Abstract
Abstract
Recently, an important role for CD47, a well-known ‘don't eat me’ signal, in the clearance of aged erythrocytes was revealed. Experimental data support the conversion of CD47 from a ‘don't eat me’ to an ‘eat me’ signal through a conformational change in CD47. Intriguingly, erythrocyte phagocytosis after this switch seems to be mediated by the same receptor that normally signals inhibition of phagocytosis, SIRPα. In this review, the possible molecular mechanisms leading to this conformational change in CD47 as well as the possible signal transduction events leading to phagocytosis after this switch are discussed. Lastly, the consequences of this newly identified mode of erythrocyte phagocytosis for the clearance of aged erythrocytes during normal turnover and after erythrocyte transfusion are addressed.
KeyWords: CD47, Aged erythrocytes, Phagocytosis, SIRPα
Abstract
Zusammenfassung
Vor kurzem ist eine wichtige Rolle für CD47, einem bekannten ‘Don't-Eat-Me’-Signal, bei der Beseitigung gealterter Erythrozyten aufgedeckt worden. Experimentelle Daten belegen die Umwandlung von CD47 von einem ‘Don't-Eat-Me’- zu einem ‘Eat-Me’-Signal durch konformative Veränderungen. Interessanterweise scheint die Erythrozytenphagozytose nach der Umwandlung von dem gleichen Rezeptor (SIRPα) vermittelt zu werden, der normalerweise die Hemmung der Phagozytose signalisiert. In dieser Übersichtsarbeit werden sowohl die potentiellen molekularen Mechanismen, die zu dieser Umwandlung von CD47 führen, als auch die möglichen Signaltransduktionsvorgänge, die nach der Umwandlung die Phagozytose einleiten, diskutiert. Des Weiteren werden die Konsequenzen dieses neu identifizierten Modus der Erythrozytenphagozytose für die Beseitigung gealterter Erythrozyten während des normalen Turnovers sowie nach Erythrozytentransfusion beleuchtet.
Introduction
Erythrocyte clearance has been studied for many years in the context of normal turnover [1, 2], enhanced clearance due to defects in erythrocyte metabolism or changes in membrane composition [3, 4, 5], and in blood transfusion [6]. Traditionally, erythrocyte phagocytosis has been proposed to be the result of the accumulation of ‘eat me’ signals on the membrane of the ageing erythrocyte. Several ‘eat me’ signals have been identified to be important for the clearance of aged erythrocytes by macrophages residing in the red pulp of the spleen, or alternatively, in the liver [1, 7, 8]. Two possible mechanisms that would lead to erythrocyte clearance are i) autoantibody binding to Band 3 after conformational changes in this protein due to ageing (the so-called ‘senescent cell antigen’) [9, 10], and ii) expression of phosphatidylserine (PS) on the outer leaflet of the erythrocyte membrane [11]. Both of these mechanisms are discussed in detail in this issue of TRANSFUSION MEDICINE AND HEMOTHERAPY.
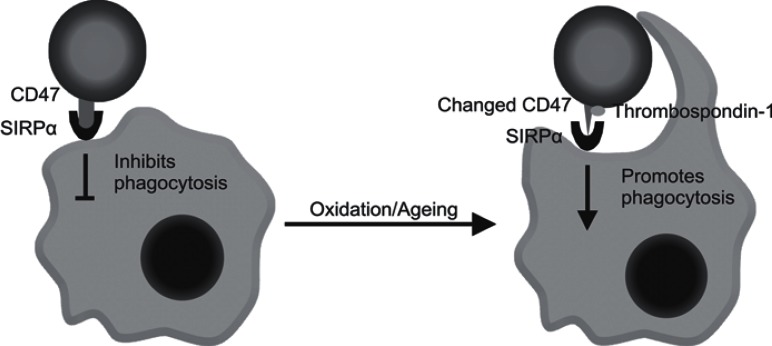
However, in 2000, Oldenborg et al. [12, 13] identified the membrane protein CD47 to be an important additional factor regulating phagocytosis of erythrocytes. In contrast to the ‘eat me’ signals as mentioned above, CD47 was shown to inhibit phagocytosis of erythrocytes by macrophages [12, 13, 14, 15, 16] (fig. 1). Moreover, the expression of CD47 by erythrocytes was found to be essential to prevent their uptake in the spleen by macrophages; erythrocytes from CD47-deficient mice were rapidly cleared when transfused into wild-type animals [13, 17]. Thus, clearance of erythrocytes was now believed to be determined by the balance between the accumulation of ‘eat me’ signals and the expression of the ‘don't eat me’ signal CD47. Loss of CD47 expression during erythrocyte ageing was proposed as an alternative mechanism for erythrocyte clearance in the normal ageing process [18, 19]. CD47 exerts its inhibitory effect through binding to SIRPα on the macrophage, which induces inhibitory signaling by the immunoreceptor tyrosinebased inhibition motifs (ITIMs) residing in the cytoplasmic tail of SIRPα [20, 21]. Upon ligation of SIRPα by CD47, the tyrosine phosphatases SHP-1 and SHP-2 are recruited to the ITIMs and activated, which in turn regulates, generally in a negative fashion, downstream signaling pathways and effector functions.
Fig. 1.
Oxidation and ageing cause a conformational change in CD47 upon which TSP-1 can bind. Under normal conditions, CD47 on the erythrocyte inhibits phagocytosis by interaction with SIRPα on the phagocyte. After the conformational change in CD47, TSP-1 is able to bind upon which interaction between CD47 and SIRPα leads to phagocytosis.
In a recent study, in which we attempted to identify the mechanism(s) that underlie the well-described clearance of a significant percentage of donor erythrocytes in the first 24 h after transfusion, CD47 was identified to be able to function as an ‘eat me’ signal after undergoing a conformational change (fig. 1) [22]. Strikingly, SIRPα was found to be the counter-receptor that recognized and bound this form of CD47 as well, revealing another layer of complexity of this ligand/receptor pair. Thus, next to its well-known role as inhibitor of phagocytosis, we propose that CD47 can switch its conformation and thereby its function to an ‘eat me’ signal on aged erythrocytes. In this review, we discuss this finding and the possible molecular mechanisms that are responsible for it.
Evidence for a Role of CD47 in the Phagocytosis of Aged Erythrocytes
Intriguingly, evidence exists in the literature that the CD47-SIRPα interaction, besides its inhibitory function on phagocytosis, can also promote cell-cell interactions [23] and even mediate phagocytosis of apoptotic cells [24, 25, 26]. First, SIRPα can mediate trans-endocytosis of CD47-containing membranes of adjacent cells, as reported by Kusakari et al. [23]. This work revealed that CD47-SIRPα signaling can, inversely to its known inhibitory effect on phagocytosis, actually lead to internalization of parts of the membrane of adjacent cells through SIRPα signaling. Furthermore, another report indicated that CD47-SIRPα interaction is very important for proper recognition and phagocytosis of apoptotic cells. Expression of CD47 in a lymphoma cell line, which did not express CD47 endogenously, was essential to allow phagocytosis of this cell line after the induction of apoptosis [25]. The parental cell line, i.e. without expression of CD47, was not phagocytosed after induction of apoptosis. Furthermore, the effect of expression of CD47 was abrogated when the lymphoma cells were treated with an antibody recognizing SIRPα, thereby blocking the interaction with CD47. These data were the first indication that CD47 and SIRPα are more than just negative regulators of phagocytosis, but might be involved in the efficient removal of apoptotic cells as well. These findings led us to hypothesize that the CD47-SIRPα interaction might play a role in the removal of aged erythrocytes. Using SIRPα-transfected cell lines, we observed that aged erythrocytes, either isolated from fresh blood or experimentally aged by oxidative treatment, were bound and phagocytosed through CD47-SIRPα interaction [22]. In addition, experimentally aged erythrocytes were found to be phagocytosed by human red pulp macrophages. Strikingly, human red pulp macrophages were unable to phagocytose anti-Dopsonized or PS-exposing erythrocytes (Burger et al., data unpublished), which is an indication that these mechanisms do not play a dominant role in the phagocytosis of aged erythrocytes by this particular cell type.
Conformational Change in CD47 Forms the Basis for Recognition by SIRPα as an ‘Eat Me’ Signal
It has been known for some time that CD47 can exist in different conformations on erythrocytes. Evidence for this was provided by Brittain et al. [27] who had used a set of antibodies directed against CD47 to study the conformational status of CD47 on sickle erythrocytes. CD47 expression levels did not vary between erythrocytes of normal controls and sickle patients, but the conformation of this protein, as determined by binding of antibodies against particular conformation-dependent epitopes, seemed to differ between the 2 groups. In addition, thrombospondin-1 (TSP-1), a well-known ligand for CD47 [28], was able to bind to CD47 on sickle erythrocytes but not to CD47 on erythrocytes of healthy controls, suggesting that the conformation of CD47 also determines whether or not it binds to TSP-1. Intriguingly, binding of TSP-1 to apoptotic cells has been reported to enhance phagocytosis of the apoptotic cell without inducing the secretion of pro-inflammatory cytokines [29]. Furthermore, TSP-1, which is a large homotrimeric glycoprotein, can mediate several cell-cell and cell-matrix interactions and can bind PS on erythrocytes [30]. Previous work by Head et al. [31] had indicated that inducing CD47 signaling on erythrocytes via antibodies but also through prolonged incubation with the TSP-1-derived peptide 4N1K leads to erythrocyte death. Together, these data indicated that the recognition of aged erythrocytes through CD47-SIRPα, which was found to be serum dependent, could be explained by a conformational change in CD47 resulting in TSP-1 binding and subsequent SIRPα recognition. In line with the experiments conducted by Brittain et al. [27], we observed that the conformation of CD47 on experimentally aged erythrocytes was found to have undergone a conformational change similar to that seen in sickle erythrocytes. Also, the TSP-1-derived peptide 4N1K was found to bind only to experimentally aged erythrocytes, i.e. to CD47 that had undergone a change in conformation. After testing binding and phagocytosis by both SIRPα-transfected cell lines as well as red pulp macrophages, we concluded that the change in conformation of CD47, followed by TSP-1 binding, marks erythrocytes for phagocytosis through SIRPα recognition.
It is presently unknown what molecular mechanism(s) induce(s) the change in conformation in CD47 leading to TSP-1 binding. Several possible mechanisms can be envisioned. First, since CD47 is part of the Band 3 complex in erythrocytes [32], changes in this membrane complex could induce conformational changes in CD47 that lead to the ‘eat me’ configuration. As discussed elsewhere in this issue of Transfusion Medicine and Hemotherapy, Band 3 itself is subject to many modifications, including clustering [33], phosphorylation [34], and cleavage [35], which lead to the generation of the senescent cell antigen. These modifications in Band 3 are well documented to occur during ageing [36, 37, 38], and this would certainly be a very elegant way to couple intracellular ageing events resulting in Band 3 modifications to the conformational change in CD47 and thus create an extracellular ‘eat me’ signal at the expense of the ‘don't eat me’ signal that CD47 normally fulfills in erythrocytes. Another interesting phenomenon that occurs on apoptotic erythrocytes is the clustering of CD47 [32]. This clustering is supposed to be the result of increased mobility of CD47, and might lead to a change in conformation and subsequent 4N1K binding which would then be recognized by SIRPα as an ‘eat me’ signal [22]. The contribution of this phenomenon to the inhibition or facilitation of clearance through SIRPα is still unexplored. Finally, it is also possible that CD47 is directly modified during ageing, i.e. through oxidation, which would directly alter the conformation of CD47 and the recognition by SIRPα. Although the latter option appears somewhat unlikely, evidence was obtained that this indeed occurs. Firstly, the conformational change in CD47 can be induced by oxidative treatment. Secondly, beads coated with the extracellular domain of CD47 were found to bind the TSP-1-derived peptide 4N1K only after oxidation and binding of oxidized CD47 to SIRPα proved to be dependent on the binding of 4N1K. Of course, the above proposed changes in CD47 leading to a conformational switch are certainly not mutually exclusive and might also occur simultaneously.
Signal Transduction by SIRPα Leading to Phagocytosis of Aged Erythrocytes
SIRPα signaling leading to the inhibition of phagocytosis is based on signaling through the 4 ITIMs residing in the cytoplasmic tail [20]. The ITIMs become phosphorylated upon CD47 binding after which the tyrosine phosphatases SHP-1 and SHP-2 are recruited and activated through another phosphorylation event. SHP-1 and SHP-2 then dephosphorylate specific protein substrates that are important for phagocytosis, and thereby exert a negative effect on this process. At present it is unknown how SIRPα signaling induced by the CD47/TSP-1 complex on aged erythrocytes induces phagocytosis. Inspired by the report that the membrane proximal region of SIRPα regulates endocytosis independent of the ITIMs [23], we explored the phagocytic capacity of different SIRPα mutants of aged erythrocytes. Phagocytosis of aged erythrocytes through CD47/SIRPα interaction proved to be dependent on the presence of the cytoplasmic tail, but did not require functional ITIMs [22]. The latter could be concluded from the fact that a SIRPα mutant, in which all tyrosines in the ITIMs were replaced with phenylalanines, was as effective as the wild-type protein. This essentially rules out a role for SHP-1 and SHP-2, but raises the question which signaling proteins are involved in this process. Interestingly, SIRPα was postulated to be a scaffold for the recruitment of signaling complexes related to integrin activity [24]. 2 different complexes were identified; one containing the tyrosine kinase Pyk2, the other SKAP55hom/R and FYB/SLAP-130 [39]. Moreover, it was shown that tyrosine phosphorylation of the ITIMs in SIRPα was not a prerequisite for the formation of these different complexes. The possibility that SIRPα cooperates with integrins to mediate phagocytosis of apoptotic cells is appealing, as many reports have already pointed out a role for integrins in the non-inflammatory removal of apoptotic cells [40, 41].
CD47/TSP-1 as an ‘Eat Me’ Signal for Normal Erythrocyte and Donor Erythrocyte Removal
Based on our previous data in which a small proportion of erythrocytes from full blood – residing in the fraction of erythrocytes that contain the oldest erythrocytes – was found to be bound and phagocytosed by SIRPα-expressing cells, we propose that CD47/TSP-1 functions as a removal signal in the normal turnover of erythrocytes (fig. 1). In line with this, human macrophages isolated from the red pulp of the spleen were found to be able to recognize and phagocytose erythrocytes expressing this removal signal. Human red pulp macrophages express high levels of SIRPα, in contrast to for instance Fc receptors which are expressed only at low levels (Burger et al., data unpublished). Human red pulp spleen macrophages were found to phagocytose anti-Dopsonized erythrocytes at very low levels, in contrast to monocyte-derived macrophages which phagocytosed erythrocytes treated in this way at high levels (Burger et al., data unpublished). Even PS-expressing erythrocytes were not phagocytosed at a level above background by red pulp macrophages which are the most relevant cell type for the removal of aged erythrocytes (Burger et al, data unpublished). In short, these results favor CD47 as a removal signal for aged erythrocytes in vivo over other potential removal signals, such as immunoglobulin binding or PS exposure. In addition, we speculate that this mechanism may also be relevant for other blood cell types, since CD47 is ubiquitously expressed on hematopoietic cells, and evidence exist in the literature that CD47 is also necessary for efficient phagocytosis of apoptotic cells of other origin [24, 25]. It is well known that transfusion of erythrocytes leads to a rapid clearance of 10–25% of the transfused cells, depending on storage time [42, 43]. The data obtained may also explain this high percentage of removal of erythrocytes after transfusion. A significant proportion of donor erythrocytes, stored for several weeks, show the conformational change in CD47 favoring TSP-1 binding when incubated in fresh blood. Short-stored erythrocytes, which are also cleared at a much lower rate after transfusion, do not show this increase. In addition, long-stored erythrocytes bind TSP-1 when diluted into whole blood, whereas short-stored cells do not. Importantly, the change in conformation and the subsequent TSP-1 binding can only be observed after dilution in whole blood and subsequent incubation at 37 °C. This finding provides a possible explanation for the high rate of clearance of long-stored erythrocytes after transfusion. Although it has been suggested that the expression of CD47 decreases during erythrocyte storage, hinting at an enhanced clearance due to diminished inhibition through SIRPα signaling, we and others have found that CD47 levels are not diminished after prolonged storage [44, 45]. Thus, a conformational change in CD47 resulting in the expression of the ‘eat me’ configuration of CD47 in long-stored erythrocytes seems to be an attractive mechanism for the high percentage of removal of donor erythrocytes.
Conclusion
CD47 undergoes a conformational change during ageing, which causes TSP-1 binding and recognition of CD47 as an ‘eat me’ signal by SIRPα. This newly identified mode of erythrocyte clearance provides a very elegant, possibly non-inflammatory, way of clearing aged erythrocytes. The conformational status of CD47 can be changed through oxidative stress or, possibly, after changes in the Band 3 complex, and is thus a potential extracellular indicator for intracellular damage and ageing. From our point of view, CD47 controls erythrocyte lifespan both positively through inhibition of phagocytosis via SIRPα, and negatively by triggering phagocytosis through SIRPα. The fact that aged erythrocytes, which can be phagocytosed through a SIRPα-mediated mechanism, can be isolated from normal blood, and primary human red pulp macrophages can phagocytose aged erythrocytes through this mechanism, strongly suggests that this mechanism is operational in vivo, but further studies are required to establish this.
Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Barnhart MI, Lusher JM. Structural physiology of the human spleen. Am J Pediatr Hematol Oncol. 1979;1:311–330. [PubMed] [Google Scholar]
- 2.Lutz HU. Innate immune and non-immune mediators of erythrocyte clearance. Cell Mol Biol (Noisy-le-grand) 2004;50:107–116. [PubMed] [Google Scholar]
- 3.Fischer T, Pescarmona GP, Bosia A, Naitana A, Turrini F, Arese P. Mechanisms of red cell clearance in favism. Biomed Biochim Acta. 1983;42:S253–S257. [PubMed] [Google Scholar]
- 4.Prasad AS, Tranchida L, Konno ET, Berman L, Albert S, Sing CF, Brewer GJ. Hereditary sideroblastic anemia and glucose-6-phosphate dehydrogenase deficiency in a Negro family. J Clin Invest. 1968;47:1415–1424. doi: 10.1172/JCI105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilairat P, Kittikalayawong A, Chaicharoen S. The thalassemic red cell membrane. Southeast Asian J Trop Med Public Health. 1992;23(suppl 2):74–78. [PubMed] [Google Scholar]
- 6.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whit-tier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 8.Weiss L. The red pulp of the spleen: structural basis of blood flow. Clin Haematol. 1983;12:375–393. [PubMed] [Google Scholar]
- 9.Kay MM, Goodman SR, Sorensen K, Whitfield CF, Wong P, Zaki L, Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983;80:1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay MM. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci U S A. 1984;81:5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci U S A. 1998;95:3077–3081. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPαlpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 14.Olsson M, Nilsson A, Oldenborg PA. Dose-dependent inhibitory effect of CD47 in macrophage uptake of IgG-opsonized murine erythrocytes. Biochem Biophys Res Commun. 2007;352:193–197. doi: 10.1016/j.bbrc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Olsson M, Oldenborg PA. CD47 on experimentally senescent murine RBCs inhibits phagocytosis following Fcgamma receptor-mediated but not scavenger receptor-mediated recognition by macrophages. Blood. 2008;112:4259–4267. doi: 10.1182/blood-2008-03-143008. [DOI] [PubMed] [Google Scholar]
- 16.Tsai RK, Rodriguez PL, Discher DE. Self inhibition of phagocytosis: the affinity of ‘marker of self’ CD47 for SIRPαlpha dictates potency of inhibition but only at low expression levels. Blood Cells Mol Dis. 2010;45:67–74. doi: 10.1016/j.bcmd.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa-Sekigami T, Kaneko Y, Okazawa H, Tomizawa T, Okajo J, Saito Y, Okuzawa C, Suga-wara-Yokoo M, Nishiyama U, Ohnishi H, Matozaki T, Nojima Y. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107:341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- 18.Anniss AM, Sparrow RL. Expression of CD47 (integrin-associated protein) decreases on red blood cells during storage. Transfus Apher Sci. 2002;27:233–238. doi: 10.1016/s1473-0502(02)00070-8. [DOI] [PubMed] [Google Scholar]
- 19.Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for the monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion. 2005;45:1496–1503. doi: 10.1111/j.1537-2995.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 20.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPαlpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Sato R, Ohnishi H, Kobayashi H, Kiuchi D, Hayashi A, Kaneko Y, Honma N, Okazawa H, Hirata Y, Matozaki T. Regulation of multiple functions of SHPS-1, a transmembrane glycoprotein, by its cytoplasmic region. Biochem Biophys Res Commun. 2003;309:584–590. doi: 10.1016/j.bbrc.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Burger P, Hilarius-Stokman P, De KD, van den Berg TK, Van BR: CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012. [DOI] [PubMed]
- 23.Kusakari S, Ohnishi H, Jin FJ, Kaneko Y, Murata T, Murata Y, Okazawa H, Matozaki T. Transendocytosis of CD47 and SHPS-1 and its role in regulation of the CD47-SHPS-1 system. J Cell Sci. 2008;121:1213–1223. doi: 10.1242/jcs.025015. [DOI] [PubMed] [Google Scholar]
- 24.Babic I, Schallhorn A, Lindberg FP, Jirik FR. SHPS-1 induces aggregation of Ba/F3 pro-B cells via an interaction with CD47. J Immunol. 2000;164:3652–3658. doi: 10.4049/jimmunol.164.7.3652. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson A, Oldenborg PA. CD47 promotes both phosphatidylserine-independent and phosphatidyl serine-dependent phagocytosis of apoptotic murine thymocytes by non-activated macrophages. Biochem Biophys Res Commun. 2009;387:58–63. doi: 10.1016/j.bbrc.2009.06.121. [DOI] [PubMed] [Google Scholar]
- 26.Tada K, Tanaka M, Hanayama R, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Tethering of apoptotic cells to phagocytes through binding of CD47 to Src homology 2 domain-bearing protein tyrosine phosphatase substrate-1. J Immunol. 2003;171:5718–5726. doi: 10.4049/jimmunol.171.11.5718. [DOI] [PubMed] [Google Scholar]
- 27.Brittain JE, Mlinar KJ, Anderson CS, Orringer EP, Parise LV. Integrin-associated protein is an adhesion receptor on sickle red blood cells for immobilized thrombospondin. Blood. 2001;97:2159–2164. doi: 10.1182/blood.v97.7.2159. [DOI] [PubMed] [Google Scholar]
- 28.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Y, Stuart L, Lindberg FP, Rosenkranz AR, Chen Y, Mayadas TN, Savill J. Nonphlogistic clearance of late apoptotic neutrophils by macrophages: efficient phagocytosis independent of beta 2 integrins. J Immunol. 2001;166:4743–4750. doi: 10.4049/jimmunol.166.7.4743. [DOI] [PubMed] [Google Scholar]
- 30.Gayen Betal S, Setty BN. Phosphatidylserine-positive erythrocytes bind to immobilized and soluble thrombospondin-1 via its heparin-binding domain. Transl Res. 2008;152:165–177. doi: 10.1016/j.trsl.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Head DJ, Lee ZE, Swallah MM, Avent ND. Ligation of CD47 mediates phosphatidylserine expression on erythrocytes and a concomitant loss of viability in vitro. Br J Haematol. 2005;130:788–790. doi: 10.1111/j.1365-2141.2005.05668.x. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian S, Tsai R, Sen S, Dahl KN, Discher DE. Membrane mobility and clustering of Integrin Associated Protein (IAP, CD47) – major differences between mouse and man and implications for signaling. Blood Cells Mol Dis. 2006;36:364–372. doi: 10.1016/j.bcmd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Bosman GJ, Willekens FL, Werre JM. Erythrocyte aging: a more than superficial resemblance to apoptosis? Cell Physiol Biochem. 2005;16:1–8. doi: 10.1159/000087725. [DOI] [PubMed] [Google Scholar]
- 34.De Almeida JP, Freitas-Santos T, Saldanha C. Evidence that the degree of band 3 phosphorylation modulates human erythrocytes nitric oxide efflux – in vitro model of hyperfibrinogenemia. Clin Hemorheol Microcirc. 2011;49:407–416. doi: 10.3233/CH-2011-1490. [DOI] [PubMed] [Google Scholar]
- 35.Mandal D, Baudin-Creuza V, Bhattacharyya A, Pathak S, Delaunay J, Kundu M, Basu J. Caspase 3-mediated proteolysis of the N-terminal cytoplasmic domain of the human erythroid anion exchanger 1 (band 3) J Biol Chem. 2003;278:52551–52558. doi: 10.1074/jbc.M306914200. [DOI] [PubMed] [Google Scholar]
- 36.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–1220. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 37.Chiarantini L, Rossi L, Fraternale A, Magnani M. Modulated red blood cell survival by membrane protein clustering. Mol Cell Biochem. 1995;144:53–59. doi: 10.1007/BF00926740. [DOI] [PubMed] [Google Scholar]
- 38.Rossi V, Leoncini S, Signorini C, Buonocore G, Paffetti P, Tanganelli D, Ciccoli L, Comporti M. Oxidative stress and autologous immunoglobulin G binding to band 3 dimers in newborn erythrocytes. Free Radic Biol Med. 2006;40:907–915. doi: 10.1016/j.freeradbiomed.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Timms JF, Swanson KD, Marie-Cardine A, Raab M, Rudd CE, Schraven B, Neel BG. SHPS-1 is a scaffold for assembling distinct adhesion-regulated multi-protein complexes in macrophages. Curr Biol. 1999;9:927–930. doi: 10.1016/s0960-9822(99)80401-1. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Park SY, Kim SY, Bae DJ, Pyo JH, Hong M, Kim IS: Cross-talk between engulfment receptors, stabilin-2 and integrin alphavbeta5 orchestrates engulfment of phosphatidylserine exposed erythrocytes. Mol Cell Biol 2012. [DOI] [PMC free article] [PubMed]
- 41.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 42.Hess JR, Greenwalt TG. Storage of red blood cells: new approaches. Transfus Med Rev. 2002;16:283–295. doi: 10.1053/tmrv.2002.35212. [DOI] [PubMed] [Google Scholar]
- 43.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–1485. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 44.Sparrow RL, Healey G, Patton KA, Veale MF. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus Apher Sci. 2006;34:15–23. doi: 10.1016/j.transci.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Holovati JL, Wong KA, Webster JM, Acker JP. The effects of cryopreservation on red blood cell microvesiculation, phosphatidylserine externalization, and CD47 expression. Transfusion. 2008;48:1658–1668. doi: 10.1111/j.1537-2995.2008.01735.x. [DOI] [PubMed] [Google Scholar]