Abstract
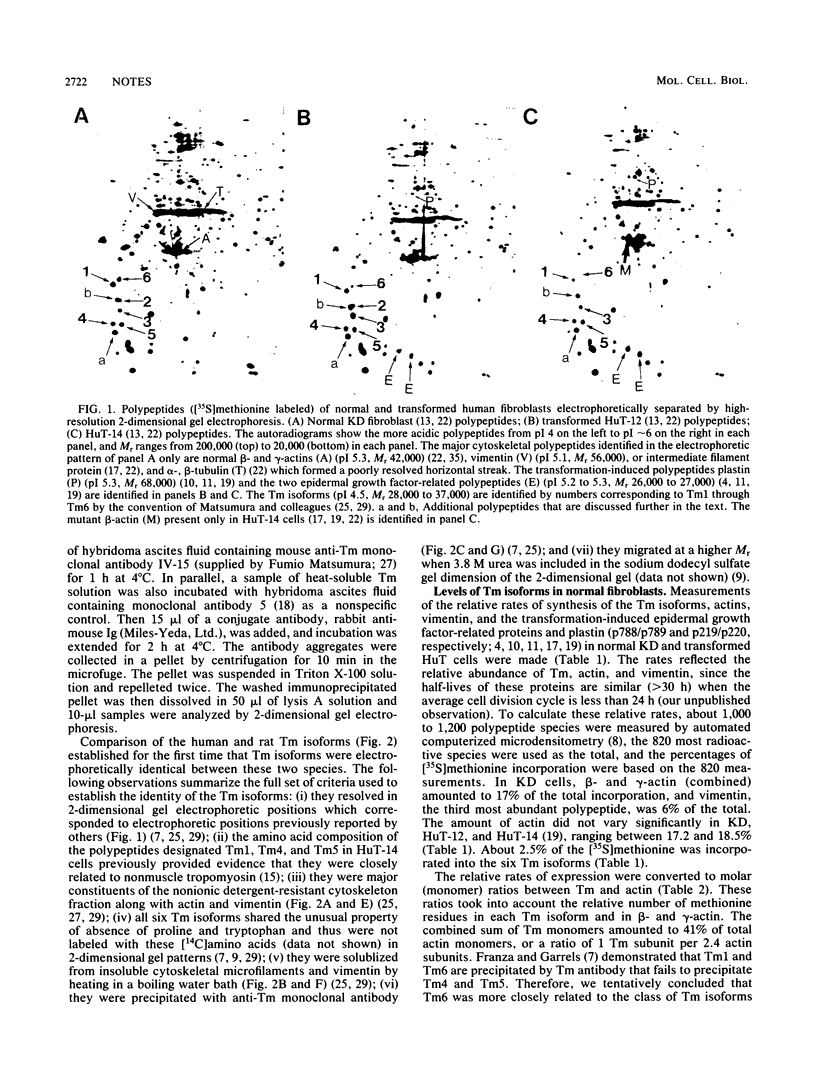
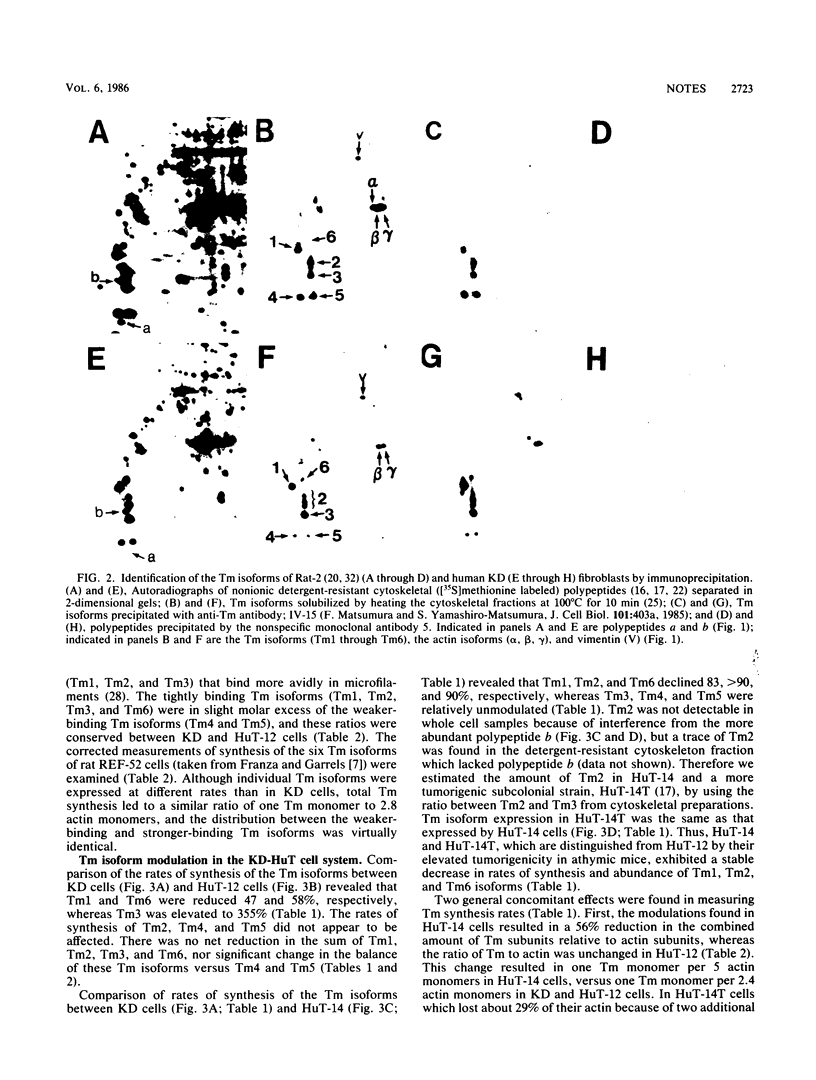
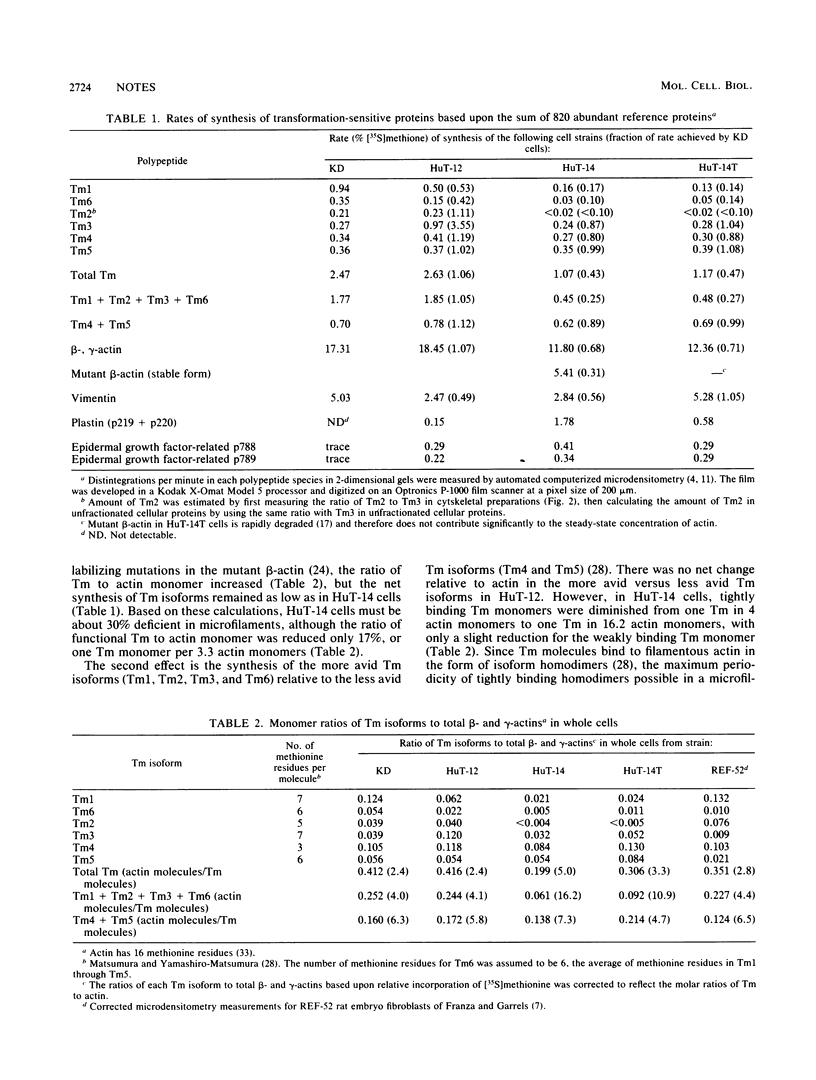
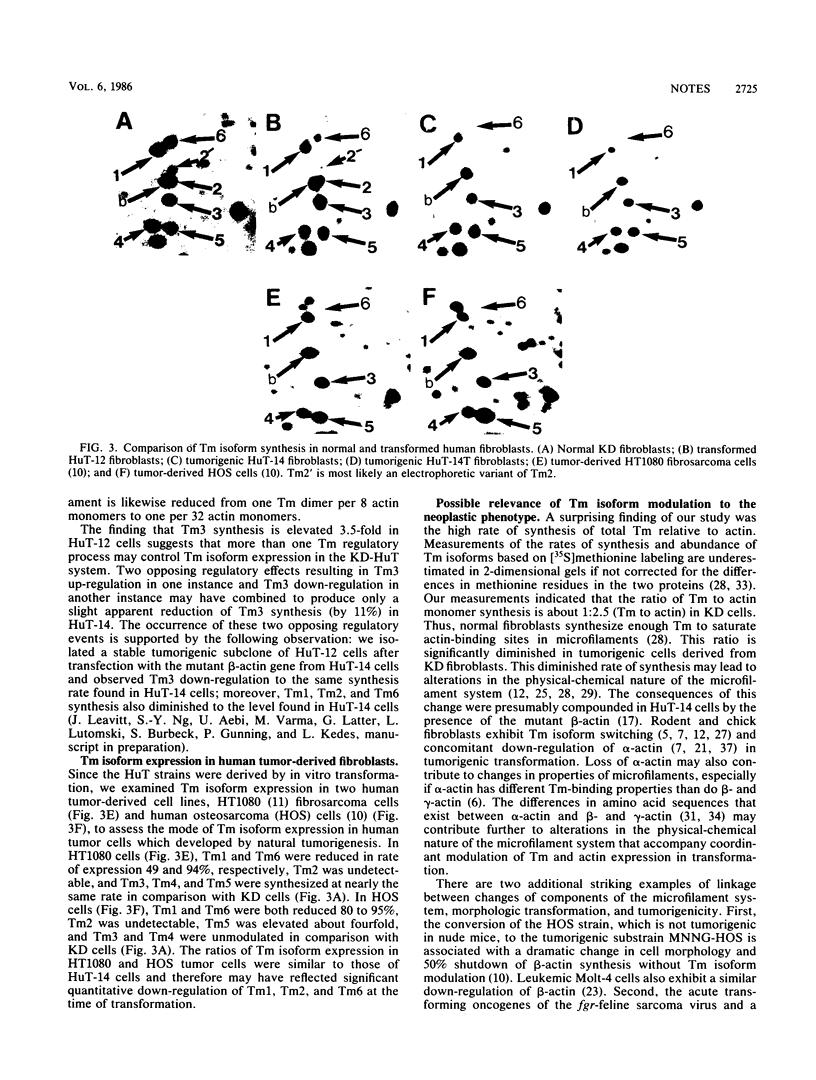
We identified six tropomyosin (Tm) isoforms in diploid human fibroblasts. We used computerized microdensitometry of 2-dimensional protein profiles to measure the relative rates of synthesis and abundance of the individual Tm isoforms and actin, the two major structural constituents of microfilaments. In carcinogen-transformed human fibroblasts (HuT-14), the rates of synthesis of three Tm isoforms (Tm1, Tm2, and Tm6) were greatly decreased relative to normal diploid parental fibroblasts and to actin. In contrast, related nontumorigenic HuT fibroblasts which are "immortalized" and anchorage independent exhibited both slight down-regulation of Tm1 and Tm6 and 3.5-fold up-regulation of Tm3. Thus, Tm isoform switching from the predominance of the larger more avid Tm isoforms (Tm1, Tm2, Tm3, and Tm6) to the smaller, less avid Tm isoforms (Tm4 and Tm5) in microfilaments was a transformation-induced change correlated with tumorigenicity in human fibroblasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. L., Taylor J., Scandora A. E., Coulter B. P., Anderson N. G. The TYCHO system for computer analysis of two-dimensional gel electrophoresis patterns. Clin Chem. 1981 Nov;27(11):1807–1820. [PubMed] [Google Scholar]
- Bartholdi M., Travis G., Cram L. S., Porreca P., Leavitt J. Flow karyology of neoplastic human fibroblasts. Ann N Y Acad Sci. 1986;468:339–349. doi: 10.1111/j.1749-6632.1986.tb42051.x. [DOI] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Bellatin J., Larsen P. M., Arevalo J., Celis J. E. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981 Dec;136(2):311–319. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Burbeck S., Latter G., Metz E., Leavitt J. Neoplastic human fibroblast proteins are related to epidermal growth factor precursor. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5360–5363. doi: 10.1073/pnas.81.17.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saleh S. C., Thieret R., Johnson P., Potter J. D. Modification of Lys-237 on actin by 2,4-pentanedione. Alteration of the interaction of actin with tropomyosin. J Biol Chem. 1984 Sep 10;259(17):11014–11021. [PubMed] [Google Scholar]
- Garrels J. I. Changes in protein synthesis during myogenesis in a clonal cell line. Dev Biol. 1979 Nov;73(1):134–152. doi: 10.1016/0012-1606(79)90143-x. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Goldstein D., Djeu J., Latter G., Burbeck S., Leavitt J. Abundant synthesis of the transformation-induced protein of neoplastic human fibroblasts, plastin, in normal lymphocytes. Cancer Res. 1985 Nov;45(11 Pt 2):5643–5647. [PubMed] [Google Scholar]
- Goldstein D., Leavitt J. Expression of neoplasia-related proteins of chemically transformed HuT fibroblasts in human osteosarcoma HOS fibroblasts and modulation of actin expression upon elevation of tumorigenic potential. Cancer Res. 1985 Jul;45(7):3256–3261. [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga T., Leavitt J., Hamada H. A mutation in actin associated with neoplastic transformation. Fed Proc. 1984 May 15;43(8):2275–2279. [PubMed] [Google Scholar]
- Kakunaga T. Neoplastic transformation of human diploid fibroblast cells by chemical carcinogens. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1334–1338. doi: 10.1073/pnas.75.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latter G. I., Burbeck S., Fleming J., Leavitt J. Identification of polypeptides on two-dimensional electrophoresis gels by amino acid composition. Clin Chem. 1984 Dec;30(12 Pt 1):1925–1932. [PubMed] [Google Scholar]
- Leavitt J., Bushar G., Kakunaga T., Hamada H., Hirakawa T., Goldman D., Merril C. Variations in expression of mutant beta actin accompanying incremental increases in human fibroblast tumorigenicity. Cell. 1982 Feb;28(2):259–268. doi: 10.1016/0092-8674(82)90344-0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Goldman D., Merril C., Kakunaga T. Changes in gene expression accompanying chemically-induced malignant transformation of human fibroblasts. Carcinogenesis. 1982;3(1):61–70. doi: 10.1093/carcin/3.1.61. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Kedes L., Jariwalla R. Smooth muscle alpha-action is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. 1985 Aug 29-Sep 4Nature. 316(6031):840–842. doi: 10.1038/316840a0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Kakunaga T. Expression of a variant form of actin and additional polypeptide changes following chemical-induced in vitro neoplastic transformation of human fibroblasts. J Biol Chem. 1980 Feb 25;255(4):1650–1661. [PubMed] [Google Scholar]
- Leavitt J., Leavitt A., Attallah A. M. Dissimilar modes of expression of beta- and gamma-actin in normal and leukemic human T lymphocytes. J Biol Chem. 1980 Jun 10;255(11):4984–4987. [PubMed] [Google Scholar]
- Leavitt J. Tumorigenic potential of human fibroblasts as a function of ability to express a novel form of influenza A nucleocapsid protein. Carcinogenesis. 1983 Oct;4(10):1229–1237. doi: 10.1093/carcin/4.10.1229. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Ng S. Y., Gunning P., Kedes L., Leavitt J. Identification and order of sequential mutations in beta-actin genes isolated from increasingly tumorigenic human fibroblast strains. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6995–6999. doi: 10.1073/pnas.82.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S., Lin J. J. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983 May 25;258(10):6636–6644. [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S. Purification and characterization of multiple isoforms of tropomyosin from rat cultured cells. J Biol Chem. 1985 Nov 5;260(25):13851–13859. [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Stone D., Smillie L. B. The amino acid sequence of rabbit skeletal alpha-tropomyosin. The NH2-terminal half and complete sequence. J Biol Chem. 1978 Feb 25;253(4):1137–1148. [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Leavitt J., Kakunaga T., Weber K. Coexpression of a mutant beta-actin and the two normal beta- and gamma-cytoplasmic actins in a stably transformed human cell line. Cell. 1980 Dec;22(3):893–899. doi: 10.1016/0092-8674(80)90566-8. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]






