Abstract
Many studies have shown that minocycline, an antibacterial tetracycline, suppresses experimental pain. While minocycline’s positive effects on pain resolution suggest that clinical use of such drugs may prove beneficial, minocycline’s antibiotic actions and divalent cation (Ca2+; Mg2+) chelating effects detract from its potential utility. Thus, we tested the antiallodynic effect induced by a non-antibacterial, non-chelating minocycline derivative in a model of neuropathic pain and performed an initial investigation of its anti-inflammatory effects in vitro. Intraperitoneal minocycline (100 mg/kg) and 12S-hydroxy-1,12-pyrazolinominocycline (PMIN; 23.75, 47.50 or 95.00 mg/kg) reduce the mechanical allodynia induced by chronic constriction injury of mouse sciatic nerve. PMIN reduces the LPS-induced production of PGE2 by primary microglial cell cultures. Human embryonic kidney cells were transfected to express human toll-like receptors 2 and 4, and the signaling via both receptors stimulated with PAM3CSK4 or LPS (respectively) was affected either by minocycline or PMIN. Importantly, these treatments did not affect the cell viability, as assessed by MTT test. Altogether, these results reinforce the evidence that the anti-inflammatory and experimental pain suppressive effects induced by tetracyclines are neither necessarily linked to antibacterial nor to Ca2+ chelating activities. This study supports the evaluation of the potential usefulness of PMIN in the management of neuropathic pain, as its lack of antibacterial and Ca2+ chelating activities might confer greater safety over conventional tetracyclines.
Keywords: 12S-hydroxy-1,12-pyrazolinominocycline; chemically modified tetracycline; neuropathic pain; PGE2; TLR2; TLR4
1. Introduction
Tetracyclines induce pleiotropic effects unrelated to their broad antibacterial actions, including anti-inflammatory and neuroprotective effects [4]. Of the tetracyclines, minocycline has been the focus of the most studies given that it is the derivative which crosses the blood-brain barrier to the greatest extent [1]. Experimental studies have shown beneficial effects induced by minocycline in animal models of inflammatory and neuropathic pain [4].
While thousands of tetracycline derivatives have been synthesized, there has been surprisingly little effort put toward developing compounds that would maintain clinically relevant effects such as pain control, while ridding the compound of antibacterial actions that would be detrimental for such uses. One strategy for developing such a candidate compound would be to modify the portion of the tetracycline molecule that binds Mg2+, resulting in loss of its antibacterial activity [26]. Indeed, Lertvorachon et al. [20] reacted tetracyclines with hydrazine to create 1,12-substituted tetracyclines. One of these “pentacyclines”, 12S-hydroxy-1,12-pyrazolinominocycline (PMIN), is a potent antioxidant [8, 20], which neither inhibits the growth of Escherichia coli or Staphylococcus aureus nor chelates Ca2+ [2, 20]. Despite this profile, PMIN reduces formalin-induced licking behavior and carrageenan-induced edema in mice, and these effects are comparable to those of minocycline [2]. Other chemically modified tetracyclines (CMTs) devoid of antibacterial activity may also be obtained after different modifications such as the removal of 4-dimethylamino group [12]. However, as far as we know, such compounds have not been evaluated in pain models.
The interest in CMTs derives from their potential superior safety, as derivatives devoid of antibacterial activity would not induce the development of tetracycline-resistant microorganisms after prolonged use, plus would be expected to avoid gastrointestinal disturbances and candidiasis associated with tetracycline-induced alterations in gut, mouth or vaginal flora [13]. Further, deleting Ca2+ chelation is important given its detrimental effects on developing bones and teeth, associated with teeth discoloration, gum dysplasia, dental hypoplasia or bone deformities [4].
Only one study of a CMT in pain models seems to have been conducted to date [2]. While this work provided evidence of efficacy exhibited by PMIN in nociceptive and inflammatory pain, it did not examine neuropathic pain. Given the prevalence of unresolved neuropathic pain worldwide, a neuropathic pain model is included for study, and in vitro anti-inflammatory effects were also investigated, such as suppression of prostaglandin (PG) E2 production and human toll-like receptors (TLR) 2 or 4 signaling. PGE2 is a key pain mediator [16]. We also tested the effects induced by minocycline and PMIN on TLR2 or TLR4 signaling because there is a quickly emerging literature, especially within the last decade, showing the versatile roles played by these receptors in the development and persistence of neuropathic pain [23].
2. Material and methods
2.1. Animals
Twelve-week-old adult female C57BL/6 mice were used. Efforts were made to minimize both animal distress and the number of animals used. The animals had free access to food and water and were maintained in a room with a 12 h light-dark cycle. The experiments were carried out at room temperature between 26 and 34 °C, which corresponds to the thermoneutral zone for mice [11]. All experiments were performed according to the ethical guidelines for the investigation of experimental pain in conscious animals [27] and approved by the Ethics Committee on Animal Experimentation of the Federal University of Minas Gerais. The works done in Boulder (USA) and Freiburg (Germany) were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder and by the Ethics Committee of the Medical School of the University of Freiburg, respectively. Each experiment was conducted in a separate group of animals. Experimenters were blinded to treatments.
2.2. Surgery
Chronic constriction injury (CCI) of the right sciatic nerve was aseptically performed as previously described [7], adapted to mice, under ketamine (100 mg/kg; Vetbrands, São Paulo, Brazil) plus xylazine (10 mg/kg; Vetbrands) intraperitoneal (i.p.) anesthesia. Three ligatures of 5-0 chromic gut (Brasuture, São Paulo, Brazil) were tightly tied around the sciatic nerve at the level of the midthigh. In sham-operated mice, the same surgical procedure was followed until the nerve was exposed, but ligatures were not performed.
2.3. Electronic von Frey test for mechanical allodynia
Mechanical allodynia was measured by using an electronic von Frey apparatus, as previously described [5], adapted to mice. After habituating the mice for 60 min to the experimental apparatus for 4 days, baseline behavioral measures were recorded, CCI or sham surgery was then performed, and behavioral responses to mechanical stimuli were tested at day 21 after surgery. At this time point, a single administration of minocycline (100 mg/kg, i.p.), or equivolume saline was given in groups tested for mechanical allodynia 2, 4, 6 and 8 h after drug administration. Based on this result, a separate group of CCI mice was divided up into 4 groups, which received a single administration of PMIN (23.75, 47.50 or 95.00 mg/kg, i.p.) or saline. Sham mice were injected only with the highest dose of PMIN (95 mg/kg, equimolar to minocycline 100 mg/kg) or minocycline (100 mg/kg).
2.4. Cell culture and in vitro assays
Primary microglial cell cultures were established from cerebral cortices of 1-day neonatal Wistar rats, as previously described [3]. ED-1 (Serotech, Raleigh, NC, USA; catalog number MCA341R; 1:1000) immunocytochemical marker was used to determine microglial purity (>98%). Supernatants were harvested, centrifuged at 10000 g for 10 min and concentrations of PGE2 in the media were measured by enzyme immunoassay (Biotrend, Cologne, Germany) according to the manufacturer’s instructions.
As previously described [14], a human embryonic kidney-293 (HEK293) cell line stably transfected to express human TLR4 was used to assess TLR4 activity. This HEK293 cell line expresses high levels of TLR4 (Invivogen, San Diego, CA, USA), the required TLR4 co-signaling molecules (MD-2 and CD14) and an optimized alkaline phosphatase reporter gene under the control of a promoter inducible by several transcription factors such as nuclear factor kappa B (NF-κB) and AP-1. A parallel HEK-hTLR2 (Invivogen) cell line was also employed to examine the TLR2 activity of tetracycline derivatives. TLR2 and TLR4 activities in the cells were assessed by measuring the production of secreted alkaline phosphatase (SEAP) protein that is produced as a consequence of TLR2 or TLR4 activation [14].
The cell viability after the treatments of primary rat microglia and HEK cells described below was assessed by MTT (Sigma-Aldrich, St. Louis, MO, USA) assay, as previously described [22].
2.5. Drugs and protocols
Minocycline hydrochloride (Sigma), lipopolysaccharide (LPS; Escherichia coli 0111:B4; Sigma), PAM3CSK4 (stable synthetic tripalmitoylated lipopeptide that mimics gram-positive bacterial exotoxins; Sigma) and cell culture grade dimethyl sulfoxide (DMSO; Sigma) were used. PMIN was prepared as previously described [20]. Throughout the study, Proton Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry analyses were performed and indicated high stability of PMIN (data not shown). Solutions and suspensions were prepared in isotonic saline immediately before the i.p. injections (10 ml/kg) or in PBS before adding onto cultures. Minocycline (1, 10 or 100 μM) or PMIN (0.95, 9.50 or 95.00 μM) or PBS was added to cell media (1% v/v) 30 min prior to LPS (10 ng/ml for microglial cells or 0.01-100 ng/ml for HEK-hTLR4 cells) or PAM3CSK4 (1, 10 or 100 ng/ml; HEK-hTLR2 cells), and then the cells were kept incubated during 24 h before assays. DMSO (5% v/v), used in the cell viability tests as a positive control, was added to cell media and the incubation also lasted 24 h.
2.6. Data analysis
Data are presented as mean ± standard error of the mean (S.E.M.). The behavioral results were analyzed by two-way ANOVA followed by Bonferroni post hoc test for multiple comparisons (Fig. 1). For in vitro assays, the results from 4 independent experiments were analyzed by one-way ANOVA followed by Newman-Keuls post hoc for multiple comparisons (Fig. 2 and 3). For ease of reading, detailed statistical information was included in the figure captions.
Fig. 1.
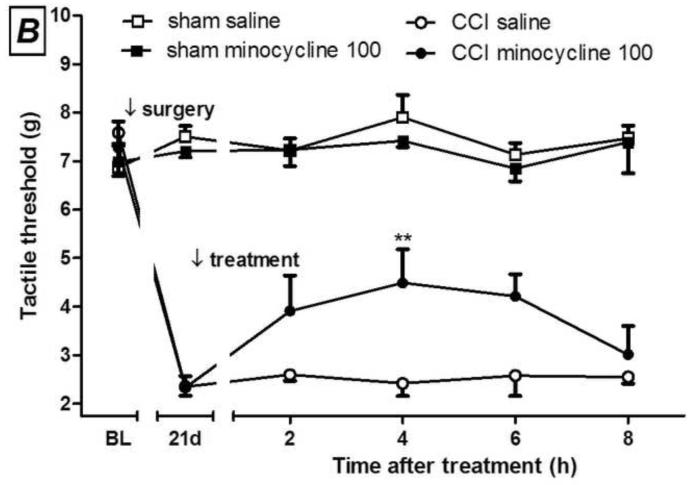
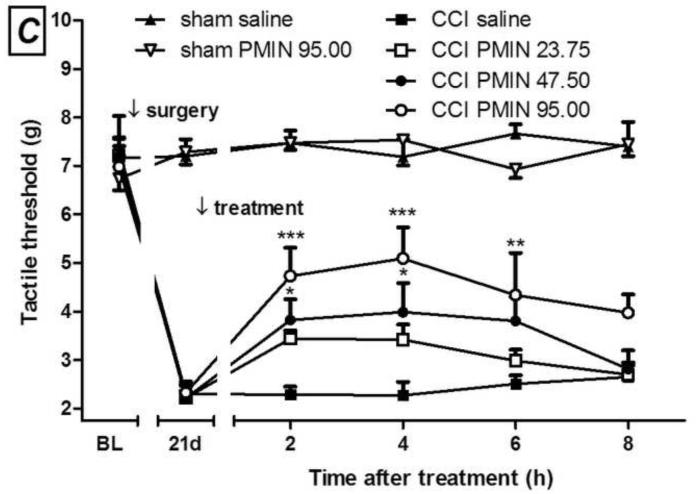
Development of mechanical allodynia after chronic constriction injury (CCI) in mice (A). I.p. minocycline (B, 100 mg/kg) or 12S-hydroxy-1,12-pyrazolinominocycline (PMIN; C, 23.75, 47.50 or 95.00 mg/kg) inhibits the mechanical allodynia induced by CCI. (A) Main effect of surgery, F(1,30)= 105.95, p < 0.0001; main effect of time, F(3,30) = 11.49, p < 0.0001; interaction, F(3,30) = 12.29, p < 0.0001. (B) Main effect of treatment, F(1,40)= 5.15, p = 0.0467; main effect of time, F(4,40) = 4.56, p = 0.0039; interaction, F(4,40) = 3.74, p = 0.0112. (C) Main effect of treatment, F(3,80)= 5.60, p = 0.0059 ; main effect of time, F(4,80) = 14.90, p < 0.0001 ; interaction, F(12,80) = 2.71, p = 0.0041. Sham animals were unaffected by treatments (all p values higher than 0.05). Data are expressed as mean ± S.E.M. (n = 6). BL = baseline. * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with the vehicle-treated group, tested by two-way ANOVA followed by Bonferroni test, taking time and surgery (A) or time and treatment (B and C) as main factors.
Fig. 2.
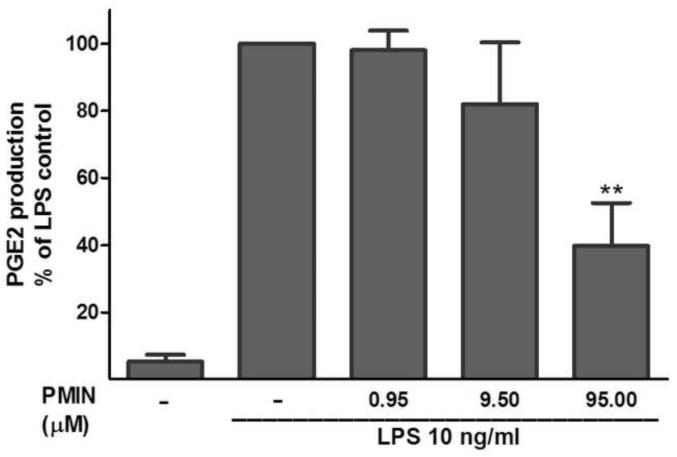
12S-hydroxy-1,12-pyrazolinominocycline (PMIN; 0.95, 9.50 or 95.00 μM) inhibits LPS-induced prostaglandin (PG) E2 microglial production, as assessed by enzyme immunoassay. PMIN (or vehicle) was added to the cell media 30 min prior to LPS (10 ng/ml, 24-h incubation). Data are expressed as mean ± S.E.M. ** p < 0.01 compared with LPS control.
Fig. 3.
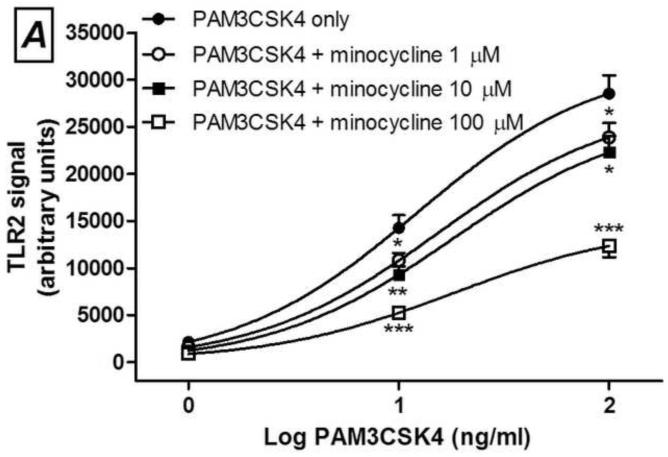
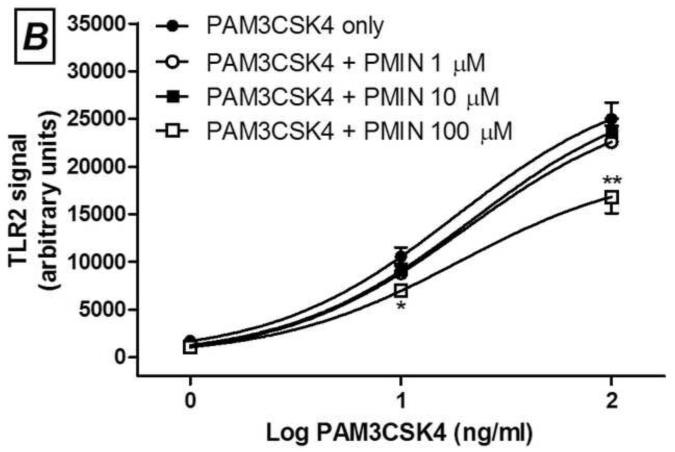
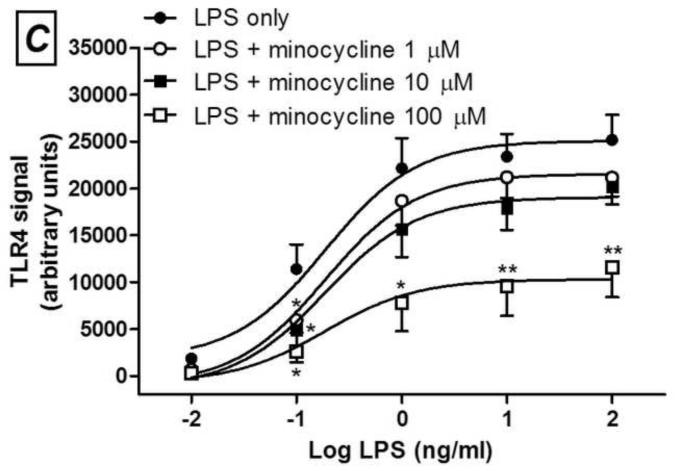
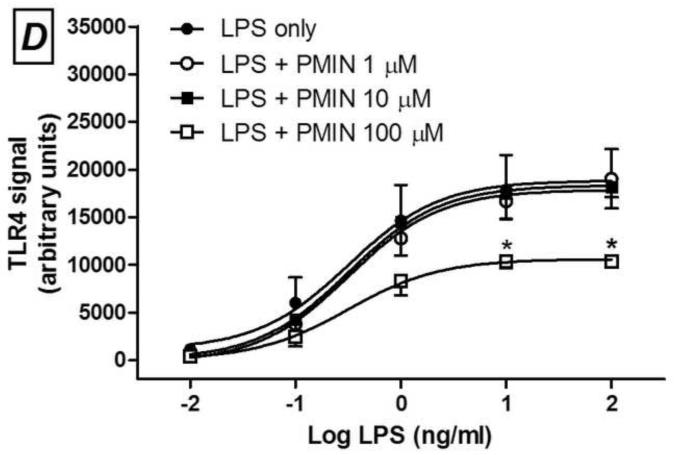
Minocycline (A and C) or 12S-hydroxy-1,12-pyrazolinominocycline (PMIN; B and D) inhibits PAM3CSK4-induced toll-like receptor (TLR) 2 signaling and LPS-induced TLR4 signaling, as assessed by SEAP assay. Minocycline or PMIN (or vehicle) was added to the HEK cell media 30 min prior to PAM3CSK4 (1, 10 and 100 ng/mL, 24-h incubation) or LPS (0.01, 0.1, 1, 10 and 100 ng/mL, 24-h incubation). Data are expressed as mean ± S.E.M. * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with LPS or PAM3CSK4 control.
3. Results
Initially, we assessed the time course of development of allodynia after mouse CCI. Allodynia is detectable 1 week after the surgery, and becomes more consistent 2 and 3 weeks afterwards (Fig. 1A). In this model of neuropathic pain, post-treatment with either minocycline (100 mg/kg, i.p., day 21 post-CCI) or PMIN (23.75, 47.50 or 95.00 mg/kg, i.p., day 21 post-CCI) induced antiallodynic effects (Fig. 1B and 2C, respectively). PMIN induced antiallodynic effect in a dose-dependent manner.
Next, we observed that PMIN (0.95, 9.50 or 95.00 μM) reduces the production of PGE2 by primary rat microglial cells stimulated with LPS in a concentration-dependent manner (Fig. 2). The effect induced by PMIN was not compared with that induced by minocycline because was already examined [3]. In addition, either minocycline (1, 10 or 100 μM) or PMIN (0.95, 9.50 or 95.00 μM) reduced either TLR2 or TLR4 signaling in HEK-hTLR2 and HEK-hTLR4 cell lines, respectively, as assessed by SEAP assay (Fig. 3). Importantly, cytotoxicity seems not to account for the anti-inflammatory effects observed in these in vitro studies because the treatments did not reduce cell viability, as assessed by the MTT assay. Not even a tendency to cytotoxicity was observed after the abovementioned treatments (p > 0.05). In contrast, DMSO (5% v/v), used as a positive control, reduced the cell viability nearly completely (p < 0.001, data not shown).
4. Discussion
We showed that PMIN, a non-antibacterial, non-chelating pyrazoline derivative of minocycline, inhibited CCI-induced allodynia in mice in a dose-dependent manner, additionally to the effects previously shown in the formalin test and carrageenan-induced edema in mice [2]. Moreover, this compound, similarly to minocycline [3], induced anti-inflammatory effect in vitro, as it inhibited the production of PGE2 induced by LPS in cultured primary rat microglial cells. Lastly, TLR2 or TLR4 signaling was inhibited either by minocycline or PMIN. Importantly, the viability of these abovementioned cell cultures was unaffected by both tetracyclines under these experimental conditions. As far as we know, the present study represents the first demonstration of the antiallodynic effect induced by a member of the CMT class in a neuropathic pain model.
I.p. PMIN partially reversed allodynia induced by CCI in mice, a model of neuropathic pain. Raghavendra et al. [25] showed that i.p. minocycline prevents the development of mechanical allodynia and thermal hyperalgesia after L5 spinal nerve ligation. However, no effect on existing mechanical allodynia and thermal hyperalgesia was observed in this study when the treatment was started from day 5 post-ligation up to day 10. In the CCI model, the same lack of effect on established allodynia was observed [19]. Recently, the antiallodynic effect induced by minocycline was shown when intrathecal injections were done 1, 3, 7 days after nerve ligation, but not 10 or 21 days afterwards [21]. In the present study, we show that a single i.p. administration of PMIN partially reversed allodynia 21 days after mouse CCI. Indeed, differences in analgesic sensitivity have been shown in different neuropathic pain models [9]. Therefore, despite methodological and drug differences, our results corroborate the more recent studies showing partial reversal of neuropathic pain with tetracycline derivatives. Obviously, in a therapeutic paradigm, it is more relevant that an analgesic drug candidate reverses pain rather than only preventing it.
The serum concentrations of minocycline 3 h and 6 h after a single i.p. injection (100 mg/kg) in mice – time points between which experimental pain suppression and anti-inflammatory effects are observed – are approximately 66 and 43 μM, respectively [2], thus falling within the concentration range used in the present in vitro experiments. Despite the big differences between the influences under which minocycline is in vivo and in vitro, serum and in vitro concentrations seem to be correlated. On the other hand, this estimation cannot yet be performed for PMIN because its pharmacokinetic profile remains to be studied.
Altogether, our previous studies [2, 6] and the present one indicate that doxycycline, minocycline and PMIN induce similar effects in experimental models of pain, regardless of exhibition of antibacterial or chelating activities by these compounds. Therefore, we hypothesize that all these semi-synthetic tetracycline derivatives share similar anti-inflammatory activities that may underlie suppression of experimental pain. Among them, minocycline is the most widely studied [4]. It has been shown that minocycline, directly or indirectly, prevents activation of many enzymes involved in the synthesis of multiple inflammatory mediators in macrophage/microglia or neuronal cell lines or in cell-free assays. Among them, phospholipase A2, different protein kinase C isoforms, multiple mitogen-activated protein kinases, phosphatidylinositol 3-kinase/Akt, inducible nitric oxide synthase, cyclooxygenase (COX)-2, microsomal prostaglandin E synthase-1, 5-lipoxygenase and poly(ADP-ribose) polymerase-1 [4]. Besides anti-inflammatory actions on macrophages, microglia and neurons, direct actions on excitability of primary afferent neurons may also explain minocycline’s suppressive effects on experimental pain [4]. Therefore, evidence supports both peripheral and central mechanisms underlying minocycline’s actions. We hypothesize that PMIN also exhibits pleiotropic anti-inflammatory activities.
Peripheral and central inhibition of PGE2 production and TLR2 and TLR4 signaling in different cells types may contribute to antiallodynic effects induced by minocycline or PMIN in experimental models of pain. PGE2 is the main inflammatory prostanoid and plays roles in experimental neuropathic pain development and persistence, acts on immune cells and neurons, both peripherally and centrally, in different relevant neuropathic pain models, though these roles are less important than those in models of inflammatory pain [16]. The fact that minocycline [3] or PMIN inhibits PGE2 would not imply that these tetracycline derivatives necessarily suppress experimental or clinical neuropathic pain. Indeed, traditional or COX-2 selective non-steroidal anti-inflammatory drugs (NSAIDs) inhibit PGE2 production but induce little-to-no suppressive effect on fully developed experimental neuropathic pain [15, 24] and have limited utility for clinical neuropathic pain management [10]. Therefore, this mechanism per se does not help explain well the effect induced by minocycline or PMIN on fully developed CCI-induced allodynia. On the other hand, inhibition of PGE2 helps explain better the previously shown suppressive effects induced by PMIN on formalin-induced licking behavior and carrageenan-induced edema in mice or by minocycline on these and other inflammatory responses [2, 4] because PGE2 plays more important roles in inflammatory or inflammation pain than in models of neuropathic pain [16].
Minocycline or PMIN inhibits also TLR2 and TLR4 signaling. TLRs are transmembrane pattern-recognition receptors, which respond to both exogenous and endogenous ligands. The exogenous ligands that are mainly recognized by TLR2 are lipoproteins from gram-positive bacteria, whereas TLR4 recognizes predominantly endotoxin (LPS) from gram-negative bacteria. More importantly, endogenous compounds such as beta-defensins, fibronectin, heat shock proteins and high mobility group box-1 – collectively known as danger-associated molecular patterns or simply “alarmins” – activate TLR receptors and are hypothesized to be the source of TLR activation under chronic pain conditions and neurodegenerative diseases. TLRs are expressed in a variety of immune cells and mediate neuroimmune interactions [23]. Studies using TLR2 knock-out mice show that the expression of this receptor is required for the development and/or persistence of mechanical allodynia, thermal hyperalgesia and experimental spontaneous pain due to peripheral nerve injury [17, 18], and this profile is associated with reduced spinal microglial activation and macrophage infiltration into dorsal root ganglia. More widely studied, the emerging roles played by TLR4 in the development and persistence of experimental pain have been shown using different genetic and pharmacological approaches, in different neuropathic pain models [23].
In conclusion, the present study shows antiallodynic and anti-inflammatory effects induced by PMIN, a novel minocycline derivative that lacks antibacterial activity and does not chelate Ca2+ or Mg2+, which might confer similar anti-inflammatory efficacy and greater safety over conventional tetracyclines. As far as we know, this is the first demonstration of antiallodynic effect induced by a CMT in a neuropathic pain model, thus representing an opportunity for drug development. Further studies are needed to better characterize the mechanisms underlying PMIN’s actions and its potential appropriateness for clinical development.
Highlights.
□12S-hydroxy-1,12-pyrazolinominocycline (PMIN) is a non-antibacterial tetracycline
□PMIN or minocycline partially reverses nerve injury-induced allodynia in mice
□PMIN or minocycline reduces LPS-induced PGE2 production by rat microglial cells
□PMIN or minocycline inhibits signaling via human TLR2 and TLR4
□PMIN might be a safe novel anti-inflammatory pain suppressive drug
Acknowledgement
The authors thank B. Guenter and U. Goetzinger-Berger for technical assistance. We acknowledge CAPES, (Brazil), CNPq (Brazil), FAPEMIG (Minas Gerais, Brazil) and Pró-Reitoria de Pesquisa (PRPq, UFMG) for support. Support was provided by NIH grants DA024044 and DE017882 to LRW.
Abbreviations
- AP-1
activator protein 1
- CCI
chronic constriction injury
- CMT
chemically modified tetracycline
- COX
cyclooxygenase
- DMSO
dimethylsulfoxide
- HEK
human embryonic kidney
- IL
interleukin
- LPS
lipopolysaccharide
- NF-κB
nuclear factor kappa B
- NSAIDs
non-steroidal anti-inflammatory drugs
- PG
prostaglandin
- PMIN
12S-hydroxy-1,12-pyrazolinominocycline
- SEAP
secreted alkaline phosphatase
- TLR
toll-like receptor
Footnotes
Conflict of interest statement Authors state no conflict of interest.
Author contributions All authors contributed to the study design. YK and SJ prepared and provided PMIN. LFSB, AMG, YZ, BCSF and RPF performed experiments. LFSB and RRM prepared the first draft of this manuscript. All authors revised and approved the final version of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Aronson AL. Pharmacotherapeutics of the Newer Tetracyclines. J. Am. Vet. Med. Assoc. 1980;176:1061–1068. [PubMed] [Google Scholar]
- [2].Bastos LFS, Angusti A, Vilaca MC, Merlo LA, Nascimento EB, Rocha LTS, Godin AM, Solano AGR, Jarussophon S, Nunan EA, Konishi Y, Coelho MM. A novel non-antibacterial, non-chelating hydroxypyrazoline derivative of minocycline inhibits nociception and oedema in mice. Br. J. Pharmacol. 2008;155:714–721. doi: 10.1038/bjp.2008.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bastos LFS, de Oliveira ACP, Schlachetzki JCM, Fiebich BL. Minocycline reduces prostaglandin E synthase expression and 8-isoprostane formation in LPS-activated primary rat microglia. Immunopharm. Immunot. 2011;33:576–580. doi: 10.3109/08923973.2010.544659. [DOI] [PubMed] [Google Scholar]
- [4].Bastos LFS, de Oliveira ACP, Watkins LR, Moraes MFD, Coelho MM. Tetracyclines and pain. Naunyn Schmiedebergs Arch. Pharmacol. 2012;385:225–241. doi: 10.1007/s00210-012-0727-1. [DOI] [PubMed] [Google Scholar]
- [5].Bastos LFS, Medeiros DC, Vieira RP, Watkins LR, Coelho MM, Moraes MFD. Intraneural dexamethasone applied simultaneously to rat sciatic nerve constriction delays the development of hyperalgesia and allodynia. Neurosci. Lett. 2012;510:20–23. doi: 10.1016/j.neulet.2011.12.062. [DOI] [PubMed] [Google Scholar]
- [6].Bastos LFS, Merlo LA, Rocha LTS, Coelho MM. Characterization of the antinociceptive and anti-inflammatory activities of doxycycline and minocycline in different experimental models. Eur. J. Pharmacol. 2007;576:171–179. doi: 10.1016/j.ejphar.2007.07.049. [DOI] [PubMed] [Google Scholar]
- [7].Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- [8].Daood MJ, Hoyson M, Watchko JF. Lipid peroxidation is not the primary mechanism of bilirubin-induced neurologic dysfunction in jaundiced Gunn rat pups. Pediatr. Res. 2012 doi: 10.1038/pr.2012.111. [DOI] [PubMed] [Google Scholar]
- [9].Decosterd I, Allchorne A, Woolf CJ. Differential analgesic sensitivity of two distinct neuropathic pain models. Anesth. Analg. 2004;99:457–463. doi: 10.1213/01.ANE.0000131967.69309.4F. [DOI] [PubMed] [Google Scholar]
- [10].Dray A. Neuropathic pain: emerging treatments. Br J Anaesth. 2008;101:48–58. doi: 10.1093/bja/aen107. [DOI] [PubMed] [Google Scholar]
- [11].Gaskill BN, Rohr SA, Pajor EA, Lucas JR, Garner JP. Some like it hot: Mouse temperature preferences in laboratory housing. Appl. Anim. Behav. Sci. 2009;116:279–285. [Google Scholar]
- [12].Golub LM, McNamara TF, D’Angelo G, Greenwald RA, Ramamurthy NS. A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J. Dent. Res. 1987;66:1310–1314. doi: 10.1177/00220345870660080401. [DOI] [PubMed] [Google Scholar]
- [13].Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br. J. Dermatol. 1996;134:693–695. doi: 10.1111/j.1365-2133.1996.tb06972.x. [DOI] [PubMed] [Google Scholar]
- [14].Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur. J. Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Inoue N, Ito S, Tajima K, Nogawa M, Takahashi Y, Sasagawa T, Nakamura A, Kyoi T. Etodolac attenuates mechanical allodynia in a mouse model of neuropathic pain. J. Pharmacol. Sci. 2009;109:600–605. doi: 10.1254/jphs.08287fp. [DOI] [PubMed] [Google Scholar]
- [16].Kawabata A. Prostaglandin E2 and pain - an update. Biol. Pharm. Bull. 2011;34:1170–1173. doi: 10.1248/bpb.34.1170. [DOI] [PubMed] [Google Scholar]
- [17].Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- [18].Kim D, You B, Lim H, Lee SJ. Toll-like receptor 2 contributes to chemokine gene expression and macrophage infiltration in the dorsal root ganglia after peripheral nerve injury. Mol. Pain. 2011;7:74. doi: 10.1186/1744-8069-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- [20].Lertvorachon J, Kim JP, Soldatov DV, Boyd J, Roman G, Cho SJ, Popek T, Jung YS, Lau PC, Konishi Y. 1,12-substituted tetracyclines as antioxidant agents. Bioorg. Med. Chem. 2005;13:4627–4637. doi: 10.1016/j.bmc.2005.04.032. [DOI] [PubMed] [Google Scholar]
- [21].Mei XP, Xu H, Xie C, Ren J, Zhou Y, Zhang H, Xu LX. Post-injury administration of minocycline: an effective treatment for nerve-injury induced neuropathic pain. Neurosci. Res. 2011;70:305–312. doi: 10.1016/j.neures.2011.03.012. [DOI] [PubMed] [Google Scholar]
- [22].Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- [23].Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp. Neurol. 2012;234:316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Padi SS, Kulkarni SK. Differential effects of naproxen and rofecoxib on the development of hypersensitivity following nerve injury in rats. Pharmacol. Biochem. Behav. 2004;79:349–358. doi: 10.1016/j.pbb.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [25].Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- [26].White JP, Cantor CR. Role of magnesium in the binding of tetracycline to Escherichia coli ribosomes. J. Mol. Biol. 1971;58:397–400. doi: 10.1016/0022-2836(71)90255-5. [DOI] [PubMed] [Google Scholar]
- [27].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]