Abstract
Bmp4 expression is tightly regulated during embryonic tooth development, with early expression in the dental epithelial placode leading to later expression in the dental mesenchyme. Msx1 is among several transcription factors that are induced by epithelial Bmp4 and that, in turn, are necessary for the induction and maintenance of dental mesenchymal Bmp4 expression. Thus, Msx1-/- teeth arrest at early bud stage and show loss of Bmp4 expression in the mesenchyme. Ectopic expression of Bmp4 rescues this bud stage arrest. We have identified Tbx2 expression in the dental mesenchyme at bud stage and show that this can be induced by epithelial Bmp4. We also show that endogenous Tbx2 and Msx1 can physically interact in mouse C3H10T1/2 cells. In order to ascertain a functional relationship between Msx1 and Tbx2 in tooth development, we crossed Tbx2 and Msx1 mutant mice. Our data show that the bud stage tooth arrest in Msx1-/- mice is partially rescued in Msx1-/-;Tbx2+/- compound mutants. This rescue is accompanied by formation of the enamel knot (EK) and by restoration of mesenchymal Bmp4 expression. Finally, knockdown of Tbx2 in C3H10T1/2 cells results in an increase in Bmp4 expression. Together, these data identify a novel role for Tbx2 in tooth development and suggest that, following their induction by epithelial Bmp4, Msx1 and Tbx2 in turn antagonistically regulate odontogenic activity that leads to EK formation and to mesenchymal Bmp4 expression at the key bud-to-cap stage transition.
Keywords: Bmp4, Msx1, Tbx2, Protein-protein interaction, Tooth
INTRODUCTION
The formation of a bud-like structure from an epithelial placode is a common occurrence in the development of many organs, including the mammary gland, lung, kidney, hair and tooth. Tooth development begins with induction and thickening of the odontogenic ectoderm to form the dental placode at ∼E12.5. This initiates an epithelial-mesenchymal signaling cascade that results in invagination of the dental epithelium to form a bud-like structure and condensation of the underlying neural crest-derived mesenchyme by E13.5 (bud stage). Subsequently, by E14.5, the bud-like structure flattens and the epithelial cells at its center condense to form the primary enamel knot (EK), which serves as a signaling center to initiate tooth morphogenesis and patterning. Meanwhile, the epithelium of the bud continues to proliferate outward and folds to surround the dental papilla mesenchyme (cap stage) (Thesleff and Mikkola, 2002; Bei, 2009).
The bud-to-cap stage transition appears to be a critical checkpoint in dental development. Mouse mutants for several transcription factors necessary for tooth development, including Msx1, Pax9, Lef1 and Runx2, manifest bud stage arrest (Satokata and Maas, 1994; van Genderen et al., 1994; Peters et al., 1998; D’Souza et al., 1999). Analysis of these mouse mutants has revealed that these transcription factors induce and maintain Bmp4 signaling, which is required for the bud-to-cap stage transition.
Bud stage arrest has been extensively studied in Msx1-/- mice, in which mesenchymal Bmp4 expression is lost entirely (Chen et al., 1996). We know that the addition of exogenous Bmp4 can rescue the Msx1-/- bud stage arrest (Bei et al., 2000; Zhao et al., 2000). However, it is still unclear how Msx1 activates or maintains Bmp4 expression. Several transcription factors, including Pax9 (Ogawa et al., 2006), Osr2 (Zhou et al., 2011) and Barx1 (Miletich et al., 2011), have been shown to regulate Bmp4 expression coordinately with Msx1. Since Msx1 functions primarily as a repressor (Catron et al., 1995; Zhang et al., 1996), it is generally believed that Msx1 activates Bmp4 indirectly. However, recent studies indicate that certain Msx1 protein-protein interactions can result in synergistic activation of target promoters directly (Ogawa et al., 2006; Zhao et al., 2013). Furthermore, O’Connell et al. (O’Connell et al., 2012) have shown that epithelial Bmp4 can diffuse to the mesenchyme and signal back to the epithelium. Consistent with this, Bmpr1a receptor knockout in the dental epithelium results in bud stage arrest (Andl et al., 2004; Liu et al., 2005; O’Connell et al., 2012). Recently, Jia et al. (Jia et al., 2013) have shown that mesenchymal Bmp4 is not required for upper molar development. However, the EK and epithelial Bmp4 are still present in these upper molars, indicating that epithelial Bmp4 diffusion and signaling are still maintained in the mesenchyme. By contrast, the lower molars lacking mesenchymal Bmp4 expression do not form an EK or show epithelial Bmp4 expression, and fail to progress beyond the bud stage. Thus, Bmp4 signaling is governed by a complex regulatory mechanism that is distinct in upper and lower molars during the bud-to-cap stage transition.
The T-box family of transcription factors has been shown to play crucial roles in the morphogenesis of many tissues, primarily the heart, bone and mammary gland (Papaioannou and Silver, 1998; Showell et al., 2004; Naiche et al., 2005). Mutations in several TBX genes have been identified in human syndromes affecting these tissues, including TBX1 mutations in DiGeorge syndrome, TBX3 mutations in ulnar-mammary syndrome (UMS) and TBX5 mutations in Holt-Oram syndrome (Packham and Brook, 2003; Naiche et al., 2005). Although some UMS patients with TBX3 mutations manifest dental abnormalities (Bamshad et al., 1999), a role for TBX genes in dental development has remained uncharacterized. Recently, a role for Tbx1 has been described in the maintenance of the epithelial stem cell compartment of the mouse incisor (Catón et al., 2009).
Here, we report Tbx2 as the first T-box transcription factor to be identified in the dental mesenchyme at bud stage. We further show that Tbx2 can physically and genetically interact with Msx1. Importantly, we show that reduction in Tbx2 gene dosage partially rescues Msx1-/- tooth arrest by restoring the EK and mesenchymal Bmp4 expression.
MATERIALS AND METHODS
Embryos and genotyping
Msx1+/- and Tbx2+/- mice were maintained on a BALB/c (Satokata and Maas, 1994) and a mixed C57BL/6×129/SvEv × ICR background (Harrelson et al., 2004), respectively. Msx1-/-;Tbx2-/- (n=0, lethal), Msx1-Tbx2 compound mutants (n=24); embryos were collected from intercrossing Msx1+/-;Tbx2+/- mice at E14.5 and E15.5. The day of plug discovery was designated E0.5. Genotyping of Msx1 and Tbx2 genes was performed as previously described (Satokata and Maas, 1994; Harrelson et al., 2004).
Histology, in situ hybridization and immunohistochemistry
For histological analysis, tissues were fixed in 4% paraformaldehyde, dehydrated in ethanol, and embedded in paraffin. Serial sections at 7 μm were stained with Hematoxylin/Eosin. Murine Tbx2 and Bmp4 cDNA fragments were used to generate antisense probes. The RNA probes were digoxigenin (DIG) labeled with DIG-UTP (Roche) using T7 RNA polymerase. In situ hybridization was performed as previously described (Bei and Maas, 1998). Immunohistochemistry was performed on 6 μm paraffin sections using a rabbit anti-Tbx2 antibody (Sigma, HPA008586) at 1/100 dilution according to the manufacturer’s protocol.
Bead implantation assay
Affi-Gel blue agarose beads (100-200 mesh, 75-150 mm diameter; Bio-Rad) and/or heparin acrylic beads (white in color; Sigma) were incubated with 100 ng/ml recombinant human BMP4 (Genetics Institute, Cambridge, MA, USA) at 37°C for 30 minutes. Control beads were soaked with 100 ng/ml BSA under the same conditions. Wild-type and Msx1-/- tooth rudiments were collected from E13.5 embryos. The dental epithelium was then removed from the rudiments following dispase treatment. Protein-soaked beads were washed in PBS and placed on top of the mesenchyme. All explants were cultured on filters, supported by metal grids in Dulbecco’s minimal essential medium (D-MEM) supplemented with 10% fetal bovine serum (FBS) and 10% chick embryo extract at 37°C for 48 hours. After culture, the explants were processed for standard in situ hybridization using the Tbx2 probe.
GST-pulldown assay
GST-Msx1 protein was prepared from bacterial culture carrying pGEX4T-Msx1 plasmid. 35S-labeled Tbx2 protein was prepared using an in vitro transcription and translation kit (Promega). GST-Msx1 or GST protein alone was immobilized on glutathione Sepharose beads (Amersham Pharmacia Biotech) and incubated with 35S-labeled Tbx2 protein. Following incubation, the beads were centrifuged and washed three times with buffer comprising 50 mM Tris (pH 7.0) and 140 mM NaCl. The beads were resuspended in 5× SDS sample buffer to a final 1× concentration, boiled for 5 minutes, and loaded on a 12.5% SDS-polyacrylamide gel for electrophoresis. Proteins were transferred to Immobilon-P polyvinylidene difluoride filters (Millipore) and exposed to autoradiography film to detect the bound 35S-labeled Tbx2 protein.
Cell culture and co-immunoprecipitation assays
C3H10T1/2 cells (ATCC) were cultured in high-glucose Dulbecco’s modified Eagle medium supplemented with 10% (v/v) FBS, 10 units/ml penicillin and 10 μg/ml (Invitrogen) under 5% CO2. Approximately 1.5×106 C3H10T1/2 cells were cultured in 100 mm dishes for 24 hours. Cells were lysed in 500 μl modified RIPA buffer (1% SDS, 20 mM Tris-HCl pH 8.0, 1% NP40, 131 mM NaCl, 10% glycerol, 2 mM EDTA) containing Protease Inhibitor Cocktail (Roche). The lysate was then pre-cleared for 30 minutes with protein A-agarose beads (Pierce) prior to incubation with anti-Msx1 antibody (Sigma, M0944) overnight. Then, protein A-agarose beads were added to the lysate, incubated for 2 hours, washed three times with lysis buffer and resuspended in SDS sample buffer. Samples were loaded onto an 11% SDS-polyacrylamide gel for electrophoresis. Western blots were performed with the anti-Tbx2 antibody (Sigma) to detect immunoprecipitated Tbx2 protein using ECL reagents (Pierce) according to the manufacturer’s protocol.
Lentiviral knockdown and qPCR analysis
Lentiviral particles expressing shRNA against Tbx2 were purchased from Sigma (TRCN0000084471). C3H10T1/2 cells were grown in 24-well plates and transduced at a multiplicity of infection of 5 for 24 hours. RNA was collected from cells after three passages following transduction. Quantitative (q) PCR analysis was performed using a Roche LightCycler 480 following the manufacturer’s instructions. Primers used were (5′-3′, forward and reverse): Tbx2, CAACACTGTGGGGGTGGCCTC and CCTGGGATGCTTTCCGAAGGAACAC; Msx1, ACCCATGATCCAGGGCTGTCTCG and CCGAGTGGCAAAGAAGTCATAGCAGC; Bmp4, TTGAGTACCCGGAGCGTCCCG and CAGAGCTCTCACTGGTCCCTGGG.
RESULTS AND DISCUSSION
Tbx2 is expressed in the dental mesenchyme and can be induced by Bmp4
Tbx2 expression was detected in the dental mesenchyme in both upper and lower molars from E12.5 to E16.5 using immunohistochemistry (Fig. 1A-D). At E13.5 bud stage, the expression domain largely overlaps with that known for Msx1 (Fig. 1B). There appears to be reduced Tbx2 expression on the lingual aspect of the developing upper and lower molars (Fig. 1, arrows). In Msx1-/- samples, Tbx2 expression is maintained (Fig. 1E,F), showing that Msx1 is not required for Tbx2 expression in bud stage dental mesenchyme. The expression domain of Tbx2 appears dispersed in the Msx1-/- arrested tooth bud towards the lingual side (Fig. 1F), which might indicate an Msx1-dependent suppression of Tbx2 expression ligually, or it could be a consequence of loss of condensed dental mesenchyme surrounding the mutant bud. Tbx2 is the first T-box factor to be identified in the developing dental mesenchyme at bud stage, with an expression pattern similar to that of Msx1. Previously, only Tbx1 had been shown to function in the mouse lower incisor epithelium, where it maintains the incisor stem cell niche, called the cervical loop (Catón et al., 2009). The persistence of Tbx2 expression in the Msx1-/- arrested tooth buds shows that Tbx2 expression is not dependent on that of Msx1.
Fig. 1.
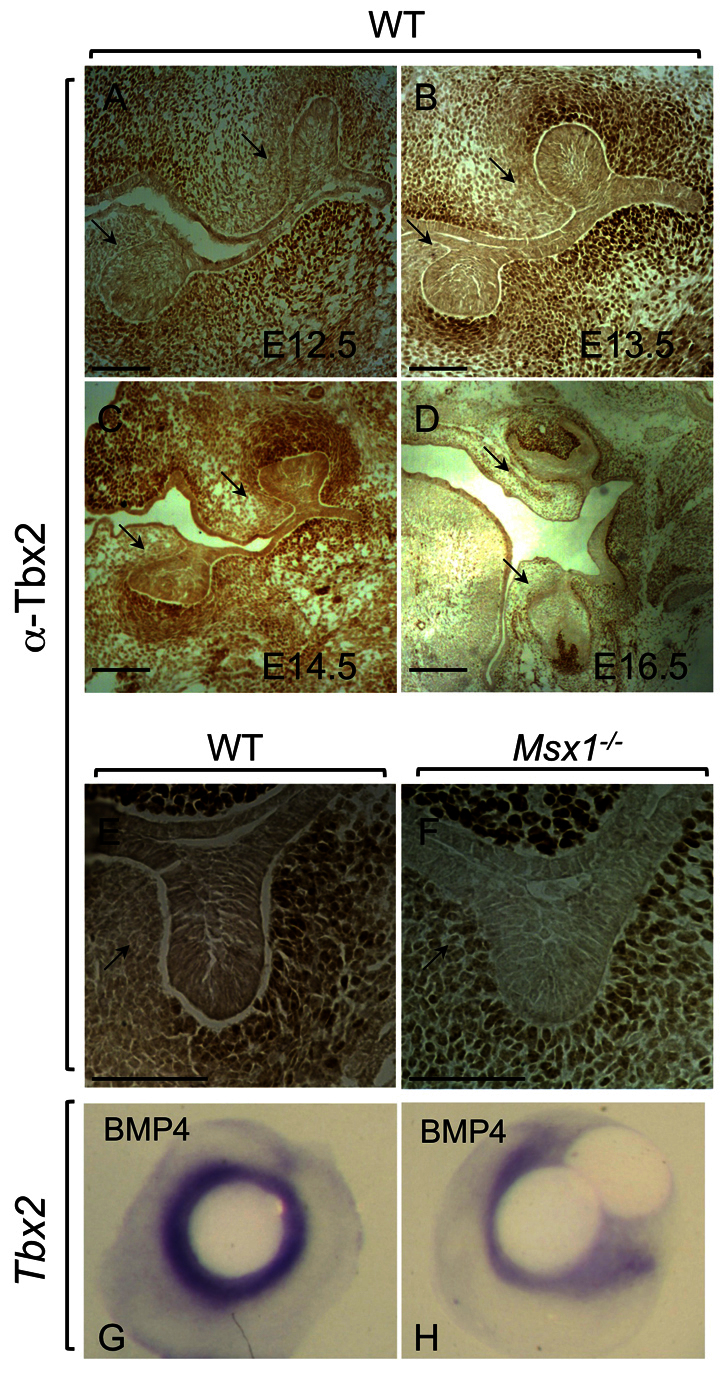
Tbx2 expression in mouse dental mesenchyme can be induced by Bmp4. (A-D) Immunohistochemistry of E12.5 to E16.5 coronal sections using an anti-Tbx2 antibody shows expression in wild-type (WT) dental mesenchyme of the upper and lower molars. (E,F) Tbx2 expression in E13.5 wild-type (E) and Msx1-/- (F) molar tooth buds. (G,H) Bmp4-soaked bead implantation results in expression of Tbx2 both in wild-type explant (n=15/15) (G) and in Msx1-/- explant (n=4/5) (H) dental mesenchyme. Arrows indicate the expression domain of Tbx2 at the lingual side. Scale bars: 0.1 mm.
Epithelial Bmp4 induces mesenchymal Msx1 expression, which then leads to the induction and maintenance of mesenchymal Bmp4 expression, sometimes referred to as the Bmp4-Msx1-Bmp4 pathway (Bei, 2009). We determined whether epithelial Bmp4 could induce mesenchymal Tbx2 expression, similarly to that of Msx1. Indeed, bead implantation experiments using Bmp4-soaked beads resulted in activation of Tbx2 expression in the dental mesenchyme (Fig. 1G). This activation was also present in Msx1-/- tissue (Fig. 1H), showing that Msx1 is not required to mediate the Bmp4 effect. Analysis of several other T-box factors also indicates activation downstream of initial Bmp signaling (Yamada et al., 2000; Naiche et al., 2005; Behesti et al., 2006; Abrahams et al., 2010). Thus, our data are consistent with the model in which Bmp4 from the dental epithelial placode activates Tbx2, similarly to Msx1, in the surrounding dental mesenchyme.
Tbx2 physically interacts with Msx1
To determine whether co-expression of Msx1 and Tbx2 also results in a physical interaction between the two proteins, we performed GST-pulldown and co-immunoprecipitation assays (Fig. 2). GST-pulldown was carried out using GST-tagged Msx1 protein in the presence of 35S-labeled Tbx2 (Fig. 2A). In the presence of GST-Msx1, 35S-Tbx2 is retained, demonstrating a physical interaction in vitro. As a control, incubation with GST protein or beads alone did not retain any 35S-Tbx2.
Fig. 2.
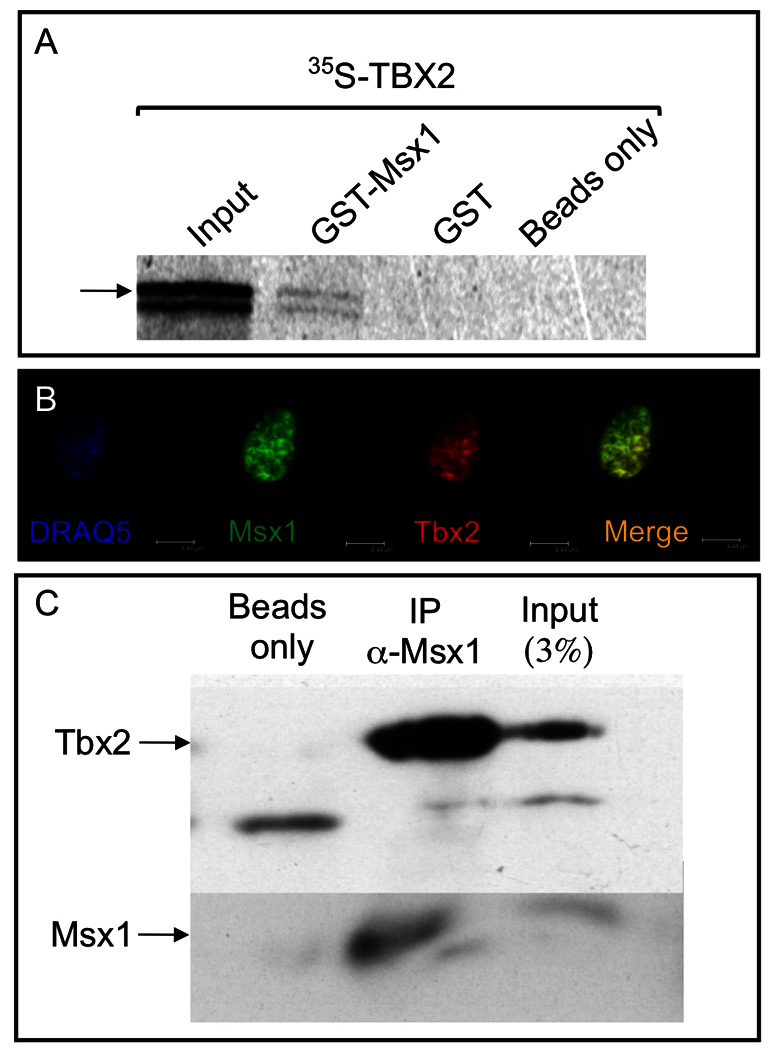
Msx1 and Tbx2 proteins can physically interact in vitro. (A) GST-Msx1 was able to pull down 35S-labeled Tbx2 (arrow; lane 2 from left). As a control, the GST moiety (lane 3) or beads alone (lane 4) were not able to pull down any Tbx2. (B) Msx1 and Tbx2 are endogenously expressed in C3H10T1/2 cells. The nucleus was stained using DRAQ5. (C) Co-immunoprecipitation was carried out in C3H10T1/2 cells as indicated. Tbx2 and Msx1 were assayed following immunoprecipitation with anti-Msx1 antibody. Whereas protein A beads alone did not show any Tbx2 (lane 1 from left), in the presence of anti-Msx1 antibody there is robust presence of Tbx2 (lane 2). As a control, Tbx2 and Msx1 are detected in the input sample (lane 3).
The co-immunoprecipitation analysis utilized C3H10T1/2 cells, a mammalian mesenchymal cell line that is appropriate for Msx1 protein-protein interaction studies (Miletich et al., 2011; Zhao et al., 2013). C3H10T1/2 cells behave as early odontogenic precursor cells and endogenously express most of the early odontogenic proteins including Msx1 and Tbx2 (Fig. 2B). Immunoprecipitation was performed using anti-Msx1 antibody against endogenous Msx1 (Fig. 2C). Western blot analysis was carried out with anti-Tbx2 antibody. A Tbx2 band is visible in the input and in anti-Msx1 immunoprecipitated samples, demonstrating interaction between the endogenous proteins. As a control, beads alone did not show any retention of Tbx2 signal. These data confirm a physical interaction between Msx1 and Tbx2 proteins in vitro and in C3H10T1/2 odontogenic precursor cells. Combined with the co-expression of these two transcription factors in the dental mesenchyme, these data are consistent with a co-regulatory role for the two proteins during the bud-to-cap stage transition.
Tbx2 gene dosage reduction partially rescues Msx1-/- bud stage arrest
Since Tbx2 is expressed in the dental mesenchyme at bud stage and physically interacts with Msx1, we examined whether they also interact genetically. We crossed Tbx2 and Msx1 mouse mutants, and did not note any defects or reduction in Mendelian ratios in Tbx2+/-;Msx1+/- double heterozygotes (data not shown). We intercrossed these double heterozygotes to generate compound mutants and analyzed them at E14.5 and E15.5. We did not observe any Tbx2-/- mice in our crosses, which is consistent with previous analyses in which Tbx2-/- embryos showed lethality by E12.5 (Harrelson et al., 2004; Zirzow et al., 2009). Tbx2 null mice on a severe background (Harrelson et al., 2004) are known to die during mid-gestation, between ∼E10.5 and E12.5, prior to bud stage, and so their role at bud stage cannot be directly established in these mutants. Recently, Zirzow et al. (Zirzow et al., 2009) have described a genetic interaction between Tbx2 and Tbx3 mutant mice, which results in a cleft palate phenotype with variable penetrance depending on the background used. They were able to analyze E14.5 Tbx2-/- embryos for palate defects; however, they did not report any dental anomalies (Zirzow et al., 2009).
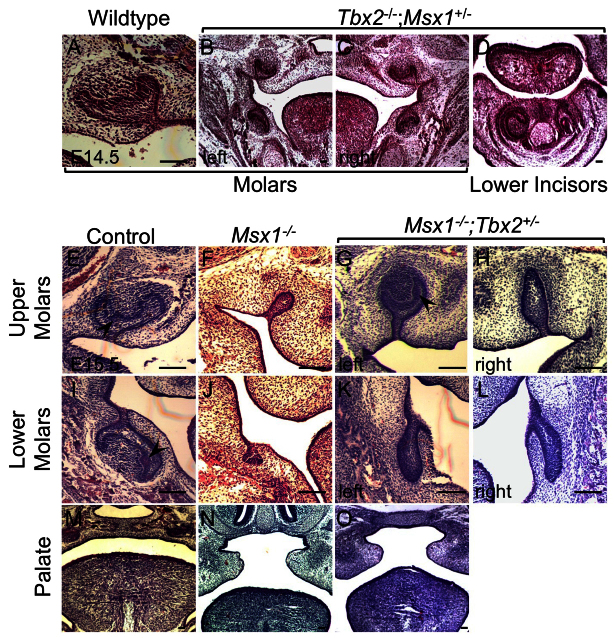
In our analysis, on a mixed severe background, we were not able to collect any Tbx2-/- embryos at E14.5 or E15.5, as expected. However, we were able to analyze several Tbx2-/-;Msx+/- compound mutants upon intercrossing Tbx2+/-;Msx1+/- double heterozygotes. These compound mutants did not show any obvious anomalies in development of the molars (compare Fig. 3A with 3B,C) or incisors (Fig. 3D). As a control we confirmed that even on our mixed background at E13.5, compared with wild type (Fig. 3E,I), the Msx1-/- mice showed the expected bud stage arrest phenotype (Fig. 3F,J). However, the formal possibility remains that reduction in Msx1 gene dosage partially ameliorates a potentially severe Tbx2 null phenotype (see below).
Fig. 3.
Msx1-/-;Tbx2+/- compound mutants show partially rescued molars. (A-D) Coronal section of a wild-type molar at E14.5 is shown as reference (A). Tbx2-/-;Msx1+/- compound mutants at E14.5 show apparently normal molars (B,C) and incisors (D). (E-O) Msx1+/-;Tbx2+/- mice were intercrossed and the progeny analyzed at E15.5. Compared with wild-type upper (E) and lower (I) molars, which have progressed to cap stage, the Msx1-/- teeth remain arrested at bud stage (F,J). Surprisingly, the Msx1-/-;Tbx2+/- embryos showed rescue of an upper molar to cap stage (G), whereas the contralateral molar showed an enlarged bud (H). Arrowheads indicate enamel knots (E,G,I). The lower molars, by contrast, show only a grossly enlarged bud (K,L). This rescue seems to be specific to the tooth because the palate (M), which fails to develop and fuse in Msx1-/- embryos (N), is not rescued (O). Scale bars: 0.1 mm.
Next, we assessed any effect of Tbx2 gene dosage reduction on the Msx1-/- phenotype. Indeed, in E15.5 Msx1-/-;Tbx2+/- embryos we noted a partial rescue of the bud stage tooth arrest (Fig. 3G,H,K,L). The upper molars in these mice showed development well beyond bud stage, frequently with progression to form a cap-like structure resembling that of the wild type (compare Fig. 3E with 3G). The cap-like structure (Fig. 3G) was seen in five out of six Msx1-/-;Tbx2+/- upper molars examined (83%), whereas the remaining one upper molar showed enlargement (Fig. 3H). In anterior and posterior sections, the rescued molar still appeared arrested at bud stage (supplementary material Fig. S1A,E), with the rescue to the cap-like structure lying mostly in the middle third of the molar (supplementary material Fig. S1B-D). Importantly, the rescued upper molar showed an EK (Fig. 3G; supplementary material Fig. S2). Surprisingly, the lower molars only showed enlargement without subsequent progression to cap stage, and appeared as elongated buds (Fig. 3K,L). This enlargement of buds in the Msx1-/-;Tbx2+/- lower molars (Fig. 3K,L) was seen in four out of six samples examined (66%); the remaining two lower molars appeared to have a bud stage arrest similar to that of Msx1-/- lower molars (Fig. 3J).
These data not only confirm a genetic interaction between Msx1 and Tbx2, but also suggest that they play antagonistic roles at bud stage. In addition, the Msx1-/- upper molars appear more sensitive than lower molars to Tbx2 gene dosage reduction. This differential response is likely to be due to the presence of distinct genetic and signaling programs for upper and lower molars. For example, Runx2 has been reported to affect the lower molars more than the upper molars (Wang et al., 2005). Similarly, Jia et al. (Jia et al., 2013) show that loss of mesenchymal Bmp4 expression leads to bud stage arrest of lower molars, but not upper molars. There is also a possibility that other Tbx factors play a role in lower molars. In contrast to the molars, the Msx1-/- cleft palate phenotype (compare Fig. 3M with 3N) remained unaffected in Msx1-/-;Tbx2+/- embryos (Fig. 3O), underscoring the specificity of the genetic interaction.
Mesenchymal Bmp4 expression is restored in Msx1-/-;Tbx2+/- rescued molars
Mesenchymal Bmp4 expression is an important marker for the bud-to-cap stage transition (Chen et al., 1996; Bei et al., 2000). Studies show that bud stage arrest in Msx1-/- and Pax9-/- mice is accompanied by loss of mesenchymal Bmp4 expression (Peters and Balling, 1999; Bei et al., 2000). We determined whether the partial rescue in Msx1-/-;Tbx2+/- compound mutants showed restoration of mesenchymal Bmp4 expression (Fig. 4A). Indeed, similar to in situ analysis in wild type at E15.5 (Fig. 4A, left), mesenchymal Bmp4 expression was present in the partially rescued upper molar (Fig. 4A, right). Interestingly, the lower molar did not show any significant mesenchymal Bmp4 expression (supplementary material Fig. S3), further supporting distinct genetic regulation of Bmp4 expression in upper versus lower molars. Restoration of Bmp4 expression in the upper molar is consistent with the rescue phenotype and implies that Msx1 and Tbx2 antagonistically regulate Bmp4 expression during the bud-to-cap stage transition.
Fig. 4.
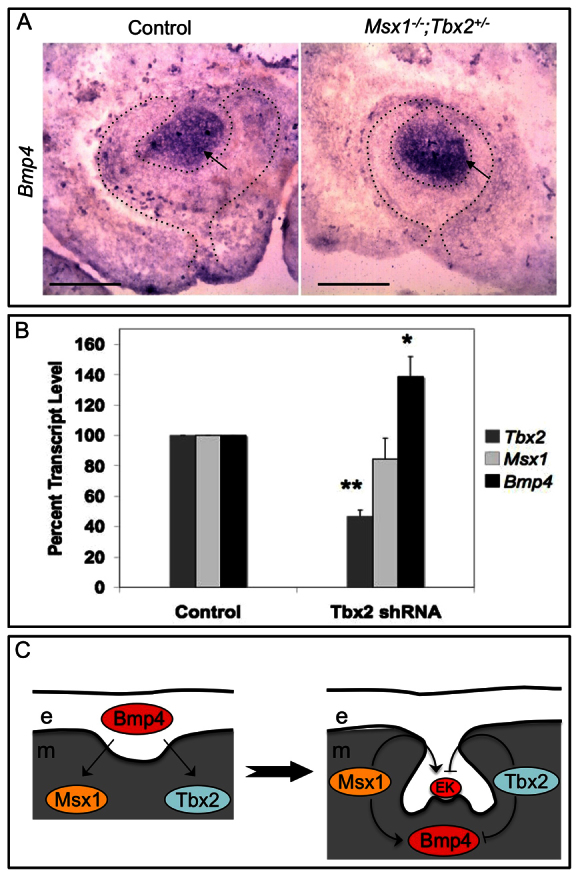
Tbx2 negatively regulates Bmp4 expression in molar mesenchyme. (A) The partially rescued Msx1-/-;Tbx2+/- upper molars were analyzed for Bmp4 expression by in situ hybridization. Bmp4 expression is detected in the mesenchyme of the rescued molar (right panel). (B) C3H10T1/2 cells were transduced with lentiviral particles containing either a control shRNA or a Tbx2 shRNA. qPCR for Tbx2, Msx1 and Bmp4 transcripts was performed on RNA isolated from four separate pellets each from two independent transduction experiments. Tbx2 expression is downregulated as expected (**P<0.00001). Msx1 expression is unaffected as in the control, whereas Bmp4 expression is significantly upregulated (*P<0.01). (C) Model depicting how epithelial Bmp4 from the dental placode is sufficient to induce mesenchymal expression of both Msx1 and Tbx2. Subsequently, these two transcription factors antagonistically regulate enamel knot formation and mesenchymal Bmp4 expression, hence maintaining a fine-tuned level of Bmp4. e, epithelium; m, mesenchyme; EK, enamel knot. Scale bars: 0.1 mm.
Restoration of the EK and of Bmp4 expression in Msx1-/-;Tbx2+/- dental mesenchyme suggests that Tbx2 negatively regulates Msx1-mediated odontogenic activity and Bmp4 expression, at least in upper molar development. To examine the latter, we knocked down Tbx2 in C3H10T1/2 mesenchymal cells using lentiviral particles expressing an shRNA against Tbx2 (Fig. 4B). Knockdown of Tbx2 (**P<0.00001) resulted in a moderate but significant upregulation of Bmp4 expression (*P<0.01). As a control, Msx1 expression remained unchanged (Fig. 4B). These results are consistent with a repressive role for Tbx2 in Bmp4 regulation.
Our genetic analysis implies that following induction by Bmp4, unlike Msx1, Tbx2 suppresses odontogenic activity and Bmp4 expression in the mesenchyme (Fig. 4C). This is consistent with the increased Bmp4 and cyclin D1 expression seen in Tbx2-deficient palatal shelves as reported previously (Zirzow et al., 2009). Rescue of the bud stage arrest and restoration of mesenchymal Bmp4 simply by reduction in Tbx2 gene dosage suggests that, in the presence of Msx1, Tbx2 repression of Bmp4 is abated either by protein-protein interaction or via recruitment of a different set of co-factors. The partial nature of the rescue indicates a further requirement of Msx1 function later in tooth development.
Together, our data suggest a model (Fig. 4C) in which initial Bmp signaling from the epithelium activates factors in the mesenchyme that not only subsequently activate and maintain Bmp signaling in odontogenesis, but also repress it, thus achieving a dynamic equilibrium that is fine-tuned for proper morphogenesis and patterning. Given the examples of interplay between Bmp signaling and Msx/Tbx factors, it is intriguing to propose that their antagonistic relationship is a general paradigm during morphogenesis in other tissues in which Msx/Tbx factors are co-expressed. These include other craniofacial regions, such as the palate where Msx1, Tbx2 and Tbx3 are co-expressed, and the mouse incisor epithelium where Msx2 and Tbx1 are co-expressed. Another example could be cardiac outflow tract morphogenesis, in which Msx1/2 and Tbx2/3 are known to be co-expressed. The latter is partly supported by the report that Msx1/2 and Tbx2/3 functionally interact in vitro in the regulation of connexin 43 in a cardiac cell line; however, in this case these factors acted coordinately to suppress connexin 43 (Boogerd et al., 2008). Interestingly, these authors also show that repression of connexin 43 by Msx1 and Msx2 requires Tbx3 expression, suggesting the formation of a functional complex. Our data are consistent with a physical interaction between Msx and T-box factors and uncover another level of complexity in the regulation of Bmp4 signaling. Further in vivo analyses in the mouse would be valuable in clarifying the relationship between different Msx and T-box factors in cardiac, craniofacial and dental morphogenesis.
Supplementary Material
Footnotes
Funding
This project was supported by the National Institutes of Health (NIH) [NIH/National Institute of Child Health and Human Development (NICHD) MERIT award HD033082 to V.E.P.; NIH/National Institute of Dental and Craniofacial Research (NIDCR) RO1 award DE019226 to M.B.; and NIH/National Institute of General Medical Sciences (NIGMS) IDeA P20GM104936 sub-award to I.S.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
M.B. and I.S. conceived and designed the experiments. M.B., I.S., P.D., M.Z., L.R., I.R. and Y.X. performed experiments. V.E.P. contributed reagents. M.B. and I.S. analyzed data. I.S. and M.B. wrote and edited the manuscript.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.088393/-/DC1
References
- Abrahams A., Parker M. I., Prince S. (2010). The T-box transcription factor Tbx2: its role in development and possible implication in cancer. IUBMB Life 62, 92–102 [DOI] [PubMed] [Google Scholar]
- Andl T., Ahn K., Kairo A., Chu E. Y., Wine-Lee L., Reddy S. T., Croft N. J., Cebra-Thomas J. A., Metzger D., Chambon P., et al. (2004). Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131, 2257–2268 [DOI] [PubMed] [Google Scholar]
- Bamshad M., Le T., Watkins W. S., Dixon M. E., Kramer B. E., Roeder A. D., Carey J. C., Root S., Schinzel A., Van Maldergem L., et al. (1999). The spectrum of mutations in TBX3: genotype/phenotype relationship in ulnar-mammary syndrome. Am. J. Hum. Genet. 64, 1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behesti H., Holt J. K., Sowden J. C. (2006). The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev. Biol. 6, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei M. (2009). Molecular genetics of tooth development. Curr. Opin. Genet. Dev. 19, 504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei M., Maas R. (1998). FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 125, 4325–4333 [DOI] [PubMed] [Google Scholar]
- Bei M., Kratochwil K., Maas R. L. (2000). BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development 127, 4711–4718 [DOI] [PubMed] [Google Scholar]
- Boogerd K. J., Wong L. Y., Christoffels V. M., Klarenbeek M., Ruijter J. M., Moorman A. F., Barnett P. (2008). Msx1 and Msx2 are functional interacting partners of T-box factors in the regulation of Connexin43. Cardiovasc. Res. 78, 485–493 [DOI] [PubMed] [Google Scholar]
- Catón J., Luder H. U., Zoupa M., Bradman M., Bluteau G., Tucker A. S., Klein O., Mitsiadis T. A. (2009). Enamel-free teeth: Tbx1 deletion affects amelogenesis in rodent incisors. Dev. Biol. 328, 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catron K. M., Zhang H., Marshall S. C., Inostroza J. A., Wilson J. M., Abate C. (1995). Transcriptional repression by Msx-1 does not require homeodomain DNA-binding sites. Mol. Cell. Biol. 15, 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bei M., Woo I., Satokata I., Maas R. (1996). Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122, 3035–3044 [DOI] [PubMed] [Google Scholar]
- D’Souza R. N., Aberg T., Gaikwad J., Cavender A., Owen M., Karsenty G., Thesleff I. (1999). Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development 126, 2911–2920 [DOI] [PubMed] [Google Scholar]
- Harrelson Z., Kelly R. G., Goldin S. N., Gibson-Brown J. J., Bollag R. J., Silver L. M., Papaioannou V. E. (2004). Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development 131, 5041–5052 [DOI] [PubMed] [Google Scholar]
- Jia S., Zhou J., Gao Y., Baek J.-A., Martin J. F., Lan Y., Jiang R. (2013). Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development 140, 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Sun X., Braut A., Mishina Y., Behringer R. R., Mina M., Martin J. F. (2005). Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132, 1453–1461 [DOI] [PubMed] [Google Scholar]
- Miletich I., Yu W. Y., Zhang R., Yang K., Caixeta de Andrade S., Pereira S. F., Ohazama A., Mock O. B., Buchner G., Sealby J., et al. (2011). Developmental stalling and organ-autonomous regulation of morphogenesis. Proc. Natl. Acad. Sci. USA 108, 19270–19275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche L. A., Harrelson Z., Kelly R. G., Papaioannou V. E. (2005). T-box genes in vertebrate development. Annu. Rev. Genet. 39, 219–239 [DOI] [PubMed] [Google Scholar]
- O’Connell D. J., Ho J. W. K., Mammoto T., Turbe-Doan A., O’Connell J. T., Haseley P. S., Koo S., Kamiya N., Ingber D. E., Park P. J., et al. (2012). A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci. Signal. 5, ra4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kapadia H., Feng J. Q., Raghow R., Peters H., D’Souza R. N. (2006). Functional consequences of interactions between Pax9 and Msx1 genes in normal and abnormal tooth development. J. Biol. Chem. 281, 18363–18369 [DOI] [PubMed] [Google Scholar]
- Packham E. A., Brook J. D. (2003). T-box genes in human disorders. Hum. Mol. Genet. 12 Spec No 1, R37–R44 [DOI] [PubMed] [Google Scholar]
- Papaioannou V. E., Silver L. M. (1998). The T-box gene family. BioEssays 20, 9–19 [DOI] [PubMed] [Google Scholar]
- Peters H., Balling R. (1999). Teeth. Where and how to make them. Trends Genet. 15, 59–65 [DOI] [PubMed] [Google Scholar]
- Peters H., Neubüser A., Kratochwil K., Balling R. (1998). Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12, 2735–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I., Maas R. (1994). Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 6, 348–356 [DOI] [PubMed] [Google Scholar]
- Showell C., Binder O., Conlon F. L. (2004). T-box genes in early embryogenesis. Dev. Dyn. 229, 201–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I., Mikkola M. (2002). The role of growth factors in tooth development. Int. Rev. Cytol. 217, 93–135 [DOI] [PubMed] [Google Scholar]
- van Genderen C., Okamura R. M., Fariñas I., Quo R. G., Parslow T. G., Bruhn L., Grosschedl R. (1994). Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8, 2691–2703 [DOI] [PubMed] [Google Scholar]
- Wang X. P., Aberg T., James M. J., Levanon D., Groner Y., Thesleff I. (2005). Runx2 (Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and prevents the budding of putative successional teeth. J. Dent. Res. 84, 138–143 [DOI] [PubMed] [Google Scholar]
- Yamada M., Revelli J. P., Eichele G., Barron M., Schwartz R. J. (2000). Expression of chick Tbx-2, Tbx-3, and Tbx-5 genes during early heart development: evidence for BMP2 induction of Tbx2. Dev. Biol. 228, 95–105 [DOI] [PubMed] [Google Scholar]
- Zhang H., Catron K. M., Abate-Shen C. (1996). A role for the Msx-1 homeodomain in transcriptional regulation: residues in the N-terminal arm mediate TATA binding protein interaction and transcriptional repression. Proc. Natl. Acad. Sci. USA 93, 1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zhang Z., Song Y., Zhang X., Zhang Y., Hu Y., Fromm S. H., Chen Y. (2000). Transgenically ectopic expression of Bmp4 to the Msx1 mutant dental mesenchyme restores downstream gene expression but represses Shh and Bmp2 in the enamel knot of wild type tooth germ. Mech. Dev. 99, 29–38 [DOI] [PubMed] [Google Scholar]
- Zhao M., Gupta V., Raj L., Roussel M., Bei M. (2013). A regulatory network operates during early tooth morphogenesis. Mol. Cell. Biol. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Gao Y., Zhang Z., Zhang Y., Maltby K. M., Liu Z., Lan Y., Jiang R. (2011). Osr2 acts downstream of Pax9 and interacts with both Msx1 and Pax9 to pattern the tooth developmental field. Dev. Biol. 353, 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirzow S., Lüdtke T. H., Brons J. F., Petry M., Christoffels V. M., Kispert A. (2009). Expression and requirement of T-box transcription factors Tbx2 and Tbx3 during secondary palate development in the mouse. Dev. Biol. 336, 145–155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.