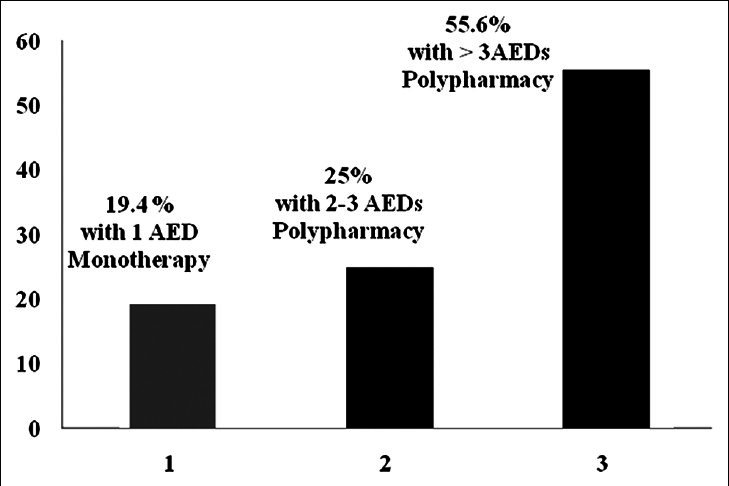
The incidence of monotherapy or polypharmacy (n=36)
Figure 2.
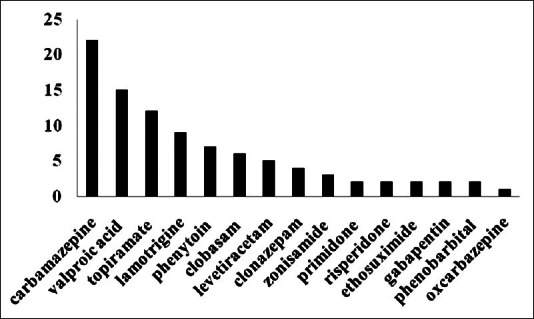
Types and frequencies of AEDs (n=36). Prescriptions were concentrated on two compounds, carbamazepine (a potent inductor of CYP isoenzymes) and valproic acid (a potent inhibitor of several CYP isoenzymes)
Table 1.
Clinical biochemistry and haematology results: All patients (n=36)
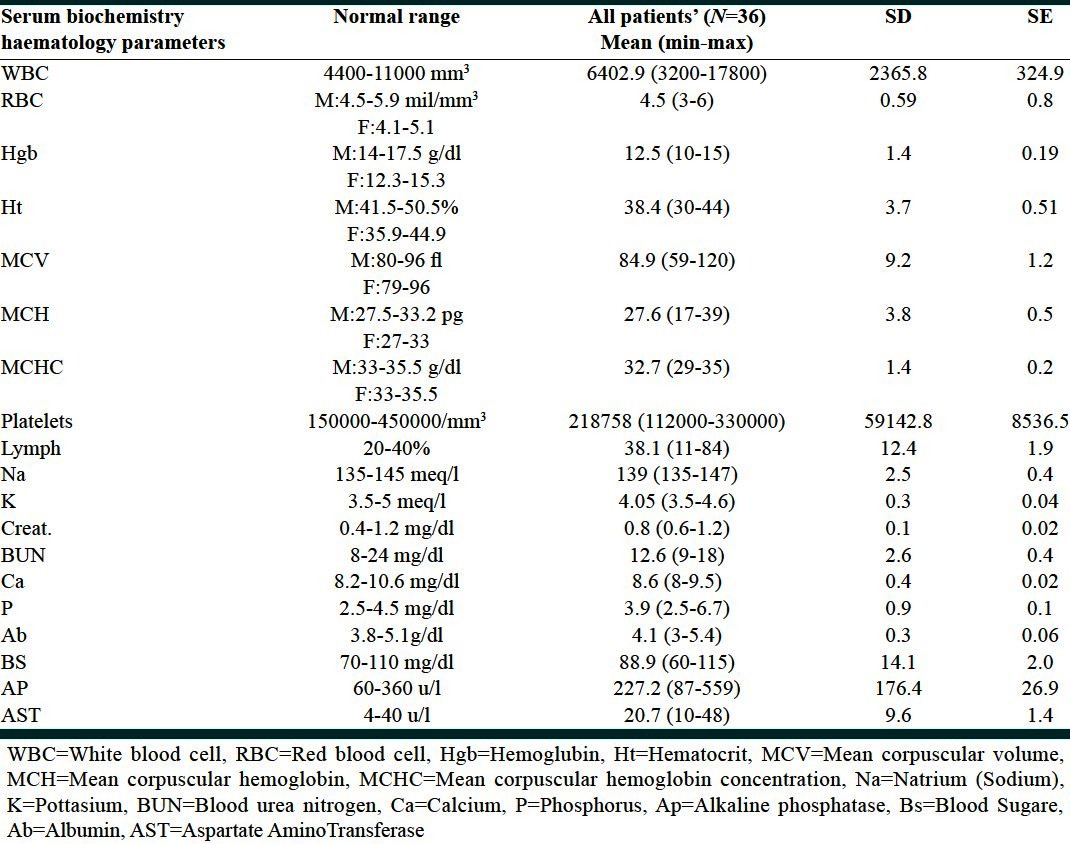
Table 2.
Drug details in patients with abnormal hematological and biochemical parameters
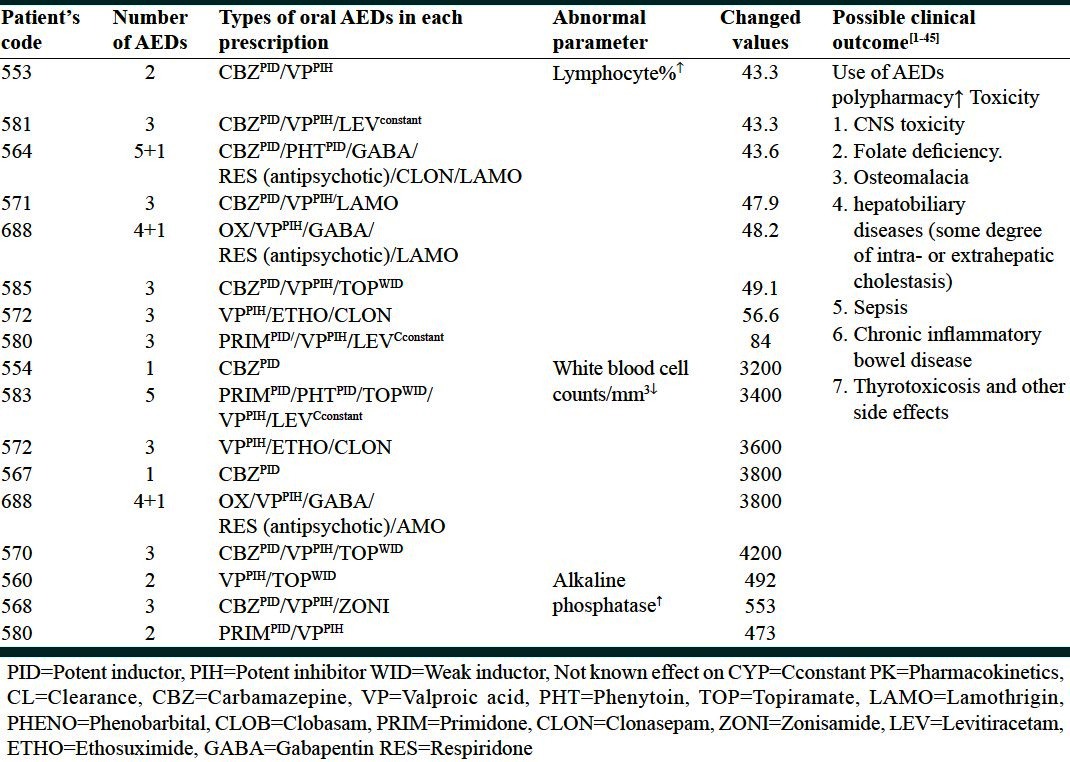
Lymph in eight patients (Code no; 553, 564, 570, 571, 580, 581, 585, 688) with a mean of 52%↑(range: 43-84%↑) was significantly (P = 0.04) higher than normal values (normal range; 20-40%). WBC counts in six patients with a mean of 3666 mm3↓ (range: 3200-4200 mm3↓) was significantly (P = 0.002) lower than normal range (4400-11,000 mm3). The number and type of AEDs used by each patient is shown in Table 2.
There was a significant decrease in Hgb. No attempt was made on information about Hgb levels, before starting the AEDs. Further analysis according to gender showed that in 70.6% of females, Hgb was significantly (P = 0.04) lower (mean 11.8 g/dl↓; [ranged; 10-14.2 g/dl↓] than normal values [normal values; 12.3-15.3 g/dl). Of the 19 males studied, in 68.4% of patients Hgb was significantly (P = 0.01) lower [mean 12.3 g/dl↓; (ranged: 9.7-13.8 g/dl↓)] than normal values (normal values; 14-17.5 g/dl).
Stepwise multiple regression analysis was used to evaluate further the contributions of these variables to each AED [Table 3]. There was a significant correlation between topiramate (n = 12) with AP↑ (P = 0.028) and phosphorus↑ (P) (P = 0.027). In a patient with (Code no; 568) also mentioned above (AP) was 553 u/l↑. In another 7-year-old boy with (Code no; 557) who was on three AEDs; topiramate (50 mg T.D.S)/valproic-acid (200 mg T.D.S)/phenobarbital (50 mg), the value for AP was 559 u/l↑. Out of 12 patients with topiramate, in three patients with (Code no; 575, 560, 585) (P) was higher (5.3, 5.1, 4.6 mg/dl↑) than normal value (2.5-4.5 mg/dl). Patient with (Code no; 575) was on four AEDs as; carbamazepine (200 mg T.D.S)/topiramate (100 mg T.D.S)/clobasam (100 mg T.D.S)/lamotrigine (100 mg T.D.S). Patient with code no 560 was on two AEDs as; topiramate (50 mg T.D.S)/valproic-acid (200 mg T.D.S). Patient with (Code no; 585) was on three AEDs as; carbamazepine (400 mg T.D.S)/topiramate (50 mg T.D.S)/valproic-acid (200 mg T.D.S). The correlation between lamotrigine (n = 9; patients with Code no; 556, 564, 566, 571, 575, 577, 578, 582, 688 with WBC counts tends to be significant (P = 0.099). The mean WBC in this group was 5225 mm3 and in one patient with (Code no; 585; a 32-year-old lady with six AEDs) was 3800 mm3↓. There was a significant correlation between phenytoin (n = 7; Code no; 564, 559, 583, 558, 688, 555, 576) with Hgb (P = 0.037) and hematocirt (HT) (P = 0.073).
Table 3.
Correlation of AEDs with clinical biochemistry and haematology results (n=36)
DISCUSSION
Heterogeneity of the number and type of AEDs in Iranian epileptic population imposes major interpretative consideration. The analysis presented here has defined some of the criteria that contribute to variability in AEDs efficacy and side-effects.
The inconsistency in number and type of AEDs observed appears to exceed previous findings, probably because of the diversity that hinders interpretation and provides indicator of factors which may influence clinical outcome. This may merit further investigation in Iranian epileptic population in terms of AEDs monotherapy or polypharmacy. From the data presented above it is clear that several dynamic variables especially, serological markers appear to be influenced by AEDs therapy.[2] Use of multiple AEDs based on combination therapy to treat epilepsy might cause difficulty in management regardless of continuing efforts to reduce their occurrence. Drug regimen based on polypharmacy was 80.6%. The number of AEDs used by each patient ranged from one to six. There appears to be agreement that AEDs polypharmacy can produce adverse effects. The limitations for judgment in order to correlate AEDs dose to C0 (trough level) was the lack of therapeutic drug monitoring results. In this study, prescriptions were dominated on two drugs, carbamazepine, and valproic acid. Carbamazepine, is a strong inductors of cythochrom P450 isoenzymes mainly glucuronyltransferases, thus declining not only its own plasma level and efficacy but also that of other AEDs and other drugs. Serum carbamazepine C0 could diminish and carbamazepine-10, 11-epoxide metabolite could increase. Afterward, the clearance of valproic acid increase and then its C0 diminish which could be clinically troublesome. In this clinical condition carbamazepine should be suspended to respond in augmented valproic acid absorptions. When two AEDs, both requiring the same enzyme for metabolism, are given concurrently, one AED may inhibit or induce metabolism of the other, and thus, an adverse drug interaction may occur. In combination therapy based on carbamazepine and valproic acid, carbamazepin C0 could raise and cause harmful property and valproic acid C0 may diminish and contribute seizure.[2,25,26] Analysis of biochemical and hematological values showed a low Hgb count in both genders. It could be related to AEDs polypharmacy. Because Hgb carries oxygen from the respiratory organs to the rest of the body, in these patients anemia could be developing. Anemia is also known to be an associated factor with folate deficiency. Little is known about the effect of each AED on changes in (Hgb) over time or about the causes of anemia. Published literature suggested that changes in (Hgb) over 12 months were inversely associated with subsequent risk of mortality and morbidity, independently of the effects of baseline anemia and other important predictors. According to previous publication, phenobarbital, phenytoin, and primidone cause a reduction in folic acid levels, predisposing patients to megaloblastic anemia. Phenytoin acts by inhibiting intestinal conjugase, thereby causing folate deficiency. Another published report indicates that use of AEDs, in particular carbamazepine and valproic acid, is associated with a 9-fold increased risk of aplastic anemia.[27–33]
AP is considered as a marker of hepatocellular strength. Increased serum AP could be seen in states of increased osteoblastic activity (hyperparathyroidism, osteomalacia, primary and metastatic neoplasms), hepatobiliary diseases characterized by some degree of (intra- or extra hepatic cholestasis) and in sepsis, chronic inflammatory bowel disease, and thyrotoxicosis. The observed increase in enzyme activity in three patients may be as a result of liver injury altered hepatocyte integrity caused by valproic acid or AEDs pharmacokinetic interactions. Previous publication reported that topiramate should be administered with cautious in hepatically impaired patients as the clearance may be decreased. Another study showed that serum biochemical changes, which indicate predisposition to development of rickets or osteomalacia appear within 90 days of starting carbamazepine or valproic acid monotherapy.[34,35] Lymphocytosis or a Lymph count over 40% that is a medical condition characterized by elevated lymphocytes has been seen in seven patients with AEDs polypharmacy. An increase in Lymph concentration might be usually a sign of acute infections, such as Epstein-Barr virus infection and viral hepatitis, leukemia, cancer of the bone marrow, or radiation therapy. In this study, changes in patients might be related to the changes in bone marrow density[36–41] due to pharmacokinetic interactions. Low WBC count, or leukopenia, is a decrease in disease-fighting cells (leukocytes). Leukopenia may occur as a result of chemotherapy, radiation therapy, or immune system disorders. A low WBC count in the present study (n = 6) might be related to long term use of AEDs polypharmacy. Published reports indicated a probable relationship between the drug-drug interaction and blood dyscrasia during lamotrigine/phenobarbital treatment.[42] Another study confirmed that patients treated with valproate alone or in combination therapy should be monitored for the occurrence of leukopenia and neutropenia due to possible pharmacokinetic and pharmacodynamic interactions.[43,45]
CONCLUSIONS
As, in an epileptic population, AEDs poly therapy might be associated to pharmacokinetic and pharmacodynamic interactions, lack of obedience to prescription, patients should be monitored for therapeutic level and ineffective AEDs should be withdrawn with cautious. Treatment with beginning one AED is an alternative in an epileptic clinic. If there is need for polypharmacy, the effect of second AED should be predicted and examined based on pharmacokinetic interactions. Therefore, poor clinical control (compliance, metabolism) should be avoided and dose-related side-effects, drug or disease interaction, routine drug levels, plus guide to dosing based on drug half-life always should be considered.
ACKNOWLEDGMENTS
We would like to gratefully acknowledge Isfahan Neurosciences Research Centre (INRC).
Footnotes
Source of Support: This article was supported by Isfahan Neurosciences Research Center. We would like to gratefully acknowledge the Research Deputy of Isfahan University of Medical Sciences for its financial support to this research (Grant No. 290296 appreciated).
Conflict of Interest: None declared.
REFERENCES
- 1.Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neuroscientist. 2012;18:360–72. doi: 10.1177/1073858411422754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johannessen SI, Landmark CJ. Antiepileptic drug interactions-principles and clinical implications. Curr Neuropharmacol. 2010;8:254–67. doi: 10.2174/157015910792246254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walia KS, Khan EA, Ko DH, Raza SS, Khan YN. Side effects of antiepileptics: A review. Pain Pract. 2004;4:194–203. doi: 10.1111/j.1533-2500.2004.04304.x. [DOI] [PubMed] [Google Scholar]
- 4.Kojima S, Sasaki J, Tomita M, Saka M, Ishizuka K, Kawakatsu H, et al. Multiple organ toxicity, including hypochromic anemia, following repeated dose oral administration of phenobarbital (PB) in rats. J Toxicol Sci. 2009;34:527–39. doi: 10.2131/jts.34.527. [DOI] [PubMed] [Google Scholar]
- 5.Laad G, Miranda MF. Eosinophilic leukemoid reaction associated with carbamazepine hypersensitivity. Indian J Dermatol Venereol Leprol. 2005;71:35–7. doi: 10.4103/0378-6323.13784. [DOI] [PubMed] [Google Scholar]
- 6.Troost RJ, Oranje AP, Lijnen RL, Benner R, Prens EP. Exfoliative dermatitis due to immunologically confirmed carbamazepine hypersensitivity. Pediatr Dermatol. 1996;13:316–20. doi: 10.1111/j.1525-1470.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 7.Ponte CD. Carbamazepine-induced thrombocytopenia, rash, and hepatic dysfunction. Drug Intell Clin Pharm. 1983;17:642–4. doi: 10.1177/106002808301700908. [DOI] [PubMed] [Google Scholar]
- 8.Sierra NM, García B, Marco J, Plaza S, Hidalgo F, Bermejo T. Cross hypersensitivity syndrome between phenytoin and carbamazepine. Pharm World Sci. 2005;27:170–4. doi: 10.1007/s11096-004-1736-z. [DOI] [PubMed] [Google Scholar]
- 9.Sénéchal A, Landry P, Deschamps R, Lessard M. Neutropenia in a patient treated with clozapine in combination with other psychotropic drugs. Encephale. 2002;28:567–9. [PubMed] [Google Scholar]
- 10.Tagawa T, Sumi K, Uno R, Itagaki Y, Fujii F, Yamaguchi H. Pure red cell aplasia during carbamazepine monotherapy. Brain Dev. 1997;19:300–2. doi: 10.1016/s0387-7604(97)00580-9. [DOI] [PubMed] [Google Scholar]
- 11.Medberry CA, 3rd, Pappas AA, Ackerman BH. Carbamazepine and erythroid arrest. Drug Intell Clin Pharm. 1987;21:439–41. doi: 10.1177/106002808702100512. [DOI] [PubMed] [Google Scholar]
- 12.Fonzari M, Bo GP, Faverio A, Benassi E. Retrospective study on side effects in 410 patients in antiepileptic therapy. Proposal for new biochemical screening. Riv Neurol. 1984;54:390–8. [PubMed] [Google Scholar]
- 13.Aouam K, Ben Romdhane F, Loussaief C, Salem R, Toumi A, Belhadjali H, et al. Hypersensitivity syndrome induced by anticonvulsants: Possible cross-reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488–91. doi: 10.1177/0091270009344985. [DOI] [PubMed] [Google Scholar]
- 14.Cetinkaya Y, Kurtulmuş YS, Tutkavul K, Tireli H. The effect of oxcarbazepine on bone metabolism. Acta Neurol Scand. 2009;120:170–5. doi: 10.1111/j.1600-0404.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 15.Linnebank M, Moskau S, Semmler A, Widman G, Stoffel-Wagner B, Weller M, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69:352–9. doi: 10.1002/ana.22229. [DOI] [PubMed] [Google Scholar]
- 16.Ozdemir O, Yakut A, Dinleyici EC, Aydogdu SD, Yarar C, Colak O. Serum asymmetric dimethylarginine (ADMA), homocysteine, vitamin B (12), folate levels, and lipid profiles in epileptic children treated with valproic acid. Eur J Pediatr. 2011;170:873–7. doi: 10.1007/s00431-010-1366-5. [DOI] [PubMed] [Google Scholar]
- 17.Dorszewska J, Winczewska-Wiktor A, Sniezawska A, Kaczmarek I, Steinborn B. Homocysteine and asymmetric dimethylarginine (ADMA) in epilepsy. Przegl Lek. 2009;66:448–52. [PubMed] [Google Scholar]
- 18.French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50:63–8. doi: 10.1111/j.1528-1167.2009.02238.x. [DOI] [PubMed] [Google Scholar]
- 19.Rho JM, Sankar R. The pharmacologic basis of antiepileptic drug action. Epilepsia. 1999;40:1471–83. doi: 10.1111/j.1528-1157.1999.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 20.Arroyo S, Perucca E. Translating monotherapy trials into clinical practice: A look into the abyss. Epilepsy Behav. 2003;4:457–63. doi: 10.1016/j.yebeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305:1669–76. doi: 10.1001/jama.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czapiñski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005;5:3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino A. Rational combinations of antiepileptic drugs for refractory epilepsy. Nihon Shinkei Seishin Yakurigaku Zasshi. 2011;31:65–72. [PubMed] [Google Scholar]
- 24.Tolou-Ghamari Z, Wendon J, Tredger JM. In vitro pentamer formation as a biomarker of tacrolimus-related immunosuppressive activity after liver transplantation. Clin Chem Lab Med. 2000;38:1209–11. doi: 10.1515/CCLM.2000.190. [DOI] [PubMed] [Google Scholar]
- 25.Tolou Ghamari Z. Antiepileptic Drugs (AEDs) polypharmacy could lead to buried pharmacokinetic interactions due to CYP450. Drug Metab Lett. 2012 doi: 10.2174/1872312811206030008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Wang KX, Wei Y, Xu MH, Su JM, Bao YG, et al. Effect of topiramate and carbamazepine on bone metabolism in children with epilepsy. Pharmacol Rep. 2011;63:372–80. [PubMed] [Google Scholar]
- 27.Arya R, Gulati S, Kabra M, Sahu JK, Kalra V. Folic acid supplementation prevents phenytoin-induced gingival overgrowth in children. Neurology. 2011;76:1338–43. doi: 10.1212/WNL.0b013e3182152844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai M, Osaka H. Acute leukoencephalopathy possibly induced by phenytoin intoxication in an adult patient with methylenetetrahydrofolate reductase deficiency. Epilepsia. 2011;52:e58–61. doi: 10.1111/j.1528-1167.2011.03064.x. [DOI] [PubMed] [Google Scholar]
- 29.Handoko KB, Souverein PC, van Staa TP, Meyboom RH, Leufkens HG, Egberts TC, et al. Risk of aplastic anemia in patients using antiepileptic drugs. Epilepsia. 2006;47:1232–6. doi: 10.1111/j.1528-1167.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 30.Acharya S, Bussel JB. Hematologic toxicity of sodium valproate. J Pediatr Hematol Oncol. 2000;22:62–5. doi: 10.1097/00043426-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Blackburn SC, Oliart AD, García Rodríguez LA, Pérez Gutthann S. Antiepileptics and blood dyscrasias: A cohort study. Pharmacotherapy. 1998;18:1277–83. [PubMed] [Google Scholar]
- 32.Davies-Jones GA. Neurology and General Medicine. 2nd ed. New York: Churchill Livingstone; 1995. Neurological manifestations of hematological disorders. The neurological aspects of medical disorders; pp. 219–45. [Google Scholar]
- 33.Bachmann T, Bertheussen KH, Svalheim S, Rauchenzauner M, Luef G, Gjerstad L, et al. Haematological side effects of antiepileptic drug treatment in patients with epilepsy. Acta Neurol Scand Suppl. 2011;124:23–7. doi: 10.1111/j.1600-0404.2011.01539.x. [DOI] [PubMed] [Google Scholar]
- 34.Krishnamoorthy G, Nair R, Sundar U, Kini P, Shrivastava M. Early predisposition to osteomalacia in Indian adults on phenytoin or valproate monotherapy and effective prophylaxis by simultaneous supplementation with calcium and 25-hydroxy vitamin D at recommended daily allowance dosage: A prospective study. Neurol India. 2010;58:213–9. doi: 10.4103/0028-3886.63796. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamoorthy G, Karande S, Ahire N, Mathew L, Kulkarni M. Bone metabolism alteration on antiepileptic drug therapy. Indian J Pediatr. 2009;76:377–83. doi: 10.1007/s12098-009-0005-5. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Ouyang DY, Geng M, Xu LH, Zhang YT, Wang FP, et al. Valproic acid exhibits biphasic effects on apoptotic cell death of activated lymphocytes through differential modulation of multiple signaling pathways. J Immunotoxicol. 2011;8:210–8. doi: 10.3109/1547691X.2011.568979. [DOI] [PubMed] [Google Scholar]
- 37.Mathieu O, Picot MC, Gelisse P, Breton H, Demoly P, Hillaire-Buys D. Effects of carbamazepine and metabolites on IL-2, IL-5, IL-6, IL-10 and IFN-γ secretion in epileptic patients: The influence of co-medication. Pharmacol Rep. 2011;63:86–94. doi: 10.1016/s1734-1140(11)70402-9. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Ren RN, Chen XM, Ye LY. An experimental study on hepatotoxicity of topiramate in young rats. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:54–8. [PubMed] [Google Scholar]
- 39.Ghozzi H, Hakim A, Sahnoun Z, Ben Mahmoud L, Atheymen R, Hammami S, et al. Relationship between plasma concentrations of valproic acid and hepatotoxicity in patients receiving high doses. Rev Neurol (Paris) 2011;167:600–6. doi: 10.1016/j.neurol.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 40.Kuntz-Simon G, Obert G. Sodium valproate, an anticonvulsant drug, stimulates human cytomegalovirus replication. J Gen Virol. 1995;76:1409–15. doi: 10.1099/0022-1317-76-6-1409. [DOI] [PubMed] [Google Scholar]
- 41.Verrotti A, Greco R, Latini G, Morgese G, Chiarelli F. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia. 2002;43:1488–92. doi: 10.1046/j.1528-1157.2002.13002.x. [DOI] [PubMed] [Google Scholar]
- 42.Scott JM, Weir DG. Drug-induced megaloblastic change. Clin Haematol. 1980;9:587–606. [PubMed] [Google Scholar]
- 43.Siniscalchi A, Gallelli L, Calabrò G, Tolotta GA, De Sarro G. Phenobarbital/Lamotrigine coadministration-induced blood dyscrasia in a patient with epilepsy. Ann Pharmacother. 2010;44:2031–4. doi: 10.1345/aph.1P335. [DOI] [PubMed] [Google Scholar]
- 44.Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43:822–30. doi: 10.1345/aph.1L617. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: A case report. J Indian Med Assoc. 2011;109:345–6. [PubMed] [Google Scholar]


