Abstract
BACKGROUND
Several previous studies have reported conflicting data on recent trends in use of initial total mastectomy (TM); the factors that contribute to TM variation are not entirely clear. Using a multi-institution database, we analyzed how practice, patient, and tumor characteristics contributed to variation in TM for invasive breast cancer.
STUDY DESIGN
We collected detailed clinical and pathologic data about breast cancer diagnosis, initial, and subsequent breast cancer operations performed on all female patients from 4 participating institutions from 2003 to 2008. We limited this analysis to 2,384 incident cases of invasive breast cancer, stages I to III, and excluded patients with clinical indications for mastectomy. Predictors of initial TM were identified with univariate analyses and random effects multivariable logistic regression models.
RESULTS
Initial TM was performed on 397 (16.7%) eligible patients. Use of preoperative MRI more than doubled the rate of TM (odds ratio [OR] = 2.44; 95% CI, 1.58–3.77; p < 0.0001). Increasing tumor size, high nuclear grade, and age were also associated with increased rates of initial TM. Differences by age and ethnicity were observed, and significant variation in the frequency of TM was seen at the individual surgeon level (p < 0.001). Our results were similar when restricted to tumors <20 mm.
CONCLUSIONS
We identified factors associated with initial TM, including preoperative MRI and individual surgeon, that contribute to the current debate about variation in use of TM for the management of breast cancer. Additional evaluation of patient understanding of surgical options and outcomes in breast cancer and the impact of the surgeon provider is warranted.
Twenty years ago, the National Institutes of Health issued a consensus statement recommending breast-conserving therapy as an appropriate alternative primary therapy to mastectomy for the majority of women with early-stage breast cancer in whom breast conservation is not contrain-dicated.1 This recommendation was based on multicenter, prospective, randomized clinical trials that established equivalent long-term survival rates for patients with early-stage invasive breast cancer treated by total mastectomy (TM) or partial mastectomy followed by radiation.2,3 In the years after issuance of the consensus statement, mastectomy rates in the United States markedly declined.4 However, several recent studies have reported conflicting data on a trend toward increasing institutional mastectomy rates, suggesting potential for inherent variation in the surgical management of breast cancer.5–9
Both clinical and nonclinical factors contribute to variability in mastectomy rates.5–9 Factors associated with the use of mastectomy include large tumor size, multicentric breast cancer, family history of breast cancer, ethnicity, age, preoperative MRI use, socioeconomic status, distance from a radiation facility, patient preference, and provider preference.7,10–17 Recent studies have also highlighted substantial variability among surgeons with respect to surgical treatment of breast cancer,18,19 and have suggested that this variability has potential to influence long-term outcomes such as local recurrence. Variability in surgical care has been attributed to characteristics including surgical volume and specialty training.20
The lack of well-accepted guidelines or any standardized reporting of breast cancer surgery outcomes can result in patients receiving widely variable surgical treatment based on geographic location or choice of hospital and surgeon.19 To date, most studies that examined underlying contributors to variability in mastectomy rates relied on administrative health care databases or the experience at single institutions.6,7,16,21 Health care administrative databases are generally limited and do not capture important clinical factors, such as known multifocal breast disease and history of breast cancer, which most surgeons have identified as contributing substantially to both the choice of initial breast cancer surgery and outcomes.16,21 In addition, surgical quality databases, such as the National Quality Measures for Breast Centers (NQMBC) program, are voluntary and outcomes from these sources might not be generalizable to community practice.22–25
In contrast to previous studies that evaluated single-institution or administrative databases, we have constructed a multi-institution Breast Cancer Surgical Outcomes (BRCASO) database that captured detailed information on both initial presenting clinical conditions and outcomes of all breast cancer operations and related pathology for each procedure performed on 4,580 women at any of the 4 collaborating institutions between 2003 and 2008. This clinical database allows for improved identification of factors contributing to selection of both initial and any subsequent procedures, which is generally not feasible through summation pathology typically available in a cancer registry or administrative dataset. These institutions vary in their geographic location and practice characteristics. Using this database, we analyzed how practice, patient, and tumor characteristics contributed to variability in the performance of TM as the initial procedure for invasive breast cancer. To better understand factors contributing to variability in initial TM rates, we excluded patients with clinical factors known to increase the likelihood of initial TM (women with history of breast cancer or chest radiation, inflammatory breast cancer, or known multifocal disease). Because not all women with noninvasive disease will undergo postoperative radiation, we limited this analysis to patients with invasive breast cancer to minimize a patient’s desire to avoid radiation therapy as a potential confounder in the selection of TM as the initial breast surgery.
METHODS
The BRCASO research consortium was developed from 3 member organizations in the Cancer Research Network (CRN) and the University of Vermont. The CRN is a consortium of 14 nonprofit research centers based in integrated health care delivery organizations within the HMO Research Network.26 The participating CRN sites included Kaiser Permanente, Colorado; Group Health, Washington; and Marshfield Clinic, Wisconsin. The BRCASO cohort has been described elsewhere.25 Briefly, BRCASO includes women with breast cancer diagnosed between January 2003 and December 2008, who are older than 18 years of age at diagnosis, and whose initial breast cancer surgery was performed by a surgeon employed by a BRCASO study site, including any of the 3 CRN sites or Fletcher Allen Health Care Center, the sole hospital affiliated with the University of Vermont. At the University of Vermont, this database was created prospectively. At the CRN sites, the data were extracted from electronic medical records to create a retrospective cohort. We are not currently adding additional patient data to this database.
Institutional Review Board approvals were obtained across sites, and a waiver of consent was obtained to collect patient and provider-level data. Detailed data were collected characterizing clinical features of initial breast cancer diagnosis and all breast cancer operations performed for the incident breast cancer.
Data collection
Trained medical abstractors with experience in abstraction of breast cancer data completed data collection via an exhaustive review of medical records, including surgical, pathology reports, radiology reports, and clinical evaluations by medical oncology. The data collection instrument included clinical factors (eg, age and history of breast cancer), demographic factors (eg, ethnicity and insurance status), tumor characteristics (eg, pathologic features and tumor size), imaging modalities (eg, use of preoperative MRI), and breast cancer treatment (eg, use of neoadjuvant chemotherapy and initial surgical procedure type). Data collection for the participating CRN sites partially relied on the CRN’s primary source of data infrastructure, electronic administrative data from their Virtual Data Warehouse and the availability of electronic medical records.
Data from 63 surgeons are included in this analysis. Volume categories were established for surgeons based on their average yearly case volume for all initial breast cancer operations identified in the entire BRCASO database (not only those included in this analysis). Low volume was defined as <10 breast cancer operations annually (n = 27 surgeons), intermediate as 10 to 24.9 (n = 31 surgeons), and high as 25 to 49.9 (n = 2 surgeons), and very high volume as ≥50 operations annually (n = 3 surgeons).
Statistical analysis
Univariate analyses relating frequency of initial TM and clinical covariates were carried out using Pearson chi-square tests of independence. Due to the greater feasibility of breast conservation with smaller tumors, analyses were conducted on all tumors and then separately for tumors <20 mm in diameter. The association between multiple predictor variables and initial TM was assessed using logistic regression, including surgeon-level random effects to account for surgeons nested within study sites. We chose to use the same multivariable model for both the “all tumors” and “tumors <20 mm in diameter” analyses to facilitate comparisons. Variables that were statistically significantly (at p ≤ 0.05) in either the all tumors or tumors <20 mm in diameter analyses were included in the multivariable models. Analyses were carried out using SAS software system, version 9.2 (SAS Institute). All statistical tests were 2-sided with reported p values unadjusted for multiple comparisons. The level for confidence intervals was set at 95% and significance was defined as p ≤ 0.05.
The magnitude of surgeon-level random effects was assessed using the median odds ratio (MOR), which transforms the random effects variance component, τ2, into an OR that is directly comparable with the ORs reported for other fixed effects. The MOR is defined as exp [√2*τ*Φ−1(0.75)], where Φ−1(0.75) is the 75th percentile of the standard normal distribution.27 The MOR describes variability in TM between surgeons at difference centers corrected for the other predictors that are included in the model. It is interpreted as the median value of the ratio of predicted odds of TM for 2 patients, with the same covariates, randomly selected from different sites. In the absence of variability among surgeons, the odds of TM predicted for patients with the same covariates would be identical, leading to a ratio of 1.0. The random effects logistic regression model was restricted to operations performed by surgeons with at least 10 cases in the entire database (n = 55 surgeons), resulting in the exclusion of 57 breast cancer operations.
RESULTS
A total of 4,684 breast cancers in 4,580 breast cancer patients are included in the BRCASO database. For this analysis, the following cases were excluded: stage 0 (n = 1,087); stage IV (n = 6); preoperative malignant diagnosis unknown (n = 278); those who received neoadjuvant chemotherapy or had inflammatory breast cancer (n = 210); known multifocal or multicentric disease (n = 424); those with history of breast cancer (n = 269) or history of chest radiation (n= 11); and 15 cases diagnosed outside the 2003 to 2008 time frame. This resulted in a total of 2,384 breast cancer cases with a confirmed invasive breast cancer diagnosis before the initial surgical procedure. Total mastectomy was performed as the first breast surgery on 397 of 2,384 cases (16.7%); of these, 319 (80.4%) were unilateral TM, 59 (14.9%) had a contralateral prophylactic TM, and 17 (4.3%) had bilateral TM for synchronous cancers. This information was unavailable for 2 patients.
Table 1 shows the frequency of initial TM by patient, tumor, and institutional characteristics, first for all tumor sizes and then for tumors <20 mm in size. Our results for tumors <20 mm were similar to our results for all tumors. Initial TM rates varied by patient age (p = 0.0005). They were highest among women younger than age 45 years (23.8%), declined with increasing age, and then increased again for women aged 75 years and older (20.1%). Asian women had a higher frequency of initial TM (32.2%) compared with 15.4% for Caucasian and 16.3% for African-American women (p = 0.0037), when comparing rates for all tumor sizes. Frequency of initial TM was 8.5% for tumors ≤10 mm in diameter and increased to >70% for patients with tumors ≥50 mm in diameter (p value for trend <0.0001). The majority of tumors (73%) were <20 mm in diameter and 10% of those cases underwent TM as the first breast surgery. Initial TM was more common for invasive lobular compared with invasive ductal carcinoma (25.1% vs 15.6%, respectively; p = 0.0001), but this might be secondary to tumor size, as this association was not significant when analysis was limited to tumors <20 mm. Greater use of initial TM was also seen for tumors of higher grade (p < 0.0001) and estrogen receptor–negative and progesterone receptor–negative tumors (p = 0.0007). Although preoperative MRI was used infrequently in our population (7.8%), the use of preoperative MRI was associated with a near doubling of initial TM (15.6% vs 29.7%; p < 0.0001). We found no statistically significant differences in TM by insurance type (p = 0.08) or by year of treatment (p = 0.25).
Table 1.
Frequency of Total Mastectomy by Practice, Patient, and Tumor Characteristics
| Variable | All tumors (n = 2,384)
|
Tumors <20 mm (n = 1,712)
|
||||
|---|---|---|---|---|---|---|
| n | % TM | p Value* | n | %TM | p Value* | |
| Age, y | ||||||
|
| ||||||
| Younger than 45 | 189 | 23.81 | 0.0005 | 109 | 16.51 | 0.0011 |
|
| ||||||
| 45–54 | 528 | 17.05 | 355 | 10.7 | ||
|
| ||||||
| 55–64 | 656 | 12.35 | 488 | 6.15 | ||
|
| ||||||
| 65–74 | 528 | 15.91 | 403 | 10.17 | ||
|
| ||||||
| 75 and older | 483 | 20.08 | 357 | 13.73 | ||
|
| ||||||
| Ethnicity | ||||||
|
| ||||||
| Caucasian | 1,859 | 15.44 | 0.0037 | 1,349 | 9.79 | 0.16 |
|
| ||||||
| African American | 49 | 16.33 | 28 | 7.14 | ||
|
| ||||||
| Asian | 59 | 32.2 | 32 | 21.88 | ||
|
| ||||||
| Hispanic | 44 | 18.18 | 29 | 6.9 | ||
|
| ||||||
| Other/unknown | 373 | 20.11 | 274 | 12.04 | ||
|
| ||||||
| Insurance | ||||||
|
| ||||||
| Commercial | 1,240 | 15.16 | 0.08 | 865 | 8.9 | 0.15 |
|
| ||||||
| Medicare | 1,012 | 17.79 | 761 | 11.56 | ||
|
| ||||||
| Medicaid | 37 | 16.22 | 22 | 4.55 | ||
|
| ||||||
| Private/other | 91 | 24.18 | 61 | 14.75 | ||
|
| ||||||
| Missing | 4 | 25.0 | 3 | 33.3 | ||
|
| ||||||
| Tumor size, mm | ||||||
|
| ||||||
| <10 | 601 | 8.5 | <0.0001† | 601 | 8.5 | 0.06 |
|
| ||||||
| 10 to <20 | 1,083 | 11.4 | 1,083 | 11.4 | ||
|
| ||||||
| 20 to <30 | 403 | 23.3 | ||||
|
| ||||||
| 30 to <40 | 156 | 36.5 | ||||
|
| ||||||
| 40 to <50 | 49 | 49.0 | ||||
|
| ||||||
| ≥50 | 64 | 71.9 | ||||
|
| ||||||
| Missing | 28 | 7.1 | ||||
|
| ||||||
| Tumor type | ||||||
|
| ||||||
| IDC | 2,129 | 15.64 | 0.0001 | 1,556 | 10.15 | 0.59 |
|
| ||||||
| ILC | 255 | 25.1 | 156 | 11.84 | ||
|
| ||||||
| Tumor grade | ||||||
|
| ||||||
| Low/medium | 1,723 | 13.52 | <0.0001 | 1,350 | 8.96 | 0.0012 |
|
| ||||||
| High | 638 | 24.76 | 343 | 14.87 | ||
|
| ||||||
| Missing | 23 | 19 | ||||
|
| ||||||
| Receptor status | ||||||
|
| ||||||
| ER/PR+ | 2,044 | 15.56 | 0.0007 | 1,512 | 9.72 | 0.0478 |
|
| ||||||
| ER/PR− | 325 | 23.08 | 188 | 14.36 | ||
|
| ||||||
| Missing | 15 | 12 | ||||
|
| ||||||
| Use of preoperative MRI | ||||||
|
| ||||||
| No | 2,199 | 15.55 | <0.0001 | 1,585 | 9.65 | 0.0025 |
|
| ||||||
| Yes | 185 | 29.73 | 127 | 18.11 | ||
|
| ||||||
| Year | ||||||
|
| ||||||
| 2003 | 440 | 17.05 | 0.25 | 312 | 11.22 | |
|
| ||||||
| 2004 | 450 | 18.22 | 302 | 11.26 | ||
|
| ||||||
| 2005 | 449 | 15.81 | 325 | 9.85 | ||
|
| ||||||
| 2006 | 501 | 13.57 | 384 | 6.77 | ||
|
| ||||||
| 2007 | 314 | 19.75 | 229 | 11.35 | 0.11 | |
|
| ||||||
| 2008 | 230 | 16.96 | 160 | 14.38 | ||
|
| ||||||
| Site | ||||||
|
| ||||||
| 1 | 549 | 10.38 | <0.0001 | 407 | 5.16 | <0.0001 |
|
| ||||||
| 2 | 849 | 20.97 | 579 | 14.16 | ||
|
| ||||||
| 3 | 763 | 17.82 | 536 | 10.63 | ||
|
| ||||||
| 4 | 223 | 11.66 | 190 | 8.42 | ||
|
| ||||||
| Surgeon volume (cases/y) | ||||||
|
| ||||||
| ≤10 | 533 | 18.2 | 0.08 | 380 | 13.16 | 0.0113 |
|
| ||||||
| 11–25 | 1,035 | 17.97 | 738 | 11.38 | ||
|
| ||||||
| 26–49 | 222 | 14.86 | 172 | 7.56 | ||
|
| ||||||
| ≥50 | 594 | 13.64 | 422 | 6.87 | ||
Chi-square p value.
For tumor size, p value is a test for trend.
ER/PR, estrogen/progesterone receptor; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; TM, total mastectomy.
Frequency of initial TM also varied by study site (p < 0.0001) and surgeon volume (defined as the average number of breast cancer cases performed yearly). The TM variation by site ranged from 10.4% to 21.0%. Lower surgeon volume was associated with higher frequency of TM as the first breast cancer operation. This difference was statistically significant for tumors <20 mm only, where surgeons with the lowest case volumes were more likely to perform initial TM than surgeons with the highest volume (13% vs 6.9%; p = 0.011).
Table 2 shows the results of our multivariable analysis, first for all tumors, and then restricted to tumors <20 mm. Several factors associated with initial TM remained significant in the multivariable analysis. Age remained a significant predictor of TM, both for all tumors (p = 0.003) and for those <20 mm (p = 0.001). Women younger than age 45 years and older than age 75 years were more likely to have initial TM than other age groups. These differences were especially pronounced for tumors <20 mm, where ORs were 2.75 (95% CI, 1.36–5.55) for women younger than 45 years and 2.81 (95% CI, 1.67–4.71) for women older than 75 years, with age 55 to 64 years as the reference group. Women of Asian descent were twice as likely as Caucasian women to undergo initial TM (OR = 2.17; 95% CI, 1.11–4.27). Tumor size (p < 0.0001) and high nuclear grade (p = 0.004) were both associated with increased frequency of initial TM, but tumor type (ductal carcinoma vs lobular carcinoma) and estrogen receptor/progesterone receptor status were no longer independently associated with practice of TM as the first breast cancer surgery. Use of preoperative MRI more than doubled the odds of initial TM for all tumors and tumors <20 mm (OR = 2.44; 95% CI, 1.58–3.77) and (OR = 2.59; 95% CI, 1.46–4.59), respectively. The effect of surgeon volume did not persist as a significant factor for mastectomy rate when controlling for other factors in multivariable analysis. We also tested for interactions by study site, but found none that were statistically significant (data not shown).
Table 2.
Multivariable Analysis of Practice, Patient, and Tumor Characteristics Associated with Initial Total Mastectomy
| Variable | All tumors
|
Tumors <20 mm
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age, y | ||||||
|
| ||||||
| Younger than 45 | 1.63 | 1.00–2.66 | 0.003 | 2.75 | 1.36–5.55 | 0.001 |
|
| ||||||
| 45–54 | 1.29 | 0.88–1.88 | 1.81 | 1.06–3.09 | ||
|
| ||||||
| 55–64 | Ref | Ref | ||||
|
| ||||||
| 65–74 | 1.36 | 0.93–1.99 | 1.67 | 0.99–2.82 | ||
|
| ||||||
| 75 or older | 2.09 | 1.43–3.05 | 2.81 | 1.67–4.71 | ||
|
| ||||||
| Ethnicity | ||||||
|
| ||||||
| Caucasian | Ref | 0.04 | Ref | 0.24 | ||
|
| ||||||
| African American | 0.59 | 0.24–1.46 | 0.27 | 0.04–2.11 | ||
|
| ||||||
| Asian | 2.17 | 1.11–4.27 | 2.35 | 0.91–6.09 | ||
|
| ||||||
| Hispanic | 0.74 | 0.27–2.06 | 0.67 | 0.14–3.13 | ||
|
| ||||||
| Other/unknown | 1.36 | 0.94–1.95 | 1.16 | 0.70–1.93 | ||
|
| ||||||
| Maximum tumor size* | 1.06 | 1.05–1.07 | <0.0001 | 1.05 | 1.01–1.10 | 0.007 |
|
| ||||||
| Tumor type | ||||||
|
| ||||||
| IDC | Ref | 0.27 | Ref | 0.48 | ||
|
| ||||||
| ILC | 1.26 | 0.84–1.89 | 1.23 | 0.69–2.19 | ||
|
| ||||||
| Tumor grade | ||||||
|
| ||||||
| Low/medium | Ref | 0.004 | Ref | 0.12 | ||
|
| ||||||
| High | 1.6 | 1.17–2.19 | 1.44 | 0.91–2.30 | ||
|
| ||||||
| Receptor status | ||||||
|
| ||||||
| ER/PR+ | Ref | 0.66 | Ref | 0.39 | ||
|
| ||||||
| ER/PR− | 1.09 | 0.74–1.60 | 1.29 | 0.73–2.28 | ||
|
| ||||||
| Use of preoperative MRI | ||||||
|
| ||||||
| No | Ref | <0.0001 | Ref | 0.001 | ||
|
| ||||||
| Yes | 2.44 | 1.58–3.77 | 2.59 | 1.46–4.59 | ||
|
| ||||||
| Site | ||||||
|
| ||||||
| 1 | Ref | 0.43 | Ref | 0.23 | ||
|
| ||||||
| 2 | 1.93 | 0.75–4.94 | 2.6 | 0.92–7.37 | ||
|
| ||||||
| 3 | 1.96 | 0.74–5.20 | 2.16 | 0.71–6.59 | ||
|
| ||||||
| 4 | 1.09 | 0.33–3.57 | 1.14 | 0.32–4.06 | ||
|
| ||||||
| Surgeon volume (cases/y) | ||||||
|
| ||||||
| ≤10 | Ref | 0.66 | Ref | 0.076 | ||
|
| ||||||
| 11–25 | 0.81 | 0.50–1.33 | 0.79 | 0.46–1.36 | ||
|
| ||||||
| 26–49 | 1.41 | 0.40–4.95 | 1.06 | 0.28–4.01 | ||
|
| ||||||
| ≥50 | 0.74 | 0.27–2.07 | 0.68 | 0.24–1.98 | ||
Modeled as a continuous variable in units of 1 mm.
ER/PR, estrogen/progesterone receptor; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; OR, odds ratio; TM, total mastectomy.
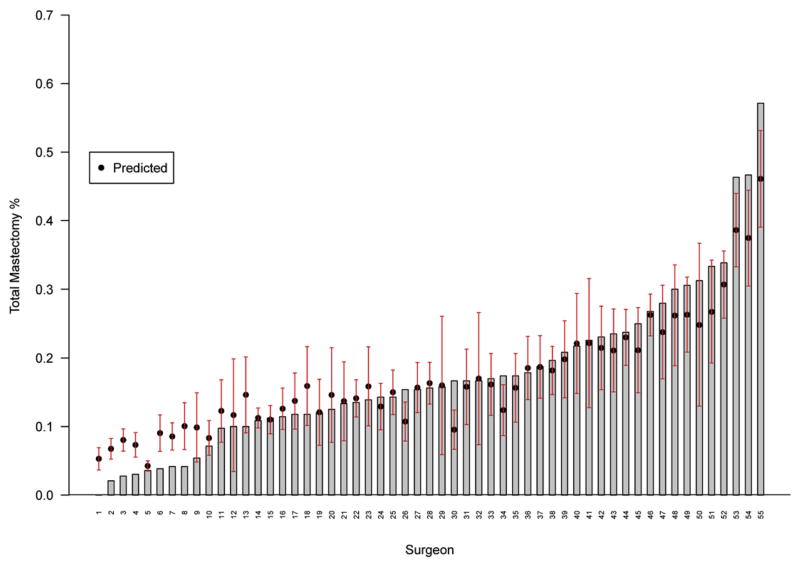
To examine variability in the performance of initial TM by individual surgeon, random effects logistic regression models were used to account for the inter-dependency of performance site and surgeon (because each surgeon only operates at 1 of the 4 sites). Figure 1 displays observed (bars) and predicted (circles) initial TM rates for individual surgeons, which ranged from 0% to 57.1%. The overall surgeon level variance component was highly significant (p <0.001). The MOR transforms this effect onto the scale of ORs reported for other effects. An MOR of 1.86 (95% CI, 1.60–2.47) was observed and corresponds to the relative odds of TM between 2 patients at different sites with the same covariates. This can be interpreted as an OR relating 2 randomly selected surgeons from different sites for 2 patients with the same characteristics. This showed a stronger effect than that of tumor type and grade, but not quite as strong as that of the use of preoperative MRI. This indicates a large individual surgeon effect, as half the ORs between 2 randomly chosen surgeons were >1.86. The range of ORs from the lower to the upper 2.5th percentile of the distribution was 12.7, indicating substantial heterogeneity among surgeons in use of initial TM that is not explained by the other covariates in the multivariable model. When this analysis was restricted to tumors <20 mm, our results were similar; the MOR was 1.75 (95% CI, 1.46–2.87) with a range of 9.98.
Figure 1.
Observed initial total mastectomy frequency for each of 55 surgeons with at least 10 patients in this study. Predicted total mastectomy rates, based on the random effects logistic regression model controlling for clinical covariates, are plotted as a circle above the encrypted surgeon identifiers on the horizontal axis.
DISCUSSION
This study included data from 4 diverse institutions and offers several important observations that contribute to the current debate about variation in use of TM for the management of breast cancer. Overall, 16.7% of invasive breast cancer cases in this analysis underwent TM as their initial breast surgery. Importantly, significant variation was observed among individual surgeons in the rate of initial TM performed for invasive breast cancer (0% to 57.1%). Use of preoperative MRI more than doubled the odds of TM (OR = 2.44; p < 0.0001). Increasing tumor size, high nuclear grade, and a bimodal distribution of age were associated with increased risk of initial TM. The highest percentage of TM occurred among the oldest and youngest women. Seventy-three percent of the tumors in this analysis were <20 mm in size, and results changed little when the analysis was restricted to only those <20 mm.
The overall frequency of 16.7% TM in this study was significantly lower than rates reported in previous studies, which generally range between 35% and 45%.5–7 The lower mastectomy rate in this study is likely attributed to several important differences from previous studies. First, this reflects the exclusion criteria, which were specifically applied to exclude clinical conditions that have previously been demonstrated to be associated with greater use of TM, such as history of breast cancer and preoperatively established multifocal disease. Without these exclusions, the overall TM frequency for invasive breast cancer was 27.5%, a figure more comparable with findings reported in previous studies (data not shown). Second, initial TM rate was reported, and most earlier reports do not distinguish mastectomy rate as initial vs subsequent. We previously reported that among patients undergoing initial partial mastectomy, an additional 8.4% subsequently underwent a TM for the incident breast cancer.19 Data presented here are comparable with those of Morrow and colleagues,8 who reported that for 17% of patients with stage 0 to II breast cancer, their surgeon recommended initial TM.
Frequency of initial TM was highest among women younger than 45 years of age (23.8%) and women aged 75 years or older (20%). The reasons for use of TM among these 2 groups might differ. Among young women, the choice of TM might be a result of factors such as genetic predisposition, family history, or higher risk of local recurrence.28,29 In addition, younger women might perceive mastectomy as an option associated with greater survival. Older women might choose TM because they are less concerned with cosmetic appearance than younger women, or to avoid radiation therapy.30 However, a recent study by Fisher and colleagues31 compared women younger than 50 to women 50 years and older and found that the 2 groups did not differ in their reasons for choosing mastectomy, with lower recurrence risk and improved survival cited as the 2 most common reasons. Whether these findings can be extrapolated to the women aged 75 years and older described here is uncertain.
This study is limited in that data on family history or genetic predisposition were not available. However, genetic predisposition is unlikely to explain the observed frequency of TM. The estimated prevalence of BRCA1 and BRCA2 mutations in the general population is approximately 1%,32 and most carriers have an early age at onset. Among the 189 women younger than age 45 years in the current study, 45 (approximately 24%) had a mastectomy. Although we can speculate that this might be driven by actual or perceived genetic risk, we can estimate that <5 women in this age group would be likely to harbor BRCA1 or BRCA2 mutations.
Frequency of initial TM also differed by ethnicity. The TM frequency was similar for Caucasian (15.4%), African-American (16.3%), and Hispanic women (18.2%). However, Asian women had a much higher frequency (32.2%) and were twice as likely to undergo TM compared with Caucasian women (OR = 2.17; 95% CI, 1.11–4.27). Similar finds have been reported previously.33–36 Suggested explanations for higher TM frequency in Asian women include unfavorable tumor to breast size ratio, patient preferences, cultural attitudes, willingness to accept radiation therapy as a component of breast-conservation therapy, and a high instance of clinical contraindications to breast conservation (eg, multiple ipsilateral breast cancer foci of breast cancer). Additional determination of the factors involved was not possible given the limitations of the available data, including lack of information on body size, acculturation, and patient preference.
Although use of preoperative MRI was uncommon in patients included in this analysis (of 185 patients, 7.7% had MRI), it was associated with more than doubling the rate of initial TM. Several previous studies have had similar findings.7,37–39 Katipamula and colleagues7 reported 51% of patients with preoperative MRI had a mastectomy, compared with 31% of patients without MRI. In the COMICE (Comparative Effectiveness of MR Imaging in Breast Cancer) trial,38 mastectomy rate was significantly higher in women randomly assigned to receive MRI (7.1%) compared with those who did not have an MRI (1.2%). The increased frequency in TM associated with preoperative MRI is especially concerning because, to date, there is no evidence of benefit for long-term clinical outcomes,39,40 and preoperative MRI is becoming routine in many care settings.40 Magnetic resonance imaging has been proven useful for detecting multifocal and multicentric disease;40 however, these cases were excluded a priori from our analysis and cannot explain the high frequency of TM with MRI use. Our database does not include information on BRCA1/2 carrier status, family history, or breast density, which are all factors that can lead to the use of preoperative MRI; however, it is debatable whether these factors alone would account for the 2-fold increase in initial TM rate found among women who received preoperative MRI. One possible explanation for the higher TM rate associated with preoperative MRI is that MRIs might have identified additional ipsilateral and contralateral breast lesions prompting TM, even if the additional lesions were ultimately found to be benign. It is also possible that many of the patients who had MRIs had a significant family history and this might have driven their mastectomy decision. Although the trend of MRI and mastectomy is strong, the numbers are very small, limiting the ability to draw firm conclusions.
Unlike some previous studies, a temporal trend in TM frequency was not seen.6,7 Initial TM frequency was 17% in both 2003 and 2008. The lowest rate observed was 13.6% in 2006 and the highest rate was 19.8% in 2007. These results of a stable TM rate are consistent with those of Habermann and colleagues,5 who used data from Surveillance, Epidemiology, and End Results registries to examine variation in mastectomy rates across the United States. Between 2000 and 2006, they found a slight decrease in TM, from 41% to 37%.
The impact of the individual surgeon on the use of mastectomy as the initial breast cancer operation is highly significant. A nearly 2-fold (MOR = 1.86) relative odds of the performance of an initial TM was found between 2 randomly selected surgeons from different sites that was not explained by patient features, tumor characteristics, or by surgical volume included in this analysis. Other investigators have also found considerably treatment variation in the performance of breast cancer surgery for ductal carcinoma in situ by individual surgeon.18 To our knowledge, this study is the first to report an association between individual surgeon and initial surgical procedure for invasive breast cancer.
One strength of our study is the data collected on clinical and tumor characteristics classically associated with higher use of TM, such as history of breast cancer or chest radiation, inflammatory breast cancer, or known multifocal disease, were known preoperatively. This allowed us to exclude such cases from the analysis and examine factors associated with initial TM among cases who would likely be good candidates for breast-conserving therapy. This information allowed for better determination of the potential influence of the individual surgeon on TM rate, independent of other clinical factors. Additionally, the large size of our dataset made it possible to delineate variability at the individual surgeon level. However, no data are available on factors that might have contributed to the identified variation attributed to surgeons or institutions, such as specialty training, use of patient decision aids, access to reconstructive surgeons, and use of multidisciplinary tumor conferences. Additional research into these potential factors would provide additional opportunities to evaluate quality standards in the care of the newly diagnosed breast cancer patient.
The results of our study reflect initial TM rates from >2,000 breast cancer cases performed at 4 geographically diverse organizations. These institutions include managed care, university, and contracted community hospitals, and represent a broad cross-section of practice patterns in the United States. Given this wide distribution of geographic and practice-type settings, we believe our study more adequately reflects the current state of surgical intervention for breast cancer care in the United States, compared with single-institution studies or large clinical trials with data derived exclusively from academic medical centers. A considerable percentage of breast cancer surgery continues to be performed in the community by low and moderate-volume surgeons. We found less frequent use of TM than has been previously reported and rates that were stable during the study period. Tumor size, high nuclear grade, and patient age were all important predictors of mastectomy. The association of preoperative MRI with higher rates of TM raises the question of whether or not preoperative MRI is appropriately identifying disease requiring mastectomy. However, due to the relatively small numbers of patients in this study who underwent MRI in advance of TM, additional studies are required to validate this trend and delineate variables that drive this phenomenon. Finally, the significant variability in the performance of initial TM attributable to the individual surgeon is an opportunity for additional study. Improved understanding of identifiable factors that contribute to this variability in health care might better serve our patients by addressing provider related or systems issues that can optimize treatment. In this way, evaluating outcomes will help identify and ensure optimal quality of care for patients undergoing breast cancer surgery.
Acknowledgments
This study was funded under the National Institutes of Health American Recovery and Reinvestment Act of 2009 (1RC1CA145402-01).
The authors would like to acknowledge the following individuals for contributions to this project: Johanna Sheehey-Jones, RN, Fletcher Allen Health Care; Kimberly J Bischoff, MSHA, and Andrew Sterrett, PhD, Kaiser Permanent Colorado; Jessica M Engel, MS, RN, Marshfield Clinic; Gabrielle Gundersen and Janice Miyoshi, Group Health Research Institute. The authors also thank Marie Fleisner of the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication for editorial assistance with the manuscript.
Abbreviations and Acronyms
- BRCASO
Breast Cancer Surgical Outcomes
- CRN
Cancer Research Network
- MOR
median odds ratio
- TM
total mastectomy
Footnotes
Disclosure Information: Nothing to disclose.
Abstract presented at the HMO Research Network Annual Meeting, Seattle, WA, April 2012.
Author Contributions
Study conception and design: Feigelson, James, Single, Onitilo, Aiello Bowles, Barney, Bakerman, McCahill
Acquisition of data: Feigelson, James, Single, Onitilo, Aiello Bowles, Barney, McCahill
Analysis and interpretation of data: Feigelson, James, Single, Onitilo, Bakerman, McCahill
Drafting of manuscript: Feigelson, James, Single, Onitilo, McCahill
Critical revision: Feigelson, James, Single, Onitilo, Aiello Bowles, Barney, Bakermann, McCahill
References
- 1.NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;2653:391–395. [PubMed] [Google Scholar]
- 2.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305:6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 4.Lazovich D, Solomon CC, Thomas DB, et al. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86:628–637. [PubMed] [Google Scholar]
- 5.Habermann EB, Abbott A, Parsons HM, et al. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28:3437–3441. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 6.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16:2682–2690. doi: 10.1245/s10434-009-0635-x. [DOI] [PubMed] [Google Scholar]
- 7.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302:1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balch CM, Jacobs LK. Mastectomies on the rise for breast cancer: “the tide is changing”. Ann Surg Oncol. 2009;16:2669–2672. doi: 10.1245/s10434-009-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez SL, France AM, Lee MM. Socioeconomic status, immigration/acculturation, and ethnic variations in breast conserving surgery, San Francisco Bay area. Ethn Dis. 2004;14:134–140. [PubMed] [Google Scholar]
- 11.Tuttle TM, Rueth NM, Abbott A, Virnig BA. United States trends in the surgical treatment of primary breast cancer. World J Surg. 2012;36:1475–1479. doi: 10.1007/s00268-012-1469-4. [DOI] [PubMed] [Google Scholar]
- 12.Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–1346. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 13.McCahill LE, Privette AR, Hart MR, James TA. Are mastectomy rates a reasonable quality measure of breast cancer surgery? Am J Surg. 2009;197:216–221. doi: 10.1016/j.amjsurg.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 14.Temple WJ, Russell ML, Parsons LL, et al. Conservation surgery for breast cancer as the preferred choice: a prospective analysis. J Clin Oncol. 2006;24:3367–3373. doi: 10.1200/JCO.2005.02.7771. [DOI] [PubMed] [Google Scholar]
- 15.Gilligan MA, Kneusel RT, Hoffmann RG, et al. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care. 2002;40:181–189. doi: 10.1097/00005650-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 17.Boscoe FP, Johnson CJ, Henry KA, et al. Geographic proximity to treatment for early stage breast cancer and likelihood of mastectomy. Breast. 2011;20:324–328. doi: 10.1016/j.breast.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Dick AW, Sorbero MS, Ahrendt GM, et al. Comparative effectiveness of ductal carcinoma in situ management and the roles of margins and surgeons. J Natl Cancer Inst. 2011;103:92–104. doi: 10.1093/jnci/djq499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307:467–475. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 20.Hershman DL, Buono D, Jacobson JS, et al. Surgeon characteristics and use of breast conservation surgery in women with early stage breast cancer. Ann Surg. 2009;249:828–833. doi: 10.1097/SLA.0b013e3181a38f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Refaie W, Kuerer HM, Khuwaja A, et al. Determinants of mastectomy in breast conservation therapy candidates. Am J Surg. 2005;190:602–605. doi: 10.1016/j.amjsurg.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Clifford EJ, De Vol EB, Pockaj BA, et al. Early results from a novel quality outcomes program: the American Society of Breast Surgeons’ Mastery of Breast Surgery. Ann Surg Oncol. 2010;17(Suppl 3):233–241. doi: 10.1245/s10434-010-1263-1. [DOI] [PubMed] [Google Scholar]
- 23.American College of Surgeons. Cancer Programs. National Accreditation Program for Breast Centers; [Accessed August 20, 2012]. Revised April 2012. Available at: http://accreditedbreastcenters.org/ [Google Scholar]
- 24.Kaufman CS, Shockney L, Rabinowitz B, et al. National Quality Measures for Breast Centers (NQMBC): a robust quality tool: breast center quality measures. Ann Surg Oncol. 2010;17:377–385. doi: 10.1245/s10434-009-0729-5. [DOI] [PubMed] [Google Scholar]
- 25.Aiello Bowles EJ, Feigelson HS, Barney T, et al. Improving quality of breast cancer surgery through development of a national breast cancer surgical outcomes (BRCASO) research database. BMC Cancer. 2012;12:136. doi: 10.1186/1471-2407-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005;(35):3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 27.Larsen K, Petersen JH, Budtz-Jorgensen E, Endahl L. Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56:909–914. doi: 10.1111/j.0006-341x.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 28.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–3891. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courdi A, Doyen J, Gal J, Chamorey E. Local recurrence after breast cancer affects specific survival differently according to patient age. Oncology. 2010;79:349–354. doi: 10.1159/000323483. [DOI] [PubMed] [Google Scholar]
- 30.Field TS, Bosco JL, Prout MN, et al. Age, comorbidity, and breast cancer severity: impact on receipt of definitive local therapy and rate of recurrence among older women with early-stage breast cancer. J Am Coll Surg. 2011;213:757–765. doi: 10.1016/j.jamcollsurg.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher CS, Martin-Dunlap T, Ruppel MB, et al. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. 2012;19:3246–3250. doi: 10.1245/s10434-012-2525-x. [DOI] [PubMed] [Google Scholar]
- 32.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 33.Gelber RP, McCarthy EP, Davis JW, Seto TB. Ethnic disparities in breast cancer management among Asian Americans and Pacific Islanders. Ann Surg Oncol. 2006;13:977–984. doi: 10.1245/ASO.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 34.Lim SE, Back M, Quek E, et al. Clinical observations from a breast cancer registry in Asian women. World J Surg. 2007;31:1387–1392. doi: 10.1007/s00268-007-9086-3. [DOI] [PubMed] [Google Scholar]
- 35.Olaya W, Wong JH, Morgan JW, et al. Disparities in the surgical management of women with stage I breast cancer. Am Surg. 2000;75:869–872. [PubMed] [Google Scholar]
- 36.Pham JT, Allen LJ, Gomez SL. Why do Asian-American women have lower rates of breast conserving surgery: results of a survey regarding physician perceptions. BMC Public Health. 2009;9:246. doi: 10.1186/1471-2458-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209:180–187. doi: 10.1016/j.jamcollsurg.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563–571. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 39.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009;59:290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 40.Painter TJ, Dipasco PJ, Misra S, Avisar E. Effect of magnetic resonance imaging on breast conservation therapy versus mastectomy: a review of the literature. Int J Surg Oncol. 2011;2011:428653. doi: 10.1155/2011/428653. [DOI] [PMC free article] [PubMed] [Google Scholar]