Abstract
Varieties of hemoglobin (Hb) forms exist in fish, which are usually well adapted to the different ecological conditions or various habitats. In the current study, Hbs from two Sturgeon species of the Southern Caspian Sea Basin were purified and studied upon interaction with n-dodecyl trimethylammonium bromide (DTAB; as a cationic surfactant) by various methods including UV-visible absorption, dynamic light scattering (DLS), and ANS fluorescence spectrophotometry. The chemometric analysis of Hbs was investigated upon interaction with DTAB under titration, using UV-visible absorption spectra. The chemometric resolution techniques were used to determine the number of the components and mole fraction of the oxidized Hbs. These results provided the evidence for the existence of three different molecular components including native (N), intermediate (I) and denatured (D) in sturgeon Hbs. According to the distribution of intermediates, which were broadened in a range of DTAB concentration, the aggregation states, DLS experiments, and thermal stability (Tm obtained by differential scanning calorimetry (DSC)), the Acipenser stellatus Hb was more stable compared to Acipenser persicus Hb. These results demonstrate a significant relationship between the stability of fish Hbs and the habitat depth requirements.
Keywords: Sturgeon hemoglobins, DTAB, Intermediates, Chemometric analysis, Aggregation, Thermal denaturation
1. Introduction
Hemoglobin multiplicity has physiological and evolutionary significance in many fish species, as well as other vertebrates. Multiple hemoglobin components, as an index for molecular adaptations, enable fish to adapt to variable ecological conditions[1]. The different Hbs, play different structural and functional roles in the animal respiratory system. Vast variations in the number and in the structural and functional properties of Hbs among species have been revealed in a number of investigations [2–8]. This multiplicity is a basis for surprising adaptation of fish to their surrounding environment [9–11]. These adaptations developed during a long evolutionary process over the course of many thousands of years to permit the need for oxygen demand, due to the different environmental conditions that need to be satisfied. Variations in oxygen tension, salinity, temperature, and pH are examples of such environmental factors [12, 13]. In the literature, there are many reports addressing different aspects of purification and characterization of Hbs from various fish species [14–22].
The thermal stability of proteins, including hemoglobin is strongly coupled with its electrostatic and hydrophobic interactions and salt bridge formations [23, 24]. The thermal stability of the Hb in fish and amphibians is much lower than birds, reptiles, and mammals [25]. The thermal stability of various types of Hbs has been studied by the differential scanning calorimetry (DSC) [26], which measures the heat changes during increases or decreases in temperature [27, 28]. The stability of a protein in dilute solution can be determined by measuring changes in the partial molar heat capacity of the protein at constant pressure (ΔCp). The ability of heat absorption for a compound can be measured by monitoring the changes in its heat capacity [29]. The hemoglobin aggregation is also studied under denaturing conditions [30, 31]. Several external factors such as temperature, ionic strength, and additives play important roles in controlling or affecting protein aggregation [32–34].
A surfactant is a substance which lowers the surface tension of a liquid while increasing the contact between the liquid and another substance. One of the important applications of surfactants is denaturation of protein structure. The use of surfactant-protein interactions is very common in different fields including medicine, chemistry, and biology [35, 36]. The presence of n-dodecyl trimethylammonium bromide (DTAB) as a cationic surfactant destabilizes a protein [37–39]. The effect of DTAB on hemoglobin has been examined in many studies [37, 40, 41]. Valuable information including thermodynamic stability, cooperativity, and the nature of the forces required to maintain tertiary structure [42, 43], and the native state of a protein can be extracted from denaturation studies of the protein [44].
The chemometric analysis was employed to interpret the Hb denaturation by interaction with DTAB. Chemometrics is the science of relating measurements made on a chemical system or process to the state of the system via application of mathematical or statistical methods. This definition can be generalized to biochemical systems as well [45]. The chemical and/or physical information can be recovered from the experimental data using chemometric resolution methods. Among the various chemometric methods, multivariate resolution methods are widely used for the analysis and interpretation of spectroscopic data obtained during monitoring of a chemical or physical process [46].
An intermediate as a molecular entity, which is formed from the reactants, has important role in identification of the reaction and it constituents. The number of intermediates present in a reacting system in the presence of surfactants can be determined using their spectra and related concentration profiles through chemometric methods [47–49]. The concentration profiles and the spectra of the protein conformations involved in the unfolding process can be recovered by employing multivariate curve resolution-alternative least square (MCR-ALS) method [48–51]. The mechanism of the unfolding process during thermal evolution of the system can be interpreted using the MCR-ALS results. Resolved spectra can reveal the nature of the conformations of intermediates and therefore, the effect of temperature on each conformation can be evaluated using the results of chemometric techniques[52].
Fluorescence analysis is also used for studying properties of the tryptophan residues in the Hb, as the most hydrophobic residue and determined the effective hydrophobicity of proteins [53, 54]. Hydrophobicity, as an important property, measures the tendency of molecules to aggregate in an aqueous solvent and shows their non-polar moieties. These structural differences in Hbs are related to their physiological role as a gas carrier under different environmental conditions [55]. In this paper, we compared the conformational stability, aggregation state and chemometric analysis of Hbs from two Acipenser species of the Caspian Sea with different habitat depths upon interaction with DTAB by various mathematical and physical techniques.
2. Materials and Methods
2.1. Materials
Fresh hemoglobin samples from Sturgeons (Acipenser persicus and Acipenser stellatus) were prepared as previously reported [27]. Sodium dodecyl sulfate (SDS), CM-cellulose, dithiotreitol (DTT), dodecyl trimethylammonium bromide (DTAB) and other chemical reagents (salts, detergents) were obtained from Sigma. Other reagents were of analytical grade. All solutions were prepared using double-distilled water. The experiments were carried out at 25°C, pH 7.3 using 10 mM phosphate buffer. The concentrations used for DTAB were under critical micelle concentration (CMC) [56].
2.2. Methods
2.2.1. Preparation and purification of hemoglobins
The experimental procedures and protocols for preparation and purification of hemoglobins were as previously described [57]. Briefly, Sturgeons (Acipenser persicus and Acipenser stellatus) hemoglobins were prepared from red blood cells of three-year-old sturgeons obtained from the Ghorogh Center of Sturgeon Aquaculture (The Iranian Fisheries Research Organization, Caspian Sea, Iran) according to the method of Williams and Tsay [58]. For Hb purification from Acipenser species, ion-exchange chromatography on CM-cellulose column equilibrated with 10 mM phosphate buffer in the pH range of 5.5 to 10 was used. The accuracy of method was confirmed by IEF and SDS-PAGE and native-PAGE analysis [57]. Only the dominant Hbs of sturgeons were used for all the experiments. The Hb concentrations were determined spectrophotometrically using a millimolar extinction coefficient of 13.5 (monomer basis) at 541 nm for oxyhemoglobin[59].
2.2.2. Aggregation assays
The aggregation of Hb samples was monitored at 360 nm by Shimadzu model UV-3100 spectrophotometer using a 10 mm path length quartz cuvette. The average time for the mixture in the cuvette necessary to reach the predetermined temperature of the cell-holder was 3 min. All measurements of absorbance were performed by incubating Hb with DTT(6.523 mM) for 60 min at 50 °C (with ± 0.1 °C error) vs. time in 10 mM sodium phosphate buffer (pH 7.3). The Hb concentration was constant (1.3 mg/ml) in all experiments.
2.2.3. UV-vis spectrophotometry
Using UV-vis spectrophotometer, Shimadzu 3100, the spectrum of Hb solutions (1.3 mg/ml) in a phosphate buffer (10 mM, pH 7.3), in the range of 200–600 nm, was obtained in the absence and presence of different concentrations of DTAB. The experiment was first base lined with buffer solutions and then Hb spectra were obtained upon titration of DTAB. The absorbance values between 250 – 600 nm were plotted versus DTAB concentration.
2.2.4. Dynamic Light Scattering (DLS) spectrophotometry
DLS is a well-established technique for measuring the protein aggregation over wide ranges [60]. Hb solutions (2.34 mg/ml) in a phosphate buffer (10 mM, pH 7.3 at 25 °C) were measured, by dynamic light scattering (DLS), using a Malvern Instrument (Brookhaven Instruments Corporation, USA). The protein samples were filtered using Whatman cellulose acetate puradisc 30 syringe filter (0.2 Micron) before performing the experiments. In these experiments for each Hb in absence or presence of DTAB (10 mM), five autocorrelation functions were recorded.
2.2.5. Fluorescence Measurements
A Cary Eclipse (Varian, Australia) spectrofluorimeter, equipped with a temperature controller bath model Cary, was used for all the fluorescence experiments. The two Hb concentrations were adjusted to be the same. 8-Anilino-1-naphthalenesulfonic acid (ANS) is a small organic compound used to probe the accessibility of hydrophobic patches in proteins. In this study, Hb solution (1.3 mg/ml in 10 mM phosphate buffer pH 7.3 at 25 °C) was prepared using 20 μl of ANS (2 mM) with DTAB (10 mM). The contribution of the DTAB/ANS solution was subtracted from Hb solutions with DTAB/ANS. Emission scans were then obtained from 365 to 600 nm using an excitation wavelength of 355 nm.
2.2.6. Differential scanning calorimery (DSC) studies
Thermal unfolding of hemoglobin was monitored by DSC (Calorimetry Sciences Corporation, CSC 6100 N-DSC II). All experiments were performed between 20–80°C. The hemoglobin concentration in the sample solution was 1.3 mg/ml in a phosphate buffer (10 mM, pH 7.3) and heating rate was 2 °C/min. The sample cell was filled with protein solution and the reference cell was filled with a phosphate buffer solution that contained all of the sample constituents, except the protein. The sample and reference vessels were equilibrated with a precision of ±0.1 mg. The temperature at each peak maximum was recorded as the transition temperature (Tm).
2.2.7. Chemometrical Analysis
The spectra information in the range of 250–600 nm obtained from UV-vis spectrophotometer (section 2.2.3) were used for chemometric analysis. The resolution study was performed while the wavelength region of 250–600 nm was applied for determination of noise levels in the system. In multivariate curve resolution using the Eq. (1), a bilinear decomposition of the experimental data matrix was performed:
| (1) |
In Eq. (1), subscripts show the dimensions of matrices. NR (number of rows) is the number of spectra, NC (number of columns) is the number of wavelengths, N is the number of considered components (different species contributing to the signal). In the current study, D is a row-wise matrix augmentation of the UV-vis spectra, D is the matrix describing how the contributions of the N species changed in the NR different rows of the data matrix (concentration profiles). ST is the matrix describing how the responses of these N species changed in the NC columns of the data matrix (pure spectral profiles). E is the residual matrix with the data variance unexplained by CST [61]. The problems to be resolve using multivariate curve resolution may be described mathematically in the following steps:
Find the number of species (N) causing the observed data matrix D.
Find the concentration profiles of these species (matrix C).
Find the pure response or the spectral profiles of these species (matrix S).
Regarding the first step, the Singular Value Decomposition (SVD) method can be used to evaluate the number of components of the system. SVD decomposes the original data matrix D to score and load matrices, which contain the orthogonal eigenvectors spanning the row and column spaces of the original data set respectively. Concerning steps 2 and 3, it is obvious that without additional information, the inherent rotational freedom and scale freedom in the solution of Eq. (1) led us to an ambiguous state for exact C and S. This problem is often referred as factor analysis ambiguity problem (there is an infinite number of possible solutions) [62]. Besides the selectivity and local rank constraints as the most important constraints to limit the number of possible solutions, there are some other restrictive constraints that are derived from the physical nature and previous knowledge of the problem under study. These can considerably reduce the infinite number of possible solutions. For instance, negative values for the concentration of components in the mixture have no physical meaning. In many spectroscopic experiments only positive values are allowed in the spectra. Unimodality is an essential constraint over concentration profiles. In reaction based systems, closure constraint and/or mass balance equations should be preserved. Under these constraints and using suitable method, unique solution for C and S can be expected. In this study, The MCR-ALS (Multivariate Curve Resolution-Alternative Least Square) algorithm [63] was used to resolve the components of the pure spectra and their related concentration profiles. Considering Eq. (1), in the MCR-ALS analysis C and S matrices are calculated in an iterative procedure such that the CST constructs the D matrix with the minimal residual error (optimal fitting), E based on following equations (Eqs. 2, 3):
| (2) |
| (3) |
The ‘+’ superscript denotes the pseudo inverse of a matrix. For ALS optimization algorithm to be started an initial estimate of the concentration profile or spectral profile of components is needed. Simple-to-use interactive self-modeling mixture Analysis (SIMPLISMA) and evolving factor analysis (EFA) are known methods to make these estimations. In the current study after comparison of both methods, the SIMPLISMA was applied. In each iteration of MCR-ALS optimization process, the non-negativity, unimodality (concentration profiles must have cumulative shapes), and closure constraints were applied to concentration profiles and non-negativity was applied to the spectral profiles. The MCR-ALS program was downloaded from the webpage of multivariate curve resolution that coded by Tauler et al. [52].
3. Results
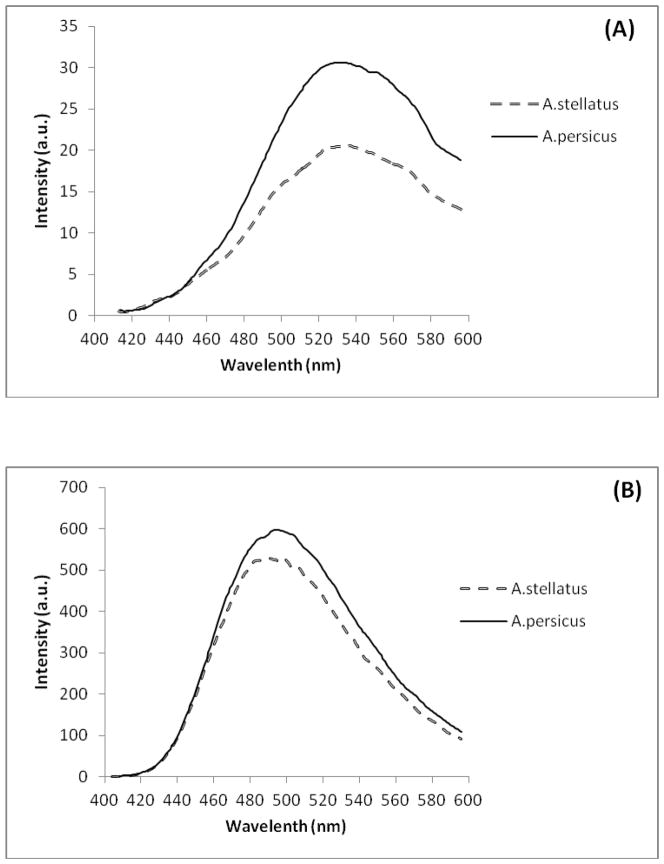
Fig. 1A shows ANS fluorescence spectra of Hb solutions, and allows comparing different Hbs with each other. This figure exhibits a large fluorescence enhancement upon ANS binding to the hydrophobic patches of the studied Hbs. Fig. 1B indicates ANS fluorescence spectra of Hb solutions in the presence of DTAB. These findings confirmed that Acipenser persicus Hb had more hydrophobic accessible surface patches than Acipenser stellatus Hb, in absence or presence of DTAB.
Fig. 1.
Fluorescence spectra of ANS binding. The Hb concentrations were 1.3 mg/ml dissolved in 10 mM phosphate buffer (pH 7.3), at the standard temperature condition (25 °C). (A)The Hb solutions with ANS. By subtracting the Hb solutions spectra from related buffer with ANS; (B) The Hb solutions with ANS and DTAB (10 mM). The contribution of the DTAB/ANS solution was subtracted from all Hb solutions.
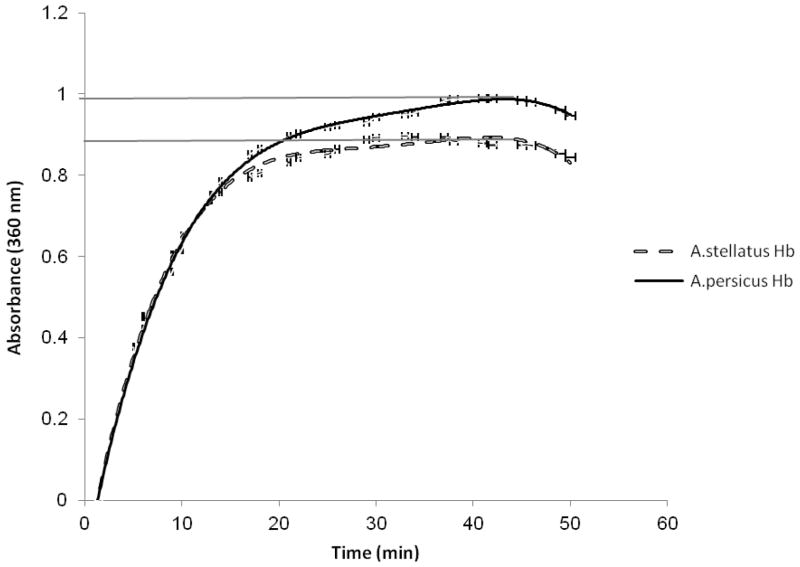
The turbid metric method as a simple and traditional instrumental method was used to monitor protein aggregation. This method, involves measuring the optical density of the sample based on light scattering in the near UV or visible region, where proteins have negligible absorption. DTT breaks up disulfide bonds formed by cysteines, and was used to unfold the Hb and eliminate the bonds that promote its folding and aggregation. The DTT can also operate as an aggregating agent. Fig. 2 shows that the aggregation of the Acipensers Hbs was started from minute two and its amount increased with time. The increase in the absorbance at 360 nm revealed that aggregation was reduced in Acipenser persicus Hb compared to Acipenser stellatus.
Fig. 2.
The increase in absorbance at 360 nm for DTT (6.523 mM) induced aggregation of Hbs solutions (1.3 mg/ml) in a phosphate buffer (10 mM, pH 7.3) at 50 °C.
Analyzing the aggregation size distributions by DLS is tabulated in Table 1. This confirmed the results of aggregation measurements. This table indicates that Acipenser persicus Hb-DTAB complex has more dissociation than Acipenser stellatus Hb. Thus, size distribution of Acipenser persicus and Acipenser stellatus Hbs had a meaningful relationship with the amount of aggregation caused by DTAB.
Table 1.
The size distribution of Acipenser persicus and Acipenser stellatus Hbs, measured by DLS has meaningful relationship with the amount of aggregation caused by DTAB (10 mM).
| Samples of Hbs | Diameter (nm) |
|---|---|
| Hb A. persicus | 7.13 |
| Hb A. stellatus | 6.16 |
| Hb A. persicus+DTAB | 10.12 |
| Hb A. stellatus+DTAB | 8.99 |
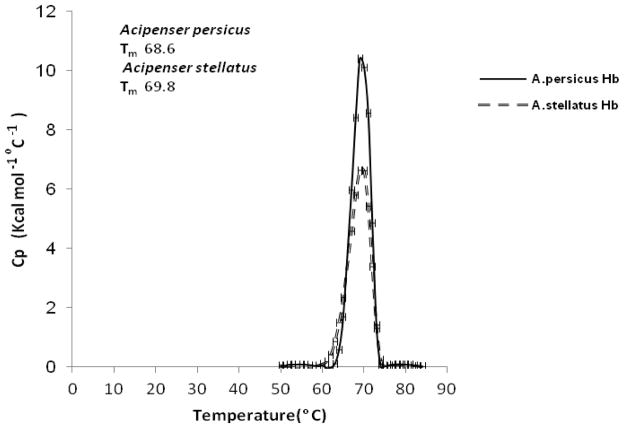
Fig. 3 shows heat capacity (Cp) profiles for the thermal denaturation of Hbs from two species of Acipensers. Different Hb derivatives showed varying thermal unfolding characteristics. The maximum point occurred at Tm in the profile.
Fig. 3.
DSC thermogram for the thermal denaturation of Hbs solutions (1.3 mg/ml) in a phosphate buffer (10 mM, pH 7.3) at 20–80 °C.
The ratio between van’t Hoff (ΔHvH) and calorimetric (ΔHcal) enthalpies can be considered as a sign for existence of intermediate states [64–65]. In the case of the current study, the ΔHcal/ΔHvH ratios are greater than 1 for the A. stellatus Hb and the A. persicus Hbs which is likely to be due to the intermediate state(s).
The row-wise UV-vis spectra were used as experimental data for the chemometric analysis. The number of total components and mole fraction in unfolding process during DTAB titration process in each of the two Hb solutions (1.3 mg/ml) in a phosphate buffer (10 mM, pH 7.3) in the range of 250–600 nm was determined (Fig. 4).
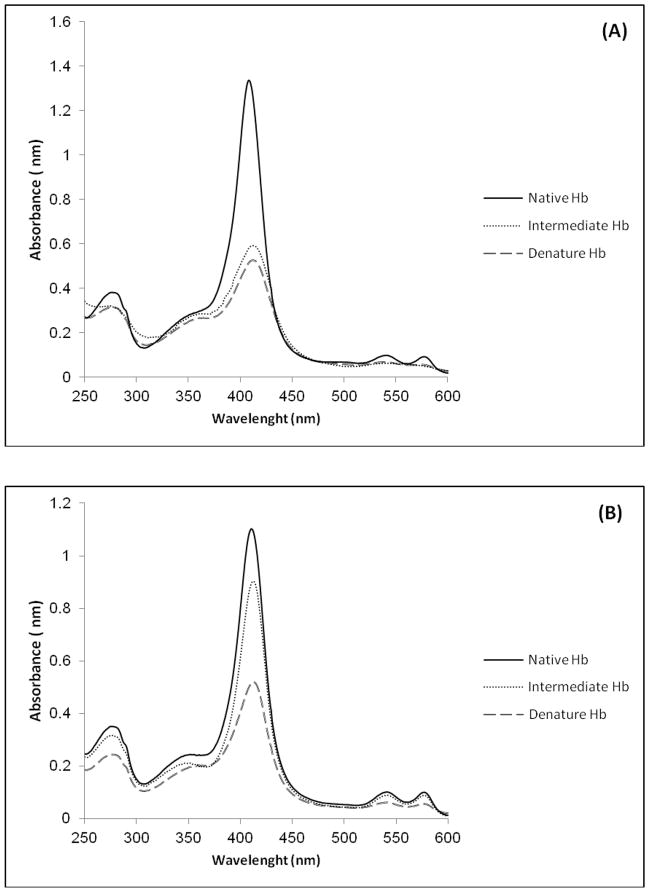
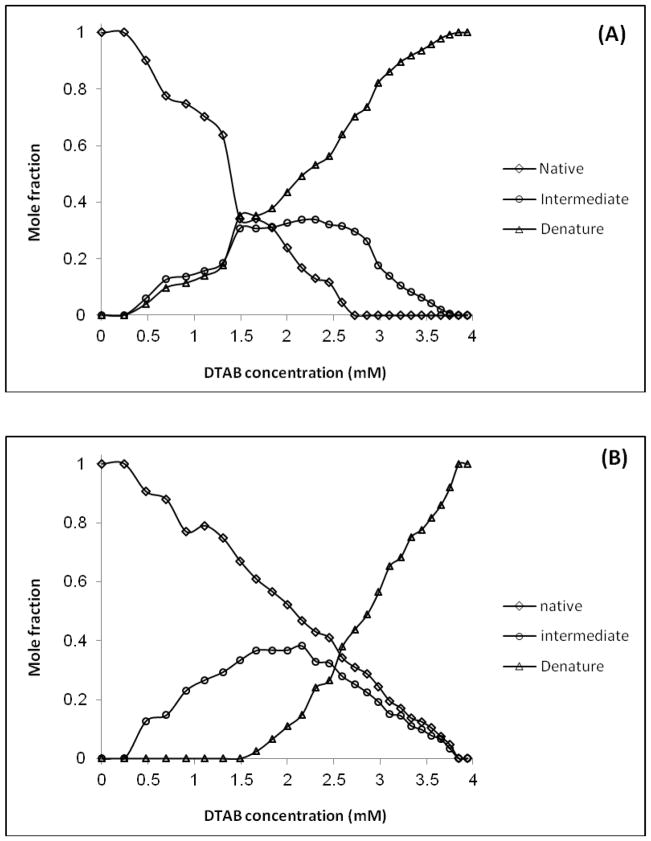
Fig. 4.
Spectral profiles of the (A) Acipenser persicus Hb (B) Acipenser stellatus Hb. N, I and D denotes to native, intermediate states.
Because of low-level noise in the data, preprocessing of the data was not necessary. Using SVD the three components were determined for each of the Hb solutions. The fact that singular values reflect the inherent properties of systems, differences between them can be interpreted as a sign of meaningful variation between the functional properties of those systems. SVD analysis showed that apart from native and denatured states, there is an intermediate state for both Hbs. This means during unfolding process three components were determined for both data sets including native (N), intermediate (I) and denature (D).
The evolution of Hb folding was also monitored by evolving factor analysis (EFA) method. The SIMPLISMA was then used to monitor the evolution of protein folding and give estimation of concentration profiles of the three components in each system under the study. Finally, the intermediate folding which appeared during Hb denaturation, was detected, identified, and quantized by applying MCR-ALS algorithm. The MCR-ALS approach provided the spectra and concentration profiles of different structural components, which are associated with analyzed processes (Figs. 4 and 5). Fig. 4A and Fig. 4B demonstrate the relationship between each component with its specific pure spectrum for Acipenser persicus and Acipenser stellatus Hbs, respectively. According to the figure, the maximum absorbance occurred in the native state of Hb and the minimum absorbance occurred in the denatured state for both Hb.
Fig. 5.
Concentration profiles of the (A) Acipenser persicus Hb (B) Acipenser stellatus Hb in 10 mM phosphate buffer (pH 7.3). The AUC for the intermediate components in (B) is 19% more than (A).
Figs. 5A and 5B depicted the concentration profiles for Sturgeon Hbs. In the figure, the mole fraction vs. DTAB concentration diagram for intermediate species showed that the Acipencer stellatus Hb had wider distribution compared to Acipenser persicus. In addition, the area under the curve (AUC) for Acipenser stellatus was about 19% wider than that of Acipenser persicus. The difference between intermediate states is meaningful and can be relevant to the habitat depth and other environmental conditions (Fig. 5). It is also apparent from Fig. 5 that the unfolding process and the appearance trend of denatured Hb species have obvious differences.
4. Discussion
Mammals and birds have relatively thermostable Hbs. The properties of their Hbs are very similar and slightly different from Hbs of the reptiles. On the other hand, the thermostability of the reptiles Hbs is sharply greater than the thermostability of fish and amphibians Hbs [25]. This fact may be partially related to the environmental conditions such as the depth of their habitats from the sea level and temperature. Most sturgeons are anadromous bottom-feeders, migrating seasonally from fresh water of the rivers around Caspian Sea, and therefore, are adapted to different salinities (from 8.59% to 14.20%) and temperatures (from 4 °C to 29°C). The Acipenser stellatus and the Acipenser persicus fish live in average depth of 150 m and 50 m from the sea level, respectively. According to Fig. 6, the Caspian Sea from the sea level to the depth of 800 m, the average water temperature and average partial oxygen pressure have decremental and incremental trends, respectively, while the average of salinity and pH is approximately constant.
Fig. 6.
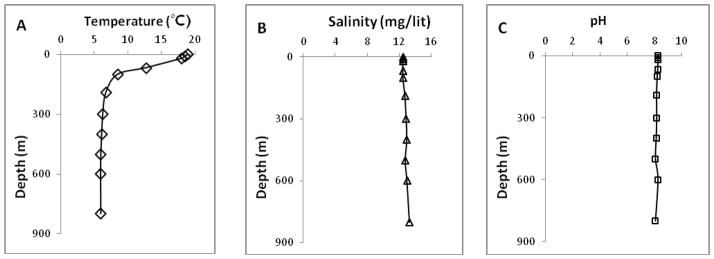
Seasonally averaged vertical profiles of the A) temperature (°C), B) salinity (mg/lit), and C) pH in the south of the Caspian Sea.
According to these facts, it is clear that the Acipenser stellatus can tolerate wider temperature and partial oxygen pressure range compared with the Acipenser persicus. Our experimental findings regarding thermostability using DSC, aggregation measurements by UV-vis and DLS showed that the Hb of Acipenser stellatus is more stable than Acipenser persicus. These facts about the environmental habitat conditions are in complete accordance with above mentioned experimental findings regarding the higher relative stability of the Acipenser stellatus in comparison with Acipenser persicus.
In previous works the chemometric studies indicated that the correspondence of distribution of the intermediate species in a range of surfactant concentration is an indicator of aggregation and instability of lysozyme [66]. In the current study, chemometric analysis using UV–vis spectrophotometery illustrated that according to the distribution of intermediates and their broadenings versus the range of DTAB concentrations, the aggregation state and stability of Hb solutions were reduced from Acipenser stellatus to Acipenser persicus. In this study, both Acipenser fish belong to the same phylogenetic species, and therefore, the observed differences in their Hb thermostability possibly does not relate to their physiological properties and instead is related to their environmental differences including temperature, partial oxygen pressure, and average depth. Fig. 4A shows that the absorbance of intermediate state for Acipenser persicus was close to the absorbance of the denatured Hb while in Fig. 4B was close to the native state for Acipenser stellatus. Comparison of intermediate states of Hbs in Fig. 5A and Fig. 5B led us to the importance of the role of intermediate state in the respiratory system of corresponding fish habitat depth. It means according to the Acipenser stellatus via DTAB concentration profile; intermediate state plays fundamental roles for deeper habitat. The distributions of intermediate mole fractions versus the range of DTAB concentration verifies that the aggregation state and instability for the Acipenser persicus Hb was greater than for the Acipenser stellatus Hb. Fluorescence analysis in Fig. 1 shows that hydrophobicity in Acipenser persicus Hb is greater than Acipenser stellatus Hb in the absence or presence of DTAB. This measurement indicates the enhanced tendency of Acipenser persicus Hb to aggregate in an aqueous solvent.
5. Conclusion
The results obtained from the DSC thermal profiles and DLS showed that the Acipenser stellatus Hb has more stability and disaggregation properties in comparison with Acipenser persicus Hb respectively. Fluorescence studies demonstrated that Acipenser persicus Hb has more hydrophobic properties than Acipenser stellatus Hb.
The chemometrical analysis of Hb denaturation induced by DTAB titration process, confirms the existence of an intermediate state as a stability indicator. This can be considered as one remarkable finding of this study. The formation of these intermediates has different trends which can be interpreted as the main reason for variation in structural properties and stability of the Hbs. The chemometric results confirm the experimental outcome and showed that intermediate state for the Acipenser stellatus as a more stable protein, which can live in deeper depths, has broader distribution and higher area under the curve than that of the Acipenser persicus.
These investigations indicate that the thermostability, hydrophobicity and disaggregation of Hbs have meaningful relationships with the state of environment and depth of the sea requirements.
Highlights.
These results indicate that the thermostability, hydrophobicity and aggregation of Hbs have meaningful relation with the state of environment and depths see requirement.
Our experimental findings about thermostability using DSC, DLS and aggregation measurements show that the Hb of Acipenser stellatus is more stable than Acipenser persicus.
In chemometric analysis using UV–vis spectrophotometery illustrates that according to the distribution of intermediates and their broadenings versus the range of DTAB concentrations, the aggregation state and instability of Hb solutions are reduced from Acipenser persicus to Acipenser stellatus.
By fluorescence study in absence and presence of DTAB, it is demonstrated that the Acipenser persicus Hb has more hydrophobic properties than Acipenser stellatus Hb.
Acknowledgments
This work was supported by University of Tehran, Iran National Science Foundation (INSF) and Center of Excellence in Biothermodynamics (CEBiotherm), University of Tehran. We also thank International Sturgeon Research Institute, Rasht, Iran for their kind provision of samples and Mr. Alipourfor his assistance.
Abbreviations
- ANS
1-aniliono-8-naphthalene sulfonate
- DTAB
n-dodecyl trimethylammonium bromide
- DTT
dithiotreitol
- Hb
Hemoglobin
- DLS
Dynamic light scattering
- DSC
differential scanning calorimetry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perutz MF. Species adaptation in a protein molecule. Mol Biol Evol. 1983;1:1–13. doi: 10.1093/oxfordjournals.molbev.a040299. [DOI] [PubMed] [Google Scholar]
- 2.Shimada T, Okihama Y, Okazaki T, Shukuya R. The multiple hemoglobins of the Japanese eel, Anguilla japonica: Molecular basis for hemoglobin multiplicity and the subunit interactions. J Biol Chem. 1980;255:7912–7917. [PubMed] [Google Scholar]
- 3.Fago A, D’Avino R, Prisco G. The hemoglobins of Notothenia angustata, a temperate fish belonging to a family largely endemic to the Antarctic Ocean. Eur J Biochem. 1992;210:963–970. doi: 10.1111/j.1432-1033.1992.tb17501.x. [DOI] [PubMed] [Google Scholar]
- 4.Fago A, Romano M, Tamburrini M, Coletta M, D’Avinno R, Prisco G. A polymerising Root effect fish hemoglobin with high subunit heterogeneity. Eur J Biochem. 1993;218:829–835. doi: 10.1111/j.1432-1033.1993.tb18438.x. [DOI] [PubMed] [Google Scholar]
- 5.Riggs A. Properties of fish hemoglobins. In: Hoar WS, Randall DJ, editors. Fish Physiology: The Nervous System, Circulation, and Respiration. Vol. 4. Academic Press; New York: 1970. pp. 209–252. [Google Scholar]
- 6.Powers DA. Structure, function, and molecular ecology of fish hemoglobins. Ann NY Acad Sci. 1974;241:472–490. doi: 10.1111/j.1749-6632.1974.tb21904.x. [DOI] [PubMed] [Google Scholar]
- 7.di Prisco G. Molecular adaptation of Antarctic fish hemoglobins. In: di Prisco G, Pisano E, Clarke A, editors. Fishes of Antarctica: a biological overview. Springer-Verlag; Italia, Milan: 1998. pp. 339–353. [Google Scholar]
- 8.Vitagliano L, Bonomi G, Riccio A, Di Prisco G, Smulevich G, Mazzarella L. The oxidation process of Antarctic fish hemoglobins. Eur J Biochem. 2004;271:1651–1659. doi: 10.1111/j.1432-1033.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 9.Clementi ME, De Rosa MC, Bertonati C, Capo C, Cataldi E, Petruzzelli R, Giardina B. Functional and structural properties of the hemoglobin components from Italian sturgeon (Acipenser naccarii) Fish Physiol Biochem. 2001;24:191–200. [Google Scholar]
- 10.di Prisco G, Tamburrini M. The hemoglobins of marine and freshwater fish: the search for correlations with physiological adaptation. Comp Biochem Physiol, Part B: Biochem Mol Biol. 1992;102:661–671. doi: 10.1016/0305-0491(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 11.Tsuyuki H, Ronald AP. Molecular basis for multiplicity of Pacific salmon hemoglobins: evidence for in vivo existence of molecular species with up to four different polypeptides. Comp Biochem Physiol, Part B: Biochem Mol Biol. 1971;39:503–510. doi: 10.1016/0305-0491(71)90196-9. [DOI] [PubMed] [Google Scholar]
- 12.Campo S, Nastasi G, D’Ascola A, Campo GM, Avenoso A, Traina P, Calatroni A, Burrascano E, Ferlazzo A, Lupidi G. Hemoglobin system of Sparus aurata: changes in fishes farmed under extreme conditions. Sci Total Environ. 2008;403:148–153. doi: 10.1016/j.scitotenv.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Pelster B, Randall D. 4 The Physiology of the Root Effect. Fish physiol. 1998;17:113–139. [Google Scholar]
- 14.di Prisco G, Eastman JT, Giordano D, Parisi E, Verde C. Biogeography and adaptation of Notothenioid fish: Hemoglobin function and globin-gene evolution. Gene. 2007;398:143–155. doi: 10.1016/j.gene.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Fago A, Forest E, Weber RE. Hemoglobin and subunit multiplicity in the rainbow trout (Oncorhynchus mykiss) hemoglobin system. Fish Physiol Biochem. 2001;24:335–342. [Google Scholar]
- 16.Giordano D, Boechi L, Vergara A, Marti MA, Samuni U, Dantsker D, Grassi L, Estrin DA, Friedman JM, Mazzarella L, Di Prisco G, Verde C. The hemoglobins of the sub-Antarctic fish Cottoperca gobio, a phyletically basal species - oxygen-binding equilibria, kinetics and molecular dynamics. FEBS J. 2009;276:2266–2277. doi: 10.1111/j.1742-4658.2009.06954.x. [DOI] [PubMed] [Google Scholar]
- 17.Giordano D, Vergara A, Lee HC, Peisach J, Balestrieri M, Mazzarella L, Parisi E, di Priseo G, Verde C. Hemoglobin structure/function and globin-gene evolution in the Arctic fish Liparis tunicatus. Gene. 2007;406:58–68. doi: 10.1016/j.gene.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Morris RJ, Neckameyer WS, Gibson QH. Multiplet T-state conformations in a fish hemoglobin-carbon-monoxide binding to hemoglobin of thunnus-thynnus. J Biol Chem. 1981;256:4598–4603. [PubMed] [Google Scholar]
- 19.Verde C, Howes BD, De Rosa MC, Raiola L, Smulevich G, Williams R, Giardina B, Parisi E, Di Prisco G. Structure and function of the Gondwanian hemoglobin of Pseudaphritis urvillii, a primitive notothenioid fish of temperate latitudes. Protein Sci. 2004;13:2766–2781. doi: 10.1110/ps.04861504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verde C, Lecointre G, di Prisco G. The phylogeny of polar fishes and the structure, function and molecular evolution of hemoglobin. Polar Biol. 2007;30:523–539. [Google Scholar]
- 21.Verde C, Parisi E, di Prisco G. The evolution of polar fish hemoglobin: A phylogenetic analysis of the ancestral amino acid residues linked to the root effect. J Mol Evol. 2003;57:S258–S267. doi: 10.1007/s00239-003-0035-y. [DOI] [PubMed] [Google Scholar]
- 22.Verde C, Parisi E, di Prisco G. Tracking adaptive evolution in the structure, function and molecular phylogeny of haemoglobin in non-Antarctic notothenioid fish species. Deep Sea Research Part II-Topical Studies in Oceanography. 2006;53:1105–1114. [Google Scholar]
- 23.Perutz MF. Electrostatic effects in proteins. Science. 1978;201:1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- 24.Kauzman W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 25.Tondo CV, Mendez HM, Reischl E. The hydrophobic effect. Comp Biochem Physiol, Part B: Biochem Mol Biol. 1980;66:151–154. [Google Scholar]
- 26.Cho KC, Choy CL. Thermal stability of hemoglobin and myoglobin: Effect of spin states. Biochim Biophys Acta, Protein Struct. 1980;622:320–330. doi: 10.1016/0005-2795(80)90043-4. [DOI] [PubMed] [Google Scholar]
- 27.Mousavy SJ, Riazi GH, Kamarei M, Aliakbarian H, Sattarahmady N, Sharifizadeh A, Safarian S, Ahmad F, Moosavi-Movahedi AA. Effects of mobile phone radiofrequency on the structure and function of the normal human hemoglobin. Int J Biol Macromol. 2009;44:278–285. doi: 10.1016/j.ijbiomac.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Reza DM, Moosavi-Movahedi AA, Norouzi P, Ghourchian H, Safarian S. Inhibition of human hemoglobin autoxidation by sodium n-dodecyl sulphate. J Biochem Mol Biol. 2002;35:364–370. doi: 10.5483/bmbrep.2002.35.4.364. [DOI] [PubMed] [Google Scholar]
- 29.Shinoda K, Hirai T. Ionic surfactants applicable in the presence of multivalent cations. Physicochemical properties. J Phys Chem. 1977;81:1842–1845. [Google Scholar]
- 30.Manno M, San Biagio PL, Palma MU. The role of pH on instability and aggregation of sickle hemoglobin solutions. Proteins: Struct, Funct, Bioinf. 2004;55:169–176. doi: 10.1002/prot.10648. [DOI] [PubMed] [Google Scholar]
- 31.Chen K, Ballas SK, Hantgan RR, Kim-Shapiro DB. Aggregation of normal and sickle hemoglobin in high concentration phosphate buffer. Biophys J. 2004;87:4113–4121. doi: 10.1529/biophysj.104.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosmaoglou M, Schwarz N, Bett JS, Cheetham ME. Molecular chaperones and photoreceptor function. Progress in Retinal and Eye Research. 2008;27:439–449. doi: 10.1016/j.preteyeres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Zvi AP, Goloubinoff P. Review: mechanisms of disaggregation and refolding of stable protein aggregates by molecular chaperones. J Struct Biol. 2001;135:84–93. doi: 10.1006/jsbi.2001.4352. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar T, Mitra G, Gupta S, Manna T, Poddar A, Panda D, Das KP, Bhattacharyya B. MAP2 prevents protein aggregation and facilitates reactivation of unfolded enzymes. Eur J Biochem. 2004;271:1488–1496. doi: 10.1111/j.1432-1033.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 35.Rigas DA, Koler RD, Cummings G, Duerst ML, Malm DR, Swisher K, Vanbellinghen P. Decreased erythrocyte survival in hemoglobin H disease as a result of the abnormal properties of hemoglobin H: the benefit of splenectomy. Blood. 1961;18:1–17. [PubMed] [Google Scholar]
- 36.Guo R, Fan GK, Liu TQ, Jiao XA. The Interaction of Methylene Blue and Bovine Serum Albumin in SDS Micelle System. Acta Phys Chim Sin. 2001;17:185–188. [Google Scholar]
- 37.Ajloo D, Moosavi-Movahedi AA, Hakimelahi GH, Saboury AA, Gharibi H. The effect of dodecyl trimethylammonium bromide on the formation of methemoglobins and hemichrome. Colloids Surf, B. 2002;26:185–196. [Google Scholar]
- 38.Moosavi-Movahedi AA, Samiee B, Hakimelahi GH. Thermal analysis of adenosine deaminase in the presence of dodecyl trimethyl ammonium bromide. J Colloid Interface Sci. 1993;161:53–56. [Google Scholar]
- 39.Meyer ML, Kauzmann W. The effects of detergents and urea on the rotatory dispersion of ovalbumin. Arch Biochem Biophys. 1962;99:348–349. doi: 10.1016/0003-9861(62)90022-x. [DOI] [PubMed] [Google Scholar]
- 40.Bordbar AK, Nasehzadeh A, Ajloo D, Omidiyan K, Naghibi H, Mehrabi M, Khajehpour H, Rezaei-Tavirani M, Moosavi-Movahedi AA. Thermodynamic elucidation of binding isotherms for hemoglobin and globin of human and bovine upon interaction with dodecyl trimethyl ammonium bromide. Bull Korean Chem Soc. 2002;23:1073–1077. [Google Scholar]
- 41.Bordbar AK, Moosavi-Movahedi AA, Amini MK. A Microcalorimetry and Binding Study on Interaction of Dodecyl Trimethylammonium Bromide with Wigeon Hemoglobin. Thermochim Acta. 2003;400:95–100. [Google Scholar]
- 42.Tanford C. Protein denaturation: Part C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- 43.Lapanje S. Physicochemical aspects of protein denaturation. Wiley; New York: 1978. pp. 207–221. [Google Scholar]
- 44.Mojtahedi M, Parastar H, Jalali-Heravi M, Chamani J, Chilaka FC, Moosavi-Movahedi AA. Comparison between two different hemichromes of hemoglobins (HbA and HbS) induced by n-dodecyl trimethylammonium bromide: Chemometric study. Colloids Surf, B. 2008;63:183–191. doi: 10.1016/j.colsurfb.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Wold S. Chemometrics; what do we mean with it, and what do we want from it? Chemom Intell Lab Syst. 1995;30:109–115. [Google Scholar]
- 46.McLennan F. In: Process Analytical Chemistry in Perspective. McLennan F, Kowalski BR, editors. Blackie Academic and Professional; Glasgow: 1995. pp. 1–13. [Google Scholar]
- 47.Jaumot J, Gargallo R, de Juan A, Tauler R. A graphical user-friendly interface for MCR-ALS: a new tool for multivariate curve resolution in MATLAB. Chemom Intell Lab Syst. 2005;76:101–110. [Google Scholar]
- 48.De Juan A, Tauler R. Chemometrics applied to unravel multicomponent processes and mixtures:: Revisiting latest trends in multivariate resolution. Anal Chim Acta. 2003;500:195–210. [Google Scholar]
- 49.Tauler R. Multivariate curve resolution applied to second order data. Chemom Intell Lab Syst. 1995;30:133–146. [Google Scholar]
- 50.Navea S, de Juan A, Tauler R. Detection and resolution of intermediate species in protein folding processes using fluorescence and circular dichroism spectroscopies and multivariate curve resolution. Anal Chem. 2002;64:6031–6039. doi: 10.1021/ac025914d. [DOI] [PubMed] [Google Scholar]
- 51.Navea S, de Juan A, Tauler R. Modeling temperature-dependent protein structural transitions by combined near-IR and mid-IR spectroscopies and multivariate curve resolution. Anal Chem. 2003;75:5592–5601. doi: 10.1021/ac0343883. [DOI] [PubMed] [Google Scholar]
- 52.Tauler R, Izquierdo-Ridorsa A, Casassas E. Simultaneous analysis of several spectroscopic titrations with self-modelling curve resolution. Chemom Intell Lab Syst. 1993;18:293–300. [Google Scholar]
- 53.Bosch Cabral C, Imasato H, Rosa JC, Laure HJ, da Silva CHT. Fluorescence properties of tryptophan residues in the monomeric d-chain of Glossoscolex paulistus hemoglobin: an interpretation based on a comparative molecular model. Biophys Chem. 2002;97:139–157. doi: 10.1016/s0301-4622(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 54.Kristinsson HG, Hultin HO, Agric J. Changes in Trout Hemoglobin Conformations and Solubility after Exposure to Acid and Alkali pH. Food Chem. 2004;52:3633–3643. doi: 10.1021/jf034563g. [DOI] [PubMed] [Google Scholar]
- 55.Gabbianelli R, Zolese G, Bertoli E, Falcioni G. Correlation between functional and structural changes of reduced and oxidized trout hemoglobins I and IV at different pHs. Eur J Biochem. 2004;271:1971–1979. doi: 10.1111/j.1432-1033.2004.04109.x. [DOI] [PubMed] [Google Scholar]
- 56.Ren B, Gao Y, Lu L, Liu X, Tong Z. Aggregates of alginates binding with surfactants of single and twin alkyl chains in aqueous solutions: Fluorescence and dynamic light scattering studies. Carbohydr Polym. 2006;66:266–273. [Google Scholar]
- 57.Ariaeenejad S, Habibi-Rezaei M, Jamili S, Fatemi MR, Poursasan N, Ahmad F, Sheibani N, Kavousi K, Moosavi-Movahedi AA. Biochemical Characterization of Hemoglobins from Caspian Sea Sturgeons (Acipenser persicus and Acipenser stellatus) Cell Biochem Biophys. 2012;62:73–81. doi: 10.1007/s12013-011-9261-x. [DOI] [PubMed] [Google Scholar]
- 58.Williams RC. A convenient chromatographic method for the preparation of human hemoglobin. Anal Biochem. 1973;54:137–145. doi: 10.1016/0003-2697(73)90256-x. [DOI] [PubMed] [Google Scholar]
- 59.Antonini E, Brunori M. Hemoglobin. Annu Rev Biochem. 1970;39:977–1042. doi: 10.1146/annurev.bi.39.070170.004553. [DOI] [PubMed] [Google Scholar]
- 60.Blaszczak Z, Pochylski M, kostka I, Ziobrowski P, Drozdowski M, Farhoud M. Brillouin scattering study of polyethylene glycol different solutions. J Mol Liq. 2005;121:75–79. [Google Scholar]
- 61.Tauler R. Calculation of maximum and minimum band boundaries of feasible solutions for species profiles obtained by multivariate curve resolution. J Chemom. 2001;15:627–646. [Google Scholar]
- 62.Lawton WH, Sylvestre EA. Self modeling curve resolution. Technometrics. 1971;13:617–633. [Google Scholar]
- 63.Diaz-Cruz MS, Mendieta J, Tauler R, Esteban M. Multivariate curve resolution of cyclic voltammetric data: Application to the study of the cadmium-binding properties of glutathione. Anal Chem. 1999;71:4629–4636. [Google Scholar]
- 64.Persikov AV, Xu Y, Brodsky B. Equilibrium thermal transitions of collagen model peptides. Protein Sci. 2004;13:893–902. doi: 10.1110/ps.03501704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saboury AA, Moosavi-Movahedi AA. Clarification of calorimetric and van’t Hoff enthalpies for evaluation of protein transition states. Biochem Educ. 1994;22:210–211. [Google Scholar]
- 66.Pirzadeh P, Moosavi-Movahedi AA, Hemmateenejad B, Ahmad F, Shamsipur M, Saboury AA. Chemometric studies of lysozyme upon interaction with sodium dodecyl sulfate and [beta]-cyclodextrin. Colloids Surf, B. 2006;52:31–38. doi: 10.1016/j.colsurfb.2006.05.019. [DOI] [PubMed] [Google Scholar]