Abstract
Affective spectrum and anxiety disorders have come to be recognized as the most prevalently diagnosed psychiatric disorders. Among a suite of potential causes, changes in mitochondrial energy metabolism and function have been associated with such disorders. Thus, proteins that specifically change mitochondrial functionality could be identified as molecular targets for drugs related to treatment for affective spectrum disorders. Here, we report generation of transgenic mice overexpressing the scaffolding and mitophagy related protein Sequestosome1 (SQSTM1/p62) or a single point mutant (P392L) in the UBA domain of SQSTM1/p62. We show that overexpression of SQSTM1/p62 increases mitochondrial energy output and improves transcription factor import into the mitochondrial matrix. These elevated levels of mitochondrial functionality correlate directly with discernible improvements in mouse behaviors related to affective spectrum and anxiety disorders. We also describe how overexpression of SQSTM1/p62 improves spatial learning and long term memory formation in these transgenic mice. These results suggest that SQSTM1/p62 provides an attractive target for therapeutic agents potentially suitable for the treatment of anxiety and affective spectrum disorders.
Keywords: Mitochondria, SQSTM1/p62, Animal models, Affective spectrum disorders, Depression, Anxiety
1. Introduction
While the molecular mechanisms behind neurodegeneration are often ambiguous, dysfunction of mitochondrial dynamics has emerged in recent years as a hallmark feature of neurodegenerative disease. Dysfunction of mitochondria leads to impaired energy metabolism, reduced ATP production, and disruption of mitochondrial calcium homeostasis and increased production of oxygen radicals [5]. Cell types with the highest energy demands, such as muscle or neurons, appear to be the most susceptible to mitochondrial dysfunction [77].
Evidence is accumulating that mitochondrial function is related to the pathophysiology and treatment of behavioral disorders [18]. In general, anxiety disorders are the most prevalent psychiatric disorders diagnosed and have been related back to altered energy metabolism, mitochondrial transport and oxidative stress. ATP production through the mitochondrial electron transport chain is necessary for the survival of neurons, and protein signaling cascades have been shown to mediate synapse changes, as well as other long term changes in neuronal structure [7]. Altered levels of proteins specifically involved in neurotransmission, energy metabolism and oxidative stress have been found in lab mice diagnosed as “high anxiety” by elevated-plus maze analysis [19]. Deletion of Bcl-2, a major modulator of mitochondria function, has been related to increased anxiolytic activity in a mouse behavior model [18]. Furthermore, monoamines, such as serotonin, have for years been included in causative hypotheses related to depression. Substantial decreases in serotonin levels in brain regions of a genetic mouse model of bipolar disorder were correlated with decreased mtDNA levels and multiple mtDNA deletions [42]. Changes in mitochondrial functionality resulting in inflammation have also been offered as an explanation for major depression disorders [20]. Collectively, such studies have revealed a strong correlation between proteins specific to mitochondrial function and behavior patterns associated with affective spectrum disorders.
The ubiquitous cytoplasmic protein Sequestosome1 (SQSTM1/p62) was originally cloned as a phosphotyrosine-independent ligand of the p56lck Src homology (SH2) domain [62] and identified as a ubiquitin binding protein [76] via its UBA domain [10]. Mutations in the C-terminal UBA domain of p62, particularly those affecting ubiquitin binding, have been associated with Paget’s Disease of Bone [9, 48]. The most common of these mutations, P392L, impairs ubiquitin binding however, the transcription factor NF-κB is constitutively activated when ubiquitin binding is absent with this mutation [65]. p62 is also implicated in multiple cellular activities including antioxidant metabolism [34], autophagy/mitophagy [23], and the ubiquitin proteasome system [69], as well as serving as a scaffold for aPKC/TRAF6 mediated activation of NF-κB [17]. Interestingly, loss of NF-κB activity in p62−/− brain contributes to increased generation of Reactive Oxidative Species (ROS) in neurons and glial cells [17] while NF-κB presence in brain cells is required for long term potentiation (LTP), neurogenesis and memory formation [2, 14]. Moreover, p62 mediates the phosphorylation of the AMPA receptor subunit GluR1 by atypical PKC further implicating that it plays a role in synaptic transmission and neuronal plasticity [35].
Growing lines of evidence support a strong correlation between p62 expression and mitochondrial function. p62 is recruited to mitochondria in PINK1/Parkin expressing cells [61], as well as being required for the completion of the mitophagy pathway [49, 61]. Recently, we have shown that p62 has an endogenous association with mitochondria where it spans the mitochondrial membrane and affects import of the mitochondrial transcription factor TFAM [70]. Loss of TFAM import into the mitochondria matrix results in decreased mitochondrial DNA (mtDNA) ultimately leading to mitochondrial dysfunction. Overexpression of p62 in Mouse Embryonic Fibroblast (MEF) cells results in increased levels of mtDNA. Further, restored expression of p62 rescues TFAM import into the mitochondrial matrix resulting in restoration of mitochondrial functionality [70].
Previously, our laboratory analyzed the behavior of mice in which the p62 gene had been deactivated (p62−/− mice). These mice were tested for their ability to complete spatio-temporal tasks as well as their susceptibility to overall affective spectrum disorders [64]. Absence of p62 from neural tissue in these mice resulted in hippocampal dependent cognitive decline and increased anxiolytic behavior. These mice also displayed a significant degree of depression when compared to wild-type (WT), a phenotype indicative of mitochondrial defects [42], while maintaining normal motor neuron function [64]. Loss of synapses and tau-pathology, as well as other signs of neurodegeneration, are also characteristic of the loss of p62 suggestive of traits related to Alzheimer’s disease [45,72]. Thus, p62 appears to be a prime candidate for a protein that changes the functionality of mitochondria and also affects changes in behavior patterns in a mouse model.
Because removal of p62 has proven to be deleterious to mitochondria function, as well as to mouse behavior, we reasoned that the converse, namely increasing levels of p62 in neural tissue, might theoretically improve mitochondrial functionality and as a direct response, abrogate atypical behaviors related to mitochondrial dysfunction. This hypothesis was the focus of the research described in this paper. Our goal was to examine the involvement of mitochondrial function in affective spectrum disorders, in learning and memory, and to explore the role that p62 plays in this context. To investigate these relationships, we designed a study to explore directed p62 overexpression in the hippocampus of a C57BL/6 mouse and the effects p62 has on mitochondrial function. Central to our question was the corresponding examination of behavioral outcomes, specifically those related to affective spectrum disorders, learning and memory. We show that by increasing the levels of p62 in the hippocampus of OEp62 mice, mitochondrial function is improved and gross behavioral patterns, especially those related to anxiety and depression, are changed. By using p62:P392L overexpressing mice, we provide evidence that p62’s ability to bind ubiquitin through its C-terminal UBA domain plays a role in controlling p62’s ability to affect both mitochondrial function and behavior in overexpressing mice.
2. Methods
2.1 Animals
Mice overexpressing p62 (OEp62) or a mutated form of the protein (p62:P392L) in the hippocampus were generated by our laboratory. C57BL/6 mice were used as the surrogate strain for the development of both OEp62 and P392L overexpressing mice. The Thy-1 vector has been widely used to obtain brain-specific expression of exogenous proteins and tends to display restricted expression to the hippocampus [25]. We generated two constructs employing the Thy 1.2 vector and insertion of an EGFP-tagged p62 cDNA (either full length p62 or mutant P392L p62 construct) by insertion into the Xho1 site of the pTSC21k Thy 1.2 expression vector. The EGFP-p62 construct encompasses the EGFP translation initiation codon, signal peptide and p62 translation initiation codon, signal peptide, termination codon and poly-A tail. The full length clone was sequenced to ensure fidelity during PCR and forward orientation. The construct encodes a 97Kd fusion protein under the influence of the Thy 1.2 promoter. Founder mice were generated at the Auburn University College of Veterinary Medicine Transgenics Facility using C57BL/6 as the donor strain and germ line transmission analyzed by EGFP-p62 expression using sequence specific primers. Donor mice were supplied by the Transgenics Facility. Once founder mice were identified, they were crossed back into the WT C57BL/6 strain and offspring characterized for EGFP-p62 expression. Mice positive for construct expression were inter-crossed to establish a strain of mice overexpressing EGFP-p62 or EGFP-p62:P392L in neural tissues, specifically the hippocampus. Mice were caged in standard barrier cages on a ventilated rack in an animal room with constant temperature (~22+/−1 °C) on a 12 hour light/dark cycle with ad lib food and water. All experiments, unless otherwise indicated, were performed with age matched (6 month) mice in the early phase of the light cycle under standard room fluorescence. There was no significant difference in maze performance between sexes of mice, thus experiments were performed using age-matched subjects only. All procedures were submitted to and approved by IACUC and were conducted following NIH guidelines. Mice used in each behavioral testing paradigm were naive and not used for other tests.
2.2 Neuronal Cell Culture
Hippocampal neurons and astrocytes were cultured as described previously [71]. Briefly, the hippocampus was dissected from day 19 embryonic mice. Hippocampi were combined in NB Media (Life Technologies, Carlsbad, CA) supplemented with 10% FBS and neuronal cells triturated. A cell count was performed and cells were plated to poly-lysine:collagen coated plates and grown for 7 days in culture at 37°C and 5% CO2. On day 7, cells were stained with 50nM MitoTracker Red (Life Technologies) for 30 minutes followed by fixation in 4% paraformaldehyde/PBS. Images were generated using a Nikon A1/T1 confocal microscope and Nikon Elements software.
2.3 Western blot and analysis
Cell lysates or isolated mitochondria were subjected to SDS-PAGE in polyacrylamide gels. Samples were Western blotted with primary antibody (phospho-AMPK/AMPK – Cell Signaling Technology, Danvers, MA; TFAM, p62 – Abcam, Cambridge, MA; p62Hum – BD Biosciences, San Jose, CA; β-actin – Sigma-Aldrich, St. Louis, MO) and HRP-tagged secondary antibody from GE Healthcare Life Sciences (Pittsburgh, PA) and processed with ECL detection reagent (GE Healthcare). Following exposure of the labeled membrane to Hyperfilm-ECL detection film, the Un-Scan-it Gel and Graph Digitizing software (Silk Scientific, Orem, UT) was used to scan and quantify the signal from the Western blot, and data were analyzed statistically (Win-SAS, Microsoft, Seattle, WA).
2.4 Mitochondria isolation
Following trypsinization, MEF cells were collected by centrifugation and mitochondria isolated essentially as described by Wieckowski, et al. 2009 [78]. Briefly, washed cells were homogenized on ice with a Teflon pestle followed by centrifugation twice at 600×g for 5 min. The post nuclear pellet was collected from the initial spin and further centrifuged at 7000×g for 10 min. The cytosolic fraction was obtained from the supernatant while the crude mitochondria pellet (C. Mito) was collected in the pellet. The pellet was washed with MRB Buffer (250mM Mannitol, 5mM HEPES, pH 7.4 and 0.5mM EDTA) before being layered over a Percol gradient. The gradient was centrifuged at 95,000xg for 30 min with a dense band containing purified mitochondria localized at the bottom of the tube. This band was collected and washed with MRB buffer before being suspended in a small volume of MRB Buffer containing protease inhibitors. Protein concentration was determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA) and subjected to SDS-PAGE and Western blot or used in further experiments as mitochondrial lysates.
2.5 ATP Assay
ATP production was measured using the ATP Determination Kit (Life Technologies, Carlsbad, CA). Briefly, MEF cells were detached from tissue culture plates by trypsin treatment and collected by brief centrifugation followed by PBS wash. Cell pellets were resuspended in isolation buffer (5mM HEPES, pH 7.2; 225mM Mannitol, 75mM Sucrose, 1mM EGTA, and protease inhibitors) followed by 5 times syringe using a 23 gauge needle. Samples were centrifuged at 1,500×g for 5 min prior to supernatants being used for ATP assay following the manufacturer’s directions and measured with a luminometer. Each sample was measured in triplicate. Results are representative of three separate experiments.
2.6 mtDNA copy number determination
Genomic DNA was isolated from brain tissue using the DNeasy Tissue Kit (Qiagen, Valencia, CA). Following elution with ddH2O, purified DNA was stored at −80°C. Relative Quantitative real time PCR was employed to determine mtDNA copy number. mtDNA was amplified with mtDNA primer: MDF: 5′-CCTATCACCCTTGCCATCAT-3′and MDR: 5′-GAGGCTGTTGCTTGTGTGAC-3′. Nuclear DNA was amplified by nuclear DNA primer: NDF: 5′-ACATCTGTTGCTCCGGCTCTCATT-3′ and NDR: 5′-GCAAGCTCAAAGGGCAAGGCTAAA-3′. RT-QPCR was conducted using an ABI 7500 Real Time PCR system (Applied Biosystems, Carlsbad, CA) using Power SYBR Green PCR Master Mix. Relative mtDNA levels were calculated by the ΔΔCt method as described [1].
2.7 Behavioral Equipment and Procedures
2.7.1 Open Field Maze
Measurement of locomotor activity and anxiety-related behavior was performed simultaneously using a multiple unit Open Field Maze consisting of four activity chambers (San Diego Instruments, CA). Each chamber measured 50cm × 50cm and a single subject animal was placed in the center of each at the beginning of a 10 minute test period. Testing was done under low lighting conditions (~30 lux) from incandescent lamps in set place in the testing room. Mice were recorded on videotape and analysis was performed using the SMART Video Tracking software (PanLab, Harvard Apparatus). The field for each maze was divided into sixteen 12.5cm × 12.5cm squares for analysis. The outer 12 squares were considered the Outer Zone while the inner 4 squares (25×25 cm) constituted the Inner Zone for analysis. Time spent in the outer area (closest to the walls) was measured as anxiety-related behavior and compared to time spent in the central area of the chamber. Total ambulatory distance was also recorded for each mouse to ensure there were no motor defects between genotypes.
2.7.2 Elevated Plus Maze
An Elevated Plus Maze (Colbourne Instruments, Allentown, PA) was used to measure anxiety-like behavior. Briefly, the maze consisted of 2 arms (50 × 10cm) open to the environment and 2 arms enclosed by darkened plastic walls (50cm × 10cm × 30cm). The arms are connected by a 10cm × 10cm central platform and elevated 50cm off the floor. Naive mice were tested in low light conditions (~30 lux) from incandescent lamps set in place in the testing room by placing the mouse into the center of the maze facing an open arm and recording explorative behavior for a period of 5 minutes using the SMART software. The number of open and closed arm entries, time spent in both types of arms, total distance traveled in each arm as well as total distance overall were analyzed.
2.7.3 Forced Swim Test
Depression-like behavior was analyzed using methods similar to the Porsalt Forced Swim Test [58]. Mice were placed in the middle of a 1 liter Pyrex beaker filled with 12cm of water and activity was videotaped for 6 minutes. Water was warmed to approximately 28°C and water depth prevented the subject from touching the bottom of the beaker. Water was completely changed between individual subjects. Activities were defined as either 1) immobility – floating either without movement or only small movements to stay above water; 2) swimming – active movement traveling within quadrants of the beaker; and finally 3) climbing – actively trying to escape, usually by attempting to climb the walls of the beaker. Behaviors were counted every 5 seconds during the test and totals were averaged within each genotype.
2.7.4 Barnes Maze
Spatial learning and memory was tested using a Barnes Maze [4, 27]. Testing occurred in a darkened room with a single bright incandescent light (~2000 lux) shining down on the surface of the maze platform. A metronome producing 85 decibels was used to further stimulate the escape response. A video tracking camera was suspended above the maze while extra-maze cues were positioned at specific places around the maze and maintained throughout the testing process. An escape hole was designated with an escape box installed beneath the surface of the maze allowing the subject to escape exposure to the adverse stimuli with a different escape hole designated for each mouse. Mice were adapted to the maze during 4 separate 3 minute trials on the first day of testing with a 15 minute intertrial interval. The mice were placed in a hinged box, released at the center of the maze and allowed to explore until the escape hole was located and the mouse entered the escape box. Upon entering the box, the metronome was turned off and the box was closed allowing the subject to remain hidden for 2 minutes prior to return to the home cage. Prior to the next subject, the maze was cleaned with a 2% ethanol solution to remove any remaining olfactory clues. The spatial acquisition phase was conducted over the next 5 days as described for day 1 and latency time was measured until the subject located and entered the escape box to determine how fast the mouse learned the location of the escape hole. Four trials per day were performed for each mouse with a 15 minute intertrial interval.
Short term memory was accessed with a probe trial on day 5, approximately 2 hours after completion of the acquisition phase. The mouse was placed in the maze as described above with light and metronome as adverse stimuli, however, the escape box was removed from the maze. Discrete zones were designated for each hole in the maze using SMART Video Tracking software (PanLab, Harvard Apparatus) and distance traveled throughout the maze was tracked for a total of 90 seconds. Time spent in the target zone (location of the prior escape hole) was indicative of how well the mouse remembered the location of the escape hole over a relatively short time. This same protocol was repeated on Day 12 for analysis of the subject’s long term ability to remember the location of the escape hole.
2.8 Statistical Methods
The overall results for all data were expressed as the mean ± S.E.M. Statistical analyses (t-tests) were performed using Excel 2010 (Microsoft, Redmond WA) and SAS 9.2 (SAS Institute, Cary NC). Corrections for confounded multiple t-tests were made using the step-up Bonferroni adjustment (Hoch option; SAS procedure MULTTEST). The step-up modification to the Bonferroni has been shown to provide additional power for controlling the post hoc family-wise error rate [32]. Individual t-tests were used throughout because the study was designed to evaluate specific comparisons between control (WT) and individual treatment (OEp62 or p62:P392L). Global comparisons among all three genotypes were not of general interest. Hard boundaries for statistical significance (i.e. p<0.05) were not used [33, 44]. t-values (degrees of freedom), raw p-values and adjusted p-values are reported for each comparison along with our interpretation for the reader’s consideration. The number of subjects (N) used in behavior testing varied between a minimum of 5 to a maximum of 28. Specific numbers of replicates are described below in the Results for each experiment and in Table 1.
Table 1.
Behavior Test Statistics
| Test | I.D. | Mean +/− S.E. | N | Comparison | df | t | p | Adjusted p |
|---|---|---|---|---|---|---|---|---|
| Open Field Maze | ||||||||
| Locomotion | WT | 615.16 +/− 34.88 | 24 | WT v. OEp62 | 34 | 0.202 | 0.421 | 0.421 |
| OEp62 | 600.45 +/− 76.59 | 12 | WT v. p62:P392L | 50 | 1.462 | 0.075 | 0.225 | |
| p62:P392L | 555.79 +/− 22.83 | 28 | OEp62 v. p62:P392L | 38 | 0.738 | 0.232 | 0.421 | |
| Thigmotaxis | ||||||||
| Outer Zone (%) | WT | 82.78 +/− 1.27 | 24 | WT v. OEp62 | 34 | 10.005 | 5.75E-12 | <0.0001 |
| OEp62 | 41.78 +/− 5.33 | 12 | WT v. p62:P392L | 50 | 6.673 | 9.76E-09 | <0.0001 | |
| p62:P392L | 57.46 +/− 3.34 | 28 | OEp62 v. p62:P392L | 38 | −2.59 | 0.007 | 0.007 | |
| Inner Zone (%) | WT | 17.22 +/− 1.27 | 24 | WT v. OEp62 | 34 | −9.933 | 6.93E-12 | <0.0001 |
| OEp62 | 58.22 +/− 5.35 | 12 | WT v. p62:P392L | 50 | −6.673 | 9.76E-09 | <0.0001 | |
| p62:P392L | 42.54 +/− 3.34 | 28 | OEp62 v. p62:P392L | 38 | 2.562 | 0.007 | 0.007 | |
|
| ||||||||
| Elevated Plus Maze | ||||||||
| Anxiety | WT | 0.52 +/− 0.046 | 20 | WT v. OEp62 | 32 | 2.079 | 0.023 | 0.058 |
| OEp62 | 0.34 +/− 0.078 | 12 | WT v. p62:P392L | 26 | 0.87 | 0.196 | 0.196 | |
| p62:P392L | 0.61 +/− 0.111 | 6 | OEp62 v. p62:P392L | 20 | −1.997 | 0.029 | 0.059 | |
|
| ||||||||
| Forced Swim Test | ||||||||
| Immobility Time (%) | WT | 26.79 +/− 3.72 | 17 | WT v. OEp62 | 24 | 0.543 | 0.296 | 0.386 |
| OEp62 | 29.92 +/− 4.45 | 13 | WT v. p62:P392L | 19 | 0.293 | 0.386 | 0.386 | |
| p62:P392L | 25 +/− 2.03 | 8 | OEp62 v. p62:P392L | 15 | 0.782 | 0.223 | 0.386 | |
| Swimming | WT | 17.11 +/− 2.73 | 8 | WT v. OEp62 | 18 | −3.101 | 0.003 | 0.007 |
| OEp62 | 35.09 +/− 2.25 | 12 | WT v. p62:P392L | 12 | −3.529 | 0.002 | 0.006 | |
| p62:P392L | 31.17 +/− 1.51 | 6 | OEp62 v. p62:P392L | 16 | 0.345 | 0.367 | 0.367 | |
| Climbing | WT | 36.11 +/− 5.96 | 8 | WT v. OEp62 | 18 | 4.17 | 0.0003 | 0.0009 |
| OEp62 | 15.27 +/− 3.14 | 12 | WT v. p62:P392L | 12 | 2.464 | 0.015 | 0.029 | |
| p62:P392L | 22.83 +/− 1.42 | 6 | OEp62 v. p62:P392L | 16 | −1.769 | 0.048 | 0.048 | |
|
| ||||||||
| Barnes Maze | ||||||||
| Acquisition Day 5 | WT | 31.51 +/− 9.15 | 8 | WT v. OEp62 | 11 | 1.458 | 0.086 | 0.173 |
| OEp62 | 13.71 +/− 4.18 | 5 | WT v. p62:P392L | 15 | 1.746 | 0.051 | 0.152 | |
| p62:P392L | 14.96 +/− 3.75 | 9 | OEp62 v. p62:P392L | 12 | −0.209 | 0.419 | 0.419 | |
| Probe Trial 1 (Short Term) | WT | 36.6 +/− 4.85 | 8 | WT v. OEp62 | 11 | 0.247 | 0.405 | 0.405 |
| OEp62 | 34.98 +/− 2.64 | 5 | WT v. p62:P392L | 15 | −0.926 | 0.185 | 0.369 | |
| p62:P392L | 42.64 +/− 4.37 | 9 | OEp62 v. p62:P392L | 12 | −1.219 | 0.123 | 0.369 | |
| Probe Trial 2 (Long Term) | WT | 22.86 +/− 1.98 | 7 | WT v. OEp62 | 10 | −3.676 | 0.002 | 0.006 |
| OEp62 | 41.46 +/− 5.54 | 5 | WT v. p62:P392L | 14 | 4.78 | 0.32 | 0.32 | |
| p62:P392L | 25.34 +/− 4.28 | 9 | OEp62 v. p62:P392L | 12 | 2.333 | 0.019 | 0.039 | |
Adjusted p = Step-up Bonferroni Adjustment for Multiple Comparisons
3. Results
3.1 Generation of OEp62 and p62:P392L overexpression mice
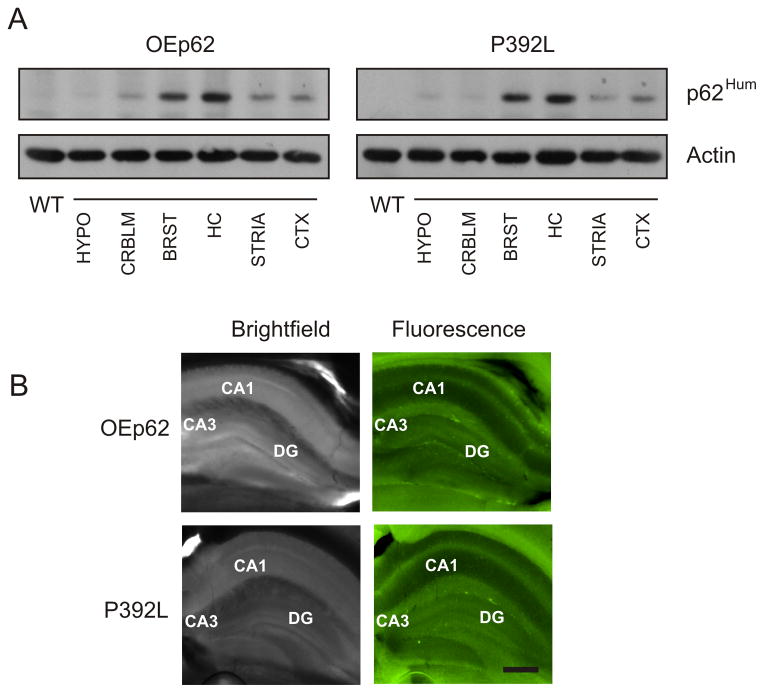
Mice overexpressing p62 specifically in neural tissue were generated as described in Materials and Methods. Human EGFP-p62 cDNA, either native or containing the P392L substitution, was inserted in the Thy 1.2 expression vector and founder mice generated. These founders were backcrossed into the C57BL/6 background and colonies of each genotype established. The Thy 1.2 expression vector directed p62 overexpression specifically to neural tissue (Fig. 1A). Human specific antibody recognized only the EGFP-tagged p62 expressed as a 97Kd fusion protein in various sub-regions of the brain. p62 expression was increased mainly in the hippocampal region of the mouse brain, however, expression was also modest in the brain stem region as well as seen to a lesser extent in the striatum and cortex. Because expression was highest in the hippocampal region, slices of 6 month adult brains were analyzed under fluorescent microscopy to verify EGFP-62 expression in this region of the brain (Fig. 1B). The expressed fusion protein was identifiable in the hippocampus of slice cultures when excited at 488nm.
Fig. 1.
Demonstration that EFGP tagged proteins were effectively expressed in mouse brain. A) Overexpressed EGFP-p62 protein was mainly localized to the hippocampus in transgenic mice. Mouse brain from either OEp62 or p62:P392L expressing mice were dissected into regions as indicated (HYPO=hypothalamus; CRBLM=cerebellum; BRST=brain stem; HC=hippocampus; STRIA=striatum; CTX=cortex) and Western blotted with p62Hum antibody to detect human specific overexpressing p62 protein. β-actin levels were used as control to indicate loading. B) Sagittal plane slices (200μm) were made through the hippocampal region of mouse brains from OEp62 and p62:P392L mice and examined under a fluorescent microscope at 488nm to show localization of EGFP tagged proteins in the hippocampal region. Bright field images are provided for structural reference. CA1, CA3 and DG regions in the hippocampus are labeled for orientation. Scale bar = 300μm.
3.2 p62 overexpression increases the functionality of mitochondria in the hippocampus
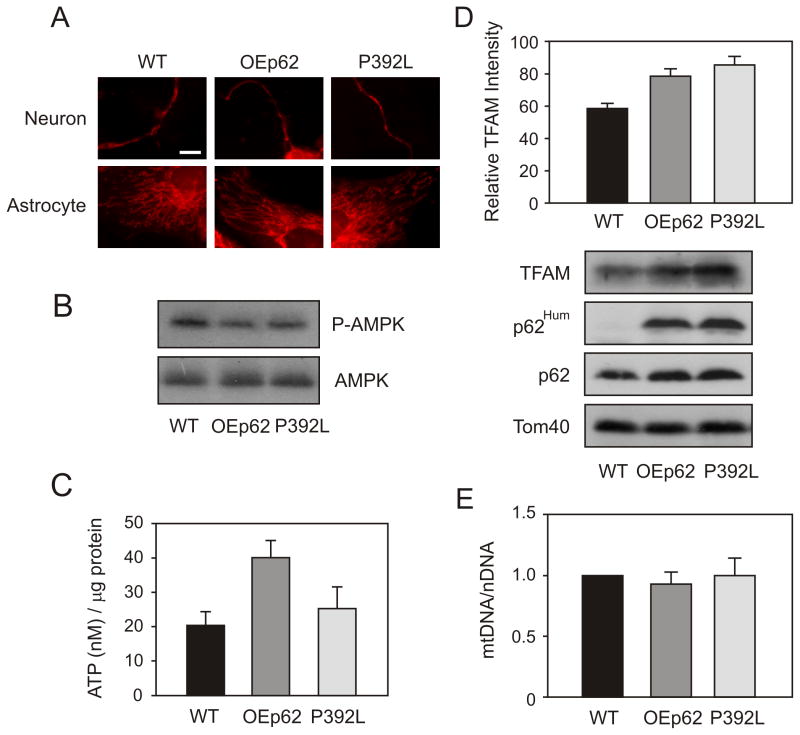
To examine the effects of elevated p62 levels on mitochondrial functionality, we first looked at mitochondrial morphology in hippocampal neurons. Neurons were isolated from day 19 embryonic pups and grown for 7 days in culture. Mitochondria were stained with MitoTracker Red (Life Technologies, Carlsbad, CA) and visualized using confocal microscopy (Fig. 2A). Overexpression of p62 showed an increase in “tubulo-reticular” structure in OEp62 mice when compared to WT however, mitochondria from neurons expressing the P392L mutant appeared to be very similar to WT morphology.
Fig. 2.
p62 overexpression increases the functionality of mitochondria in the hippocampus. A) Mitochondrial morphology was altered in OEp62 mice compared to WT and p62:P392L. Primary neuronal cells were cultured from dissected embryonic Day 19 hippocampus. At culture day 7, mitochondria were visualized by staining with MitoTracker Red and examined using a confocal microscope. B) Activated AMPK levels were decreased in p62 overexpressing tissue. Hippocampal lysates from WT, OEp62 and p62:P392L mice were separated on SDS-PAGE and phospho-AMPK levels compared to total AMPK examined by Western blot. C) Overexpression of p62 results in increased ATP production. Total ATP levels in hippocampal lysates were measured by luciferase assay and compared to WT. D) Mitochondrial import was increased with p62 overexpression. Mitochondria were isolated from hippocampal lysates and import of TFAM examined by SDS-PAGE and Western blot. Presence of overexpressed protein was confirmed with p62Hum antibody and compared to native protein. E) p62 overexpression did not show increased mtDNA levels. Total mitochondrial DNA copy number was quantitated by RT-PCR.
As mitochondrial morphology drives mitochondrial function, we next examined aspects of ATP production in hippocampal tissue from overexpressing mice. AMPK acts as a metabolic switch for ATP production in the mitochondria [60] with increases in the ratio of AMP:ATP affecting the activation state of AMPK [74]. As a cellular energy response element, low levels of ATP positively activate AMPK which then results in downstream regulation of signaling pathways and cyclic replenishing of cellular ATP levels. Over expression of p62 caused a decrease in phosphorylated AMPK compared to WT (Fig. 2B). These results suggest that cellular ATP levels in hippocampal tissue could be higher due to increased p62 levels enhancing mitochondrial homeostasis. We therefore examined ATP levels in hippocampal tissues of overexpressing mice and compared them to WT (Fig. 2C). OEp62 mice showed increased levels of ATP correlating with the decreased expression of activated AMPK. Interestingly, mutation of P392L in the ubiquitin binding domain (p62:P392L), yielded ATP levels more consistent with the lower levels seen in WT suggesting a pathway by which p62 could regulate mitochondrial ATP production potentially based on its ability to bind to ubiquitin or poly-ubiquitinated substrates.
The mitochondrial transcription factor A (TFAM) has been reported to protect the mitochondrial genome once imported into mitochondria [30]. TFAM promotes mtDNA replication/transcription [24] and aids in the repair of oxidatively damaged DNA [39, 81]. As we observed an increase in mitochondrial functionality as measured by ATP production, we next examined import of this important transcription factor. Mitochondria were isolated from the hippocampal region of the mouse brain and levels of TFAM associated specifically with the mitochondria measured by Western blot (Fig. 2D). TFAM expression localized to the mitochondria was increased in both OEp62 and p62:P392L mice hippocampi when compared to WT. As post translational modification (maturing) of TFAM occurs following import into mitochondria [47], increases in mature TFAM protein indicate an increase in mitochondrial import capability specific to p62 expression levels. mtDNA copy number is often used as a direct correlation of mitochondrial TFAM levels [24, 38, 39] and as a measure of functional mitochondrial energetics [66]. Thus, we measured total mitochondrial DNA present in the hippocampus of our overexpressing mice. Levels of mtDNA were essentially consistent with WT (Fig. 2E).
3.3 p62 overexpression changes behavior patterns related to affective spectrum disorders
Recent studies have suggested that Bcl-2 protein imbedded in the inner mitochondrial membrane is a major modulator of mitochondrial function [54] and that overexpression of the Bcl-2 gene reduces anxiety-like behaviors in a mouse model [67]. Mitochondrial function has been related to anxiety disorders caused by the deletion of Bcl-2 [18]. Genetic inactivation of p62 in mice leads to increases in anxiogenic behavior [64] accompanied by mitochondrial dysfunction [70]. Because we demonstrate here that increased expression of p62 results in increased mitochondrial functionality, we sought to examine behaviors in p62 overexpressing mice related to affective spectrum disorders. We hypothesized that increased mitochondrial function could in fact provide relief in anxiety related behaviors. Results and statistical analysis from behavior testing are summarized in Table 1.
3.3.1 Mice overexpressing p62 show no change in locomotor activity in the Open Field Maze
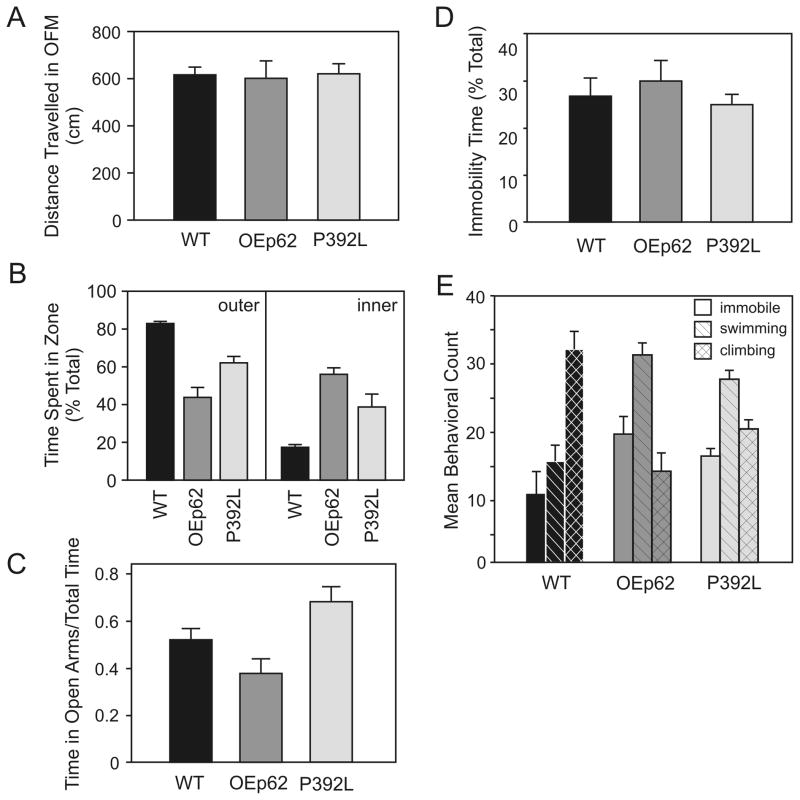
OEp62 (N=12) and p62:P392L (N=28) mice were observed in an Open Field Maze for any difference gene expression levels would have on general locomotor activity (Fig. 3A). Distances traveled in the maze during 10 minute trials were essentially the same for overexpressing mice when compared to WT (N=24). However, p62 overexpressing mice spent significantly more time exploring the center of the open field (Fig. 3B), a measure reflecting decreased thigmotaxis and anxiety related behavior [73].
Fig. 3.
p62 overexpression influences affective disorder behavior patterns. A) Behavior in the Open Field Maze: there was no significant difference in distance traveled in the maze between genotypes. B) Open field behavior analyzed by zone: p62 overexpression resulted in increased maze exploration toward to the center and away from the walls by genotypes. C) Anxiety measure in the Elevated Plus Maze: overexpression of p62 substantially reduced the time spent in open arms of the maze, however, expression of P392L protein increased time spent on open arms. D) Depression measure in the Forced Swim Test: no difference in behavior was indicated when using immobility time as a measure. E) Mean behavior exhibited during the Forced Swim Test: overexpression of p62 changed the overall behavior patterns resulting in increased swimming activity and decrease climbing compared to WT. Refer to Table 1 for analysis of statistics.
3.3.2 Mice overexpressing p62 have variable differences in measured anxiety in the Elevated Plus Maze
An Elevated Plus Maze was used to measure anxiogenic behavior in overexpressing mice. OEp62 mice (N=12) showed a decrease in anxiety-related behavior by spending more time on the open arms of the Elevated Plus Maze than comparable WT mice (N=20). Conversely, p62:P392L mice (N=6) showed a significant increase in anxiogenic behavior (Fig. 3C) raising the possibility of ubiquitin binding by p62 playing a role in both mitochondria functionality (Fig. 2) and behavioral pattern modification.
3.3.3 Mice overexpressing p62 show different behavior patterns in the Forced Swim Test
A modified Forced Swim Test was used to access depression in overexpressing mice compared to WT [11]. Immobility time of OEp62 (N=11) and p62:P392L (N=6) mice did not measurably differ from that of WT (N=15) (Fig. 3D). However, other behavioral traits did show variation during the testing period. Specifically, there were significant differences in swimming and climbing behaviors between WT and overexpressing mice (Fig. 3E). WT mice exhibited climbing behavior greater that what was seen in the overexpressing genotypes while conversely, overexpressing mice spent much more time in swimming behavior when compared to WT.
3.4 p62 overexpression effects on spatial learning and memory tasks in mice
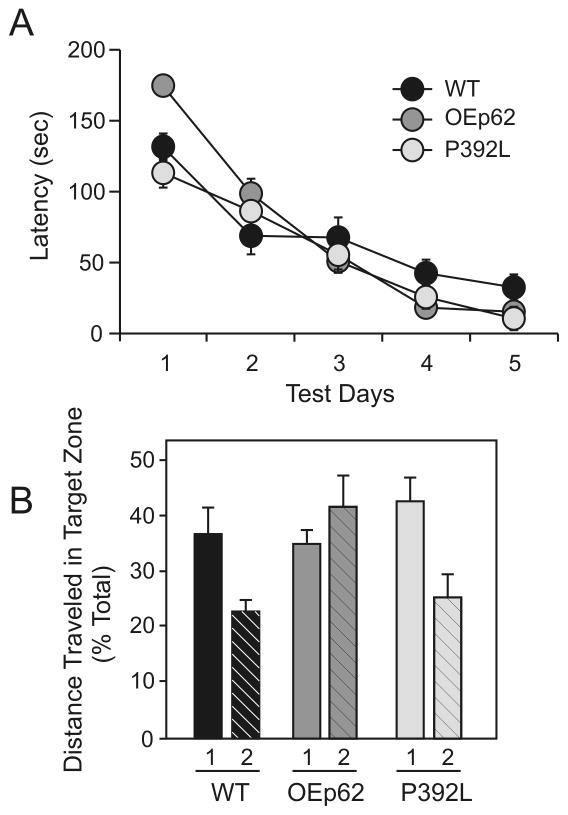
Spatial learning in p62 overexpressing mice was measured using a Barnes Maze. While all three genotypes exhibited equal search times for the hidden escape box in Day 3 of the acquisition, both OEp62 and p62:P392L mice continued to show slight improvement in latency to escape compared to WT during Days 4 and 5 of the acquisition phase. At the end of the 5 day acquisition phase for the maze, both genotypes of p62 overexpressing mice (OEp62, N=5; p62:P392L, N=9) performed better in latency measurements than did WT mice (N=8; Fig. 4A).
Fig. 4.
p62 overexpression affects spatial learning and memory tasks. A) Latency times to complete the Barnes Maze: overexpression of p62 improved latency times compared to WT on last day of Acquisition period. B) Probe Trials in the Barnes Maze (1=Probe Trial 2 hours post Day 5 acquisition; 2=Probe Trial 6 days post Day 5 acquisition): no significant differences in short term memory measure during Probe Trial 1 were observed between genotypes, however overexpression of p62 improved long term memory in Probe Trial 2 compared to WT and p62:P392L. Refer to Table 1 for analysis of statistics.
To measure effects of p62 overexpression on memory, both short and long term probe trials were undertaken using the Barnes Maze after the acquisition phase. Short term memory was assessed two hours after the final acquisition measurements were taken and showed no significant differences in short term memory between genotypes (Fig. 4B). Long term memory measurements were undertaken six days after the last acquisition measure. At this time, OEp62 mice showed significantly greater ability to remember escape box locations compared to both WT and p62:P392L (Fig. 4B).
4. Discussion
Mitochondrial dysfunction has recently been associated with the pathophysiology of affective spectrum and anxiety disorders [20]. Patients exhibiting major depression display significantly decreased mitochondrial function [21] while treatment with anxiolytic as well as anti-depressant drugs has been shown to improve mitochondrial respiration thus increasing anti-oxidant activity [75]. Mitochondrial dysfunction can be caused by numerous deficiencies related to the electron transport chain and thus to energy production. We have previously shown that shutdown of the SQSTM1/p62 gene can result in decreases in mitochondrial energy production [70], as well as increased oxidative damage to mitochondrial DNA [15, 16]. Other key modulators of mitochondrial function include regulation of intracellular Ca+2 levels by Bcl-2 [59] and abrogation of electron transfer along the respiration chain by coenzyme Q10 (CoQ10) deficiency [51]. Overexpression of either of these proteins results in improved mitochondrial function [59, 68], an observation we have also seen with p62 overexpression in mouse embryonic fibroblasts [70].
Neurodegeneration refers to progressive death and loss of neurons in the brain. The most common form of cell death in neurodegeneration is through apoptotic pathways related to mitochondria [20]. Specifically, increases in reactive oxygen species (ROS), changes in mitochondrial fusion and fission rates, Ca+2 homeostasis, and mitochondrial membrane permeability all lead to mitochondrial disease and dysfunction [70]. In this study, by increasing the levels of p62 expressed in brain tissue, we have shown improvement in mitochondrial functionality. Morphologically, hippocampal mitochondria showed increased fusion resulting in increases in ATP production and mitochondrial import of the major mitochondrial transcription factor A (TFAM). TFAM is known to have a role in replication of mitochondrial DNA, and also in its maintenance [40]. Furthermore, overexpression of TFAM results in decreased ROS production as well as increased mitochondrial ATP production and function [29]. With increased TFAM presence in hippocampal mitochondria of OEp62 mice, an increase in mtDNA could also be expected. However, p62 also exhibits a scaffolding function for the delivery of mitochondria in the cell for disposal by mitophagy [23, 31]. Increased p62 levels would not only increase the amount of mtDNA present, but would also improve mitophagy thus playing a two stage role in regulating mtDNA levels. Increased TFAM presence in mitochondria also ameliorates age dependent impairment of cognitive functions in the brain [29]. It should also be noted that while increases in p62 resulted in increased mitochondrial functionality, the role of ubiquitin binding also appears to play a major role in ATP production. Given p62’s transmembrane presence in the mitochondria [50], it is possible that the C-terminal UBA domain of p62 is required for electron transport chain functionality on the inner mitochondrial membrane. Regardless, one can surmise that increases in mitochondrial functionality in the mouse brain appear to ameliorate neurodegeneration resulting in an associated change in learning, memory and long term potentiation.
In the present study, we observe an overall improvement in the affective spectrum disorder behavior patterns in mice exhibiting increased mitochondria functionality. This study also suggests a role for functional mitochondria in the learning and long term memory patterns of these mice. p62 overexpressing mice displayed no gross physiological changes when compared to age matched WT nor was any inhibition of locomotor ability observed between genotypes. Thus, measurements related to distance and latency in the maze could be used to compare behaviors of the mice. Two separate methods for anxiety measurement were used in this study. Movement of mice in the open field away from the walls is an indication of increased exploratory behavior and a decrease in thigmotaxis in mice [73]. The Elevated Plus Maze is the standard measure of anxiety-like behavior in rodents [8, 46]. The anxiety measures used here were of the exploratory nature and related to a mouse’s desire to examine new environments. A reduction in anxiety-like behavior was observed in the overexpressing mice when compared to WT. Interestingly, mice lacking ubiquitin binding ability of p62 displayed a decrease in anxiogenic behavior when tested on the Elevated Plus Maze. Decreased anxiety has been previously seen in mice overexpressing the ubiquitin ligase KF-1 resulting in ubiquitination and degradation of target proteins in dopaminergic neurons [28]. Conversely, increased anxiety-like behavior has been recorded where decreases in ubiquitin ligase activity of Parkin [37] and RNF41 [43] have been observed. p62 has a scaffolding function of binding to K63-ubiquitinated proteins and targeting those proteins to the proteasome for degradation [69]. The inability of p62 to associate with K63-tagged cargoes would result in an increase in aggregated proteins in the cell. How this lack scaffolding function affects anxiety-like behaviors requires further study but should not be overlooked.
A relationship between depression and mitochondrial dysfunction has been examined in a number of studies. Mutations in mitochondrial proteins have been found in the pre-frontal and frontal cortices of post-mortem samples obtained from patients diagnosed with major depression [41] while levels of electron transport chain activity were found to be decreased below control levels in a major depressive disorder study [3]. While p62 overexpressing mice showed no difference from WT in immobility time in the Forced Swim Test, which measures despair/depression, when specific behaviors were distinguished in the test, a notable difference was seen between overexpressing mice and their WT counterparts. WT mice exhibited specific climbing or thrashing behavior characterized by upward directed movements with the front paws on the side of the swim chamber. Studies have revealed that catecholaminergic agents decrease immobility in the swim chamber with a corresponding increase in climbing behavior [52]. Conversely, p62 overexpressing mice exhibited significantly increased swimming behavior over controls, a behavior associated with increases in 5-HT-related compounds [11]. Several 5-HT receptors have been suggested to play a role in mediating antidepressant behavior [53]. Furthermore, antidepressant treatment not only mediates the effects of 5-HT-related compounds on behavior [11, 12], but also affects electron transport and ATPase activity in the mitochondria [13].
Serotonergic pathways are known to play a major role in despair and depression. Multiple 5-HT receptors have been implicated in mechanisms underlying learning and memory [79]. However, the role of 5-HT receptors in learning and memory is complex. Some studies have reported improvement in cognition and memory formation [56] while others have reported limited involvement of chronic 5-HT depletion in spatial learning and memory [63]. We found in this study, that p62 overexpression improved spatial learning in the Barnes Maze while also increased the ability of the mice to retain long term memory. Activation of 5-HT1A by its agonist 8-OH-DPAT has been shown to significantly enhance learning acquisition and memory formation as well [26]. Cognitive impairments are coincident with loss of long term potentiation in p62−/− mice [64]. Furthermore, we have previously demonstrated that p62 is an AMPA receptor interacting protein and this interaction, along with aPKC, results in delivery of the GluR1 subunit to neuronal surfaces in the hippocampus mediating synaptic plasticity and long term potentiation [36]. Our results are further suggestive of p62 involvement in the mediation of 5-HT cognitive functions in the hippocampus. This new evidence for a role for p62 in abrogating behavioral responses associated with depression-related behaviors may provide a new target for development of antidepressant drugs or drugs effective in other 5-HT mediated disorders.
In conclusion, our study demonstrated that SQSTM1/p62 plays a role in mitochondrial functionality and provides a link to affective spectrum disorders such as anxiety and depression, as well as affecting cognitive learning and memory. It is possible that changes in behavior patterns can be related to p62 function outside of the mitochondria as it is ubiquitously expressed and plays a myriad of different roles in the cell [6, 22, 80]. However, p62 clearly plays a crucial role in maintaining mitochondrial homeostasis [70] and as we show here, overexpression improves functionality of the mitochondria. Given the increasing scientific support for a relationship between mitochondria and affective disorders [21, 55, 57], p62 would seem to be a novel target for potential drug discovery to treat anxiety and affective disorders.
Highlights.
p62 overexpression increases the functionality of mitochondria in the hippocampus.
p62 overexpression changes behavior patterns related to affective spectrum disorders.
p62 overexpression effects on spatial learning and memory tasks in mice.
Acknowledgments
This work was supported by NIH-2RO1NS033661 (MCW). We thank Dr. Mike Irwin and Dr. Carl Pinkert at the Auburn University Transgenic Facility for their assistance in generation of the overexpression mice. We also thank Dr. Jorge Moscat and Dr. Maria-Theresa Diaz-Meco for their fruitful discussions and continued support.
Footnotes
The authors have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Lamar Seibenhener, Email: seibemi@auburn.edu.
Ting Zhao, Email: tzhao5@emory.edu.
Yifeng Du, Email: duyifen@auburn.edu.
Luis Calderilla-Barbosa, Email: lzc0012@auburn.edu.
Jin Yan, Email: jzy0002@auburn.edu.
Jianxiong Jiang, Email: jjiang3@emory.edu.
Marie W. Wooten, Email: wootemw@auburn.edu.
References
- 1.Acevedo-Torres K, Berríos L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, Torres-Ramos CA, Ayala-Torres S. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair (Amst) 2009;8:126–136. doi: 10.1016/j.dnarep.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn HJ, Hernandez CM, Levenson JM, Lubin FD, Liou HC, Sweatt JD. c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn Mem. 2008;15:539–549. doi: 10.1101/lm.866408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- 4.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 5.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 6.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Chamoux E, Couture J, Bisson M, Morissette J, Brown JP, Roux S. The p62 P392L mutation linked to Paget’s disease induces activation of human osteoclasts. Mol Endocrinol. 2009;23:1668–1680. doi: 10.1210/me.2009-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciani B, Layfield R, Cavey JR, Sheppard PW, Searle MS. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget’s disease of bone. J Biol Chem. 2003;278:37409–37412. doi: 10.1074/jbc.M307416200. [DOI] [PubMed] [Google Scholar]
- 11.Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- 12.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 13.Curti C, Mingatto FE, Polizello AC, Galastri LO, Uyemura SA, Santos AC. Fluoxetine interacts with the lipid bilayer of the inner membrane in isolated rat brain mitochondria, inhibiting electron transport and F1F0-ATPase activity. Mol Cell Biochem. 1999;199:103–109. doi: 10.1023/a:1006912010550. [DOI] [PubMed] [Google Scholar]
- 14.Denis-Donini S, Dellarole A, Crociara P, Francese MT, Bortolotto V, Quadrato G, Canonico PL, Orsetti M, Ghi P, Memo M, Bonini SA, Ferrari-Toninelli G, Grilli M. Impaired adult neurogenesis associated with short-term memory defects in NF-kappaB p50-deficient mice. J Neurosci. 2008;28:3911–3919. doi: 10.1523/JNEUROSCI.0148-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y, Wooten MC, Gearing M, Wooten MW. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free Radic Biol Med. 2009;46:492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y, Wooten MC, Wooten MW. Oxidative damage to the promoter region of SQSTM1/p62 is common to neurodegenerative disease. Neurobiol Dis. 2009;35:302–310. doi: 10.1016/j.nbd.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res. 2005;165:172–180. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Filiou MD, Zhang Y, Teplytska L, Reckow S, Gormanns P, Maccarrone G, Frank E, Kessler MS, Hambsch B, Nussbaumer M, Bunck M, Ludwig T, Yassouridis A, Holsboer F, Landgraf R, Turck CW. Proteomics and metabolomics analysis of a trait anxiety mouse model reveals divergent mitochondrial pathways. Biol Psychiatry. 2011;70:1074–1082. doi: 10.1016/j.biopsych.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Gardner A, Johansson A, Wibom R, Nennesmo I, von Döbeln U, Hagenfeldt L, Hällström T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 22.Geetha T, Seibenhener ML, Chen L, Madura K, Wooten MW. p62 serves as a shuttling factor for TrkA interaction with the proteasome. Biochem Biophys Res Commun. 2008;374:33–37. doi: 10.1016/j.bbrc.2008.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 24.Gensler S, Weber K, Schmitt WE, Pérez-Martos A, Enriquez JA, Montoya J, Wiesner RJ. Mechanism of mammalian mitochondrial DNA replication: import of mitochondrial transcription factor A into isolated mitochondria stimulates 7S DNA synthesis. Nucleic Acids Res. 2001;29:3657–3663. doi: 10.1093/nar/29.17.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Götz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- 26.Haider S, Khaliq S, Tabassum S, Haleem DJ. Role of somatodendritic and postsynaptic 5-HT A receptors on learning and memory functions in rats. Neurochem Res. 2012;37:2161–2166. doi: 10.1007/s11064-012-0839-5. [DOI] [PubMed] [Google Scholar]
- 27.Harrison FE, Hosseini AH, MacDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto-Gotoh T, Iwabe N, Tsujimura A, Takao K, Miyakawa T. KF-1 Ubiquitin Ligase: An Anxiety Suppressor. Front Neurosci. 2009;3:15–24. doi: 10.3389/neuro.01.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, Kanki T, Kang D, Sunagawa K, Tsutsui H, Nakanishi H. Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci. 2008;28:8624–8634. doi: 10.1523/JNEUROSCI.1957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu T, Guan T, Gerace L. Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins. J Cell Biol. 1996;134:589–601. doi: 10.1083/jcb.134.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 33.Hoem JM. The reporting of statistical significance in scientific journals. Demographic Res. 2008;18:437–442. [Google Scholar]
- 34.Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, Parameshwaran K, Seibenhener ML, Kang MG, Suppiramaniam V, Huganir RL, Diaz-Meco MT, Wooten MW. AMPA receptor trafficking and synaptic plasticity require SQSTM1/p62. Hippocampus. 2009;19:392–406. doi: 10.1002/hipo.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Suppiramaniam V, Wooten MW. Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity. Neurosignals. 2006–2007;15:266–282. doi: 10.1159/000105517. [DOI] [PubMed] [Google Scholar]
- 37.Kägi G, Klein C, Wood NW, Schneider SA, Pramstaller PP, Tadic V, Quinn NP, van de Warrenburg BP, Bhatia KP. Nonmotor symptoms in Parkin gene-related parkinsonism. Mov Disord. 2010;25:1279–1284. doi: 10.1002/mds.22897. [DOI] [PubMed] [Google Scholar]
- 38.Kang D, Hamasaki N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: overview of its multiple roles. Ann N Y Acad Sci. 2005;1042:101–108. doi: 10.1196/annals.1338.010. [DOI] [PubMed] [Google Scholar]
- 39.Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Kanki T, Ohgaki K, Gaspari M, Gustafsson CM, Fukuoh A, Sasaki N, Hamasaki N, Kang D. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol. 2004;24:9823–9834. doi: 10.1128/MCB.24.22.9823-9834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karry R, Klein E, Ben Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol Psychiatry. 2004;55:676–684. doi: 10.1016/j.biopsych.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, Kato T. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry. 2006;11:577–593. doi: 10.1038/sj.mp.4001824. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Zhang S, Choi KH, Reister R, Do C, Baykiz AF, Gershenfeld HK. An E3 ubiquitin ligase, Really Interesting New Gene (RING) Finger 41, is a candidate gene for anxiety-like behavior and beta-carboline-induced seizures. Biol Psychiatry. 2009;65:425–431. doi: 10.1016/j.biopsych.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirk RE. Practical significance: A concept whose time has come. Ed and Psych Measurement. 1996;56:746–759. [Google Scholar]
- 45.Kuusisto E, Salminen A, Alafuzoff I. Early accumulation of p62 in neurofibrillary tangles in Alzheimer’s disease: possible role in tangle formation. Neuropathol Appl Neurobiol. 2002;28:228–237. doi: 10.1046/j.1365-2990.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- 46.Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J Neurosci Meth. 2008;171:48–52. doi: 10.1016/j.jneumeth.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Larsson NG, Garman JD, Oldfors A, Barsh GS, Clayton DA. A single mouse gene encodes the mitochondrial transcription factor A and a testis-specific nuclear HMG-box protein. Nature Genetics. 1996;13:296–302. doi: 10.1038/ng0796-296. [DOI] [PubMed] [Google Scholar]
- 48.Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70:1582–1588. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee M, Shin J. Triage of oxidation-prone proteins by Sqstm1/p62 within the mitochondria. Biochem Biophys Res Commun. 2011;413:122–127. doi: 10.1016/j.bbrc.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 51.Lenaz G, Fato R, Castelluccio C, Cavazzoni M, Estornell E, Huertas JF, Pallotti F, Parenti Castelli G, Rauchova H. An updating of the biochemical function of coenzyme Q in mitochondria. Mol Aspects Med. 1994;15:s29–s36. doi: 10.1016/0098-2997(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 52.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Lucki I, Singh A, Kreiss DS. Antidepressant-like behavioral effects of serotonin receptor agonists. Neurosci Biobehav Rev. 1994;18:85–95. doi: 10.1016/0149-7634(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 54.Manfredi G, Kwong JQ, Oca-Cossio JA, Woischnik M, Gajewski CD, Martushova K, D’Aurelio M, Friedlich AL, Moraes CT. BCL-2 improves oxidative phosphorylation and modulates adenine nucleotide translocation in mitochondria of cells harboring mutant mtDNA. J Biol Chem. 2003;278:5639–5645. doi: 10.1074/jbc.M203080200. [DOI] [PubMed] [Google Scholar]
- 55.Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 56.Manuel-Apolinar L, Meneses A. 8-OH-DPAT facilitated memory consolidation and increased hippocampal and cortical cAMP production. Behav Brain Res. 2004;148:179–184. doi: 10.1016/s0166-4328(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 57.Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, Catena Dell’Osso M. Mitochondrial alterations and neuropsychiatric disorders. Curr Med Chem. 2011;18:4715–4721. doi: 10.2174/092986711797379221. [DOI] [PubMed] [Google Scholar]
- 58.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci U S A. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004;63:275–278. doi: 10.1079/PNS2004339. [DOI] [PubMed] [Google Scholar]
- 61.Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, Kimura M, Sato S, Hattori N, Komatsu M, Tanaka K, Matsuda N. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells. 2010;15:887–900. doi: 10.1111/j.1365-2443.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park I, Chung J, Walsh CT, Yun Y, Strominger JL, Shin J. Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region. Proc Natl Acad Sci USA. 1995;92:12338–12342. doi: 10.1073/pnas.92.26.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piechal A, Blecharz-Klin K, Wyszogrodzka E, Kołomańska P, Rok-Bujko P, Krząścik P, Kostowski W, Widy-Tyszkiewicz E, Filip M, Stefański R. Neonatal serotonin (5-HT) depletion does not affect spatial learning and memory in rats. Pharmacol Rep. 2012;64:266–274. doi: 10.1016/s1734-1140(12)70764-8. [DOI] [PubMed] [Google Scholar]
- 64.Ramesh Babu J, Lamar Seibenhener M, Peng J, Strom AL, Kemppainen R, Cox N, Zhu H, Wooten MC, Diaz-Meco MT, Moscat J, Wooten MW. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem. 2008;106:107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 65.Rea SL, Walsh JP, Ward L, Magno AL, Ward BK, Shaw B, Layfield R, Kent GN, Xu J, Ratajczak T. Sequestosome 1 mutations in Paget’s disease of bone in Australia: prevalence, genotype/phenotype correlation, and a novel non-UBA domain mutation (P364S) associated with increased NF-kappaB signaling without loss of ubiquitin binding. J Bone Miner Res. 2009;24:1216–1223. doi: 10.1359/jbmr.090214. [DOI] [PubMed] [Google Scholar]
- 66.Rocher C, Taanman JW, Pierron D, Faustin B, Benard G, Rossignol R, Malgat M, Pedespan L, Letellier T. Influence of mitochondrial DNA level on cellular energy metabolism: implications for mitochondrial diseases. J Bioenerg Biomembr. 2008;40:59–67. doi: 10.1007/s10863-008-9130-5. [DOI] [PubMed] [Google Scholar]
- 67.Rondi-Reig L, Lemaigre Dubreuil Y, Martinou JC, Delhaye-Bouchaud N, Caston J, Mariani J. Fear decrease in transgenic mice overexpressing bcl-2 in neurons. Neuroreport. 1997;8:2429–2432. doi: 10.1097/00001756-199707280-00004. [DOI] [PubMed] [Google Scholar]
- 68.Rosenfeldt F, Marasco S, Lyon W, Wowk M, Sheeran F, Bailey M, Esmore D, Davis B, Pick A, Rabinov M, Smith J, Nagley P, Pepe S. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J Thorac Cardiovasc Surg. 2005;129:25–32. doi: 10.1016/j.jtcvs.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 69.Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol. 2004;24:8055–8068. doi: 10.1128/MCB.24.18.8055-8068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seibenhener ML, Du Y, Diaz-Meco MT, Moscat J, Wooten MC, Wooten MW. A role for sequestosome 1/p62 in mitochondrial dynamics, import and genome integrity. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbamcr.2012.11.004. pii: S0167-4889(12)00325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seibenhener ML, Wooten MW. Isolation and culture of hippocampal neurons from prenatal mice. J Vis Exp. 2012;(65) doi: 10.3791/3634. pii: 3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shao CY, Crary JF, Rao C, Sacktor TC, Mirra SS. Atypical protein kinase C in neurodegenerative disease II: PKCiota/lambda in tauopathies and alpha-synucleinopathies. J Neuropathol Exp Neurol. 2006;65:327–335. doi: 10.1097/01.jnen.0000218441.00040.82. [DOI] [PubMed] [Google Scholar]
- 73.Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 74.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 75.Thiffault C, Quirion R, Poirier J. The effect of L-deprenyl, D-deprenyl and MDL72974 on mitochondrial respiration: a possible mechanism leading to an adaptive increase in superoxide dismutase activity. Brain Res Mol Brain Res. 1997;49:127–136. doi: 10.1016/s0169-328x(97)00135-6. [DOI] [PubMed] [Google Scholar]
- 76.Vadlamudi RK, Joung I, Strominger JL, Shin J. p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins. J Biol Chem. 1996;271:20235–20237. doi: 10.1074/jbc.271.34.20235. [DOI] [PubMed] [Google Scholar]
- 77.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Ann Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc. 2009;4:1582–90. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- 79.Woods S, Clarke NN, Layfield R, Fone KC. 5-HT(6) receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br J Pharmacol. 2012;167:436–449. doi: 10.1111/j.1476-5381.2012.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wooten MW, Geetha T, Seibenhener ML, Babu JR, Diaz-Meco MT, Moscat J. The p62 scaffold regulates nerve growth factor-induced NF-kappaB activation by influencing TRAF6 polyubiquitination. J Biol Chem. 2005;280:35625–35629. doi: 10.1074/jbc.C500237200. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida Y, Izumi H, Ise T, Uramoto H, Torigoe T, Ishiguchi H, Murakami T, Tanabe M, Nakayama Y, Itoh H, Kasai H, Kohno K. Human mitochondrial transcription factor A binds preferentially to oxidatively damaged DNA. Biochem Biophys Res Commun. 2002;295:945–951. doi: 10.1016/s0006-291x(02)00757-x. [DOI] [PubMed] [Google Scholar]