Abstract
Purpose of review
When present clinically, cardiac involvement in systemic sclerosis (SSc) is a major risk factor for death. It is therefore vitally important to understand the epidemiology, screening, diagnosis, and treatment of the cardiac manifestations of SSc.
Recent findings
The epidemiology of cardiac involvement in SSc has been the subject of several recent studies. Most importantly, the prevalence of overt left ventricular (LV) systolic dysfunction and its associated risk factors have been defined, and patients with diffuse cutaneous SSc appear to be most susceptible to direct cardiac involvement. From a diagnostic and screening standpoint, tissue Doppler echocardiography and natriuretic peptides have provided fresh insight into subclinical cardiac dysfunction in SSc. Newer techniques, such as speckle-tracking echocardiography, diffuse myocardial fibrosis imaging, and absolute myocardial perfusion imaging, are poised to further advance our knowledge. Lastly, there is now consistent observational data to suggest a central role for calcium channel blockers in the treatment of microvascular ischemia and prevention of overt LV systolic dysfunction, although randomized controlled trials are lacking.
Summary
Recent studies have improved our understanding of cardiac involvement in SSc. Nevertheless, key questions regarding screening, diagnosis, and treatment remain. Novel diagnostic techniques and multicenter studies should yield important new data, which will hopefully ultimately result in improved outcomes.
Keywords: echocardiography, myocardial fibrosis, myocardial perfusion, natriuretic peptides, systemic sclerosis
Introduction
Systemic sclerosis (SSc) can cause a wide variety of cardiac abnormalities, including microvascular coronary artery disease (with resultant myocardial ischemia), myocardial fibrosis, left ventricular (LV) systolic dysfunction, LV diastolic dysfunction, pericardial disease, and conduction abnormalities (including bradyarrhythmias and tachyarrhythmias) [1–4]. In addition, cardiac involvement in SSc can be primary (i.e. direct cardiac involvement) or secondary to pulmonary arterial hypertension (PAH), interstitial lung disease (ILD), or kidney disease, all of which are potential complications of SSc [2]. Clinically evident cardiac involvement in SSc is associated with an increased risk of death [1–4]; therefore, understanding the cardiac manifestations of SSc is critically important.
Pathophysiologic aspects of cardiac manifestations of SSc, especially direct myocardial involvement, have been reviewed in detail elsewhere [1–4]. Therefore, the present review will focus on the latest data on epidemiology of SSc-induced heart disease, current and emerging tools for the detection and monitoring of cardiac involvement in SSc, and evaluation and treatment of patients with SSc who have known or suspected cardiac manifestations.
Epidemiology
Understanding the prevalence of cardiac involvement in SSc is difficult due to the multiple types of cardiac manifestations which can occur, and the wide variety of techniques used for their detection. Therefore, rates of cardiac involvement in SSc can vary widely depending on the cardiac phenotype analyzed. For example, the rate of LV systolic dysfunction has been estimated to be approximately 5% [5••] whereas the rate of abnormalities on myocardial perfusion imaging may exceed 60% [1,6].
Risk factors for cardiac involvement include presence of diffuse cutaneous SSc (dcSSc), rapid progression of skin thickness, and older age at the onset of SSc. In a study [7] of 1012 Italian patients with SSc, cardiac involvement was present in 32% of dcSSc vs. 23% in limited cutaneous SSc (lcSSc). A large study [5•• ] of LV systolic dysfunction in SSc replicated these findings by showing that SSc patients with LV systolic dysfunction were more likely to have dcSSc compared with patients with normal LV systolic function (48 vs. 32%, respectively; P = 0.001). In addition, Domsic et al. [8•] found an association between rapid skin thickness progression rate and the development of cardiac involvement at 1 year, and Manno and colleagues [9] found that late-age onset of SSc (age ≥ 65 years) was associated with increased risk of cardiac involvement [OR 2.7, 95% confidence interval (CI) 1.9–3.8].
As mentioned above, it is well known that cardiac involvement in SSc can be primary or secondary to other complications of SSc such as PAH. However, recent studies have highlighted the importance of co-existence of primary and secondary cardiac involvement in SSc. For example, recent reports [10•,11] suggest that it is not uncommon for patients with SSc-PAH to have concomitant LV perfusion defects despite normal epicardial coronary arteries. Co-existence of PAH and direct cardiac involvement of the LV may explain the increased morbidity and mortality in SSc-PAH compared with other types of PAH.
Given the frequency of cardiac involvement and coexistence with other complications of SSc, it is not surprising that cardiac manifestations are a common cause of death, as demonstrated in several recent studies [12•,13,14,15••]. In the largest study [15••] (N = 5860), 55% of deaths were due to SSc whereas 45% of deaths were thought to be unrelated to SSc. Of the SSc-related deaths, 26% were cardiac (predominantly heart failure and arrhythmias) whereas 29% of non-SSc related deaths were due to cardiac causes.
Screening and diagnosis
Given the poor prognosis associated with cardiac involvement in SSc (especially when clinically evident), screening for subclinical cardiac disease and thorough evaluation of cardiac symptoms are essential. However, unlike ILD and PAH, there are fewer data on optimal screening for direct cardiac involvement in SSc. Patients with SSc undergo routine Doppler echocardiography for pulmonary hypertension screening; therefore, screening for direct cardiac involvement should be performed simultaneously, and, if possible, the echocardiography examination should include tissue Doppler imaging. N-term-inal pro-B-type natriuretic peptide (NT-proBNP and BNP) also play a critical role in the evaluation of possible cardiac involvement in SSc, as outlined below.
Echocardiography
Many patients with SSc undergo routine annual screening with echocardiography for estimation of pulmonary artery systolic pressure with simultaneous evaluation for LV systolic dysfunction (reduced LV ejection fraction) and pericardial effusion. Although these routine assessments are important, several newer echocardiographic techniques such as tissue Doppler imaging and right ventricular quantification are available and should be utilized in all patients with SSc.
Tissue Doppler imaging
The assessment of myocardial tissue velocities using the Doppler technique has provided immense insight into longitudinal function of the heart [16–25]. As the heart is composed of longitudinal, circumferential, and radial fibers, global indices (such as ejection fraction) do not capture the full extent of myocardial dysfunction especially in patients who have subclinical cardiac involvement. Because subendocardial fibers, which are mostly longitudinal in orientation, are most susceptible to microvascular ischemia, longitudinal systolic and diastolic dysfunction are often the earliest signs of cardiac impairment in SSc [17–23,25]. Systolic longitudinal velocity (S’) can be used to identify early subclinical LV systolic dysfunction in patients with SSc whereas early diastolic tissue velocity (E’) is critically important as a marker of LV diastolic dysfunction [25].
Right ventricular quantification
In SSc, right ventricular (RV) dysfunction can be primary (due to direct myocardial involvement) or secondary (due to PAH, LV dysfunction, or ILD). Recent developments in echocardiographic RV imaging [27], along with published guidelines for the quantification of the right ventricle [28•], now allow routine evaluation of the RV in SSc. Five parameters [RV fractional area change, tricuspidb annular plane systolic excursion (TAPSE), RV free wall tissue Doppler S’, pulmonary artery systolic pressure, and the ratio of tricuspid regurgitant velocity to pulmonary artery velocity time integral (a noninvasive marker of pulmonary vascular resistance) [29]] form the cornerstone of echocardiographic RV assessment in SSc. In addition, the shape of the RV outflow tract pulse wave Doppler velocity profile can provide insight into the presence and type of pulmonary hypertension [30•].
Natriuretic peptides
The best studied cardiac biomarkers in SSc have been the natriuretic peptides [20,31–36]. Thus far, BNP and NT-proBNP have been studied primarily as biomarkers for the identification of PAH in most studies of SSc patients. However, it is well known that BNP and NT-proBNP can increase not only in the setting of RV dysfunction (e.g. in response to pulmonary hypertension) but also in LV systolic dysfunction, LV diastolic dysfunction, and myocardial ischemia [37,38]. Thus, natriuretic peptides may be better utilized as a screen for overall cardiac involvement in SSc as opposed to simply screening for pulmonary hypertension [33]. In a study of 69 consecutive patients, Allanore et al. [20] found that NT-proBNP diagnosed overall cardiac involvement (either LV systolic dysfunction, RV systolic dysfunction, or elevated pulmonary artery pressure) with an excellent area under the curve of 0.94 (95% CI 0.87–0.99) on receiver operator characteristic (ROC) analysis. These authors additionally found that an NT-proBNP cut-off of 125 pg/ml (well within the ‘normal’ range in non-SSc patients [37]) was optimal for the detection of cardiac involvement in SSc.
Most studies to date in SSc have examined NT-proBNP [20,32,33,35,39–44], which has longer half-life and increased stability [37] compared with BNP. However, in a single-center study, Cavagna et al. [45••] compared the utility of BNP and NT-proBNP for the diagnosis of PAH in SSc and found that although pulmonary artery pressure correlated with both BNP and NT-proBNP, the former was slightly superior to the latter with higher area under the ROC curve (0.74 vs. 0.63, respectively). On the basis of these recent studies, it appears that either NT-proBNP or BNP can be used for screening for primary or secondary cardiac involvement.
It is also apparent that compared with the general population, lower thresholds for BNP and NT-proBNP should be used to signify possible cardiovascular involvement in the patient with SSc. Levels of BNP greater than 60 pg/ ml or NT-proBNP greater than 125 pg/ml (values within the ‘normal limits’ for these tests) may be a sign of cardiac involvement in SSc. However, it should be noted that both cardiac and noncardiac conditions can influence natriuretic peptide levels, and these should be taken into consideration when interpreting results of BNP (or NT-proBNP) testing in SSc.
Nuclear imaging, computed tomography, and cardiac magnetic resonance
Nuclear imaging techniques such as single-photon emission computed tomography (SPECT) are currently the most commonly used method for the detection of abnormal myocardial perfusion in SSc, and have been reviewed in detail previously [2]. Cardiac computed tomography (CT) and cardiac magnetic resonance (CMR) can provide valuable information on cardiac involvement, and both provide higher spatial resolution than SPECT. Routine chest CT (which is often ordered for evaluation of ILD) can provide data on pericardial thickness and coronary calcium. Mok et al. [46•] recently demonstrated that SSc patients were more likely to have an elevated coronary artery calcium score on chest CT compared with controls. SSc disease duration was also associated with higher coronary artery calcium score. Whether these findings will be replicated in other SSc cohorts, and the clinical relevance of these findings, has yet to be determined.
CMR has been investigated in SSc and found to detect cardiac abnormalities with high sensitivity. In one study [47] of 52 patients with SSc, 75% had at least one detectable abnormality on CMR. Traditional vasodilator first-pass perfusion CMR can also reliably detect subendocardial ischemia by displaying a circumferential impairment of perfusion in the subendocardium, a finding that is thought to be due to microvascular disease in SSc [6]. Finally, CMR is emerging as a vital technique for evaluation of RV structure and function [48] and may be useful in SSc for the differentiation of primary RV involvement from secondary RV involvement due to PAH. In a CMR study of 50 SSc patients, Bezante et al. [48] found that RV ejection fraction was decreased in SSc compared with controls (47 ± 7 vs. 58±4%;P < 0.0001) and lower in dcSSc when compared with lcSSc (44 ± 6 vs. 48 ± 7%; P = 0.03).
Emerging diagnostic tools
Future tools likely to be important in SSc heart disease including speckle-tracking echocardiography for the evaluation of myocardial strain [49–52], diffuse fibrosis imaging using CMR to evaluate extent of diffuse cardiac fibrosis [53–55], and absolute perfusion CMR to evaluate for microvascular coronary ischemia [56].
Speckle-tracking echocardiography for measurement of myocardial strain
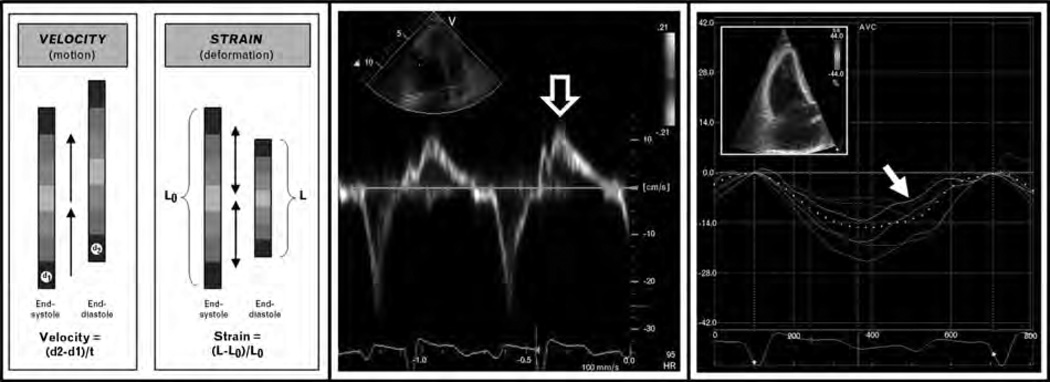
Myocardial strain (the fractional change in length of a segment of myocardium compared with its original length [49]) is a measure of cardiac deformation, which differs from tissue velocity (Fig. 1). The fundamentals of strain imaging have recently been described in detail [24,49,57]. On the basis of the association between cardiac fibrosis, circulating markers of fibrosis, and reduced myocardial strains in prior non-SSc studies [18–27,28• ,29,30• ,31–44,45•• ,46• ,47–58, measurement of strain in SSc may be a valuable tool for the assessment of preclinical myocardial involvement in SSc [59,60]. Matias et al. [59] performed speckle-tracking echocardiography of the RV in patients with SSc and compared with controls; they found that while tissue Doppler indices were similar between groups, reduced strain by speckle-tracking echocardiography was present in the SSc patients.
Figure 1. Advanced echocardiographic techniques for the evaluation of cardiac involvement in systemic sclerosis: tissue Doppler imaging vs. speckle-tracking echocardiography.
Left panel: Differences between myocardial velocity and myocardial strain. Middle panel: Example of tissue velocities of basal RV free wall in a patient with SSc [open arrow points to peak systolic (S’) tissue velocity]. Right panel: Example of systolic strain curves of the RV derived from speckle-tracking echocardiography in the same patient with SSc (inset = 2D speckle-tracking image; arrow points to peak systolic strain of the basal RV free wall). In this case, peak systolic strain (−4.4%) was reduced (consistent with intrinsic RV systolic dysfunction) whereas peak systolic tissue velocity (12.3 cm/s) was preserved. RV systolic tissue velocity was normal despite abnormal RV function due to the tethering effect of normal LV systolic function (i.e. RV longitudinal velocity, but not strain, is somewhat dependent on LV systolic function as the LV ‘pulls’ the RV base toward the apex during systole). Thus, speckle-tracking strain analysis may be advantageous (compared with tissue Doppler imaging) for the detection of subclinical cardiac involvement.
Cardiac magnetic resonance diffuse fibrosis imaging
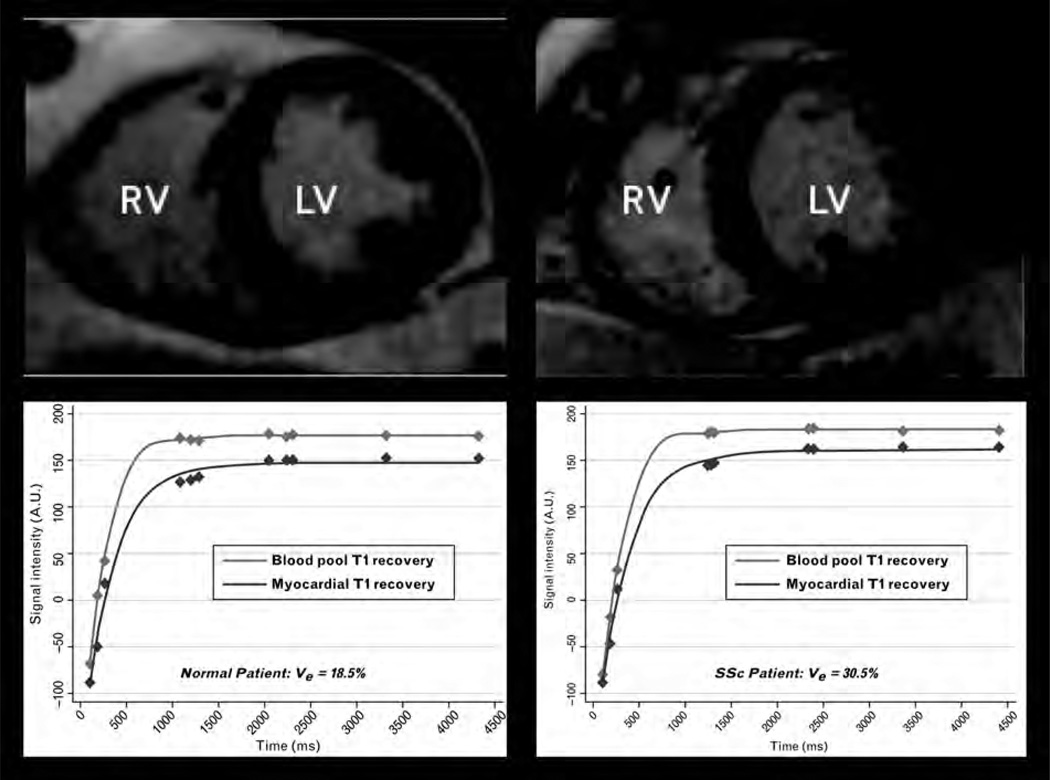
Late gadolinium enhancement imaging (LGE) has become the gold standard for detecting and quantifying focal myocardial fibrosis. However, in patients with SSc, fibrosis is relatively diffuse and therefore cannot be detected readily using traditional LGE imaging, which relies on differential uptake of gadolinium in scarred (fibrotic) and adjacent nonscarred (nonfibrotic) myocardial tissue [61]. Fortunately, recent advances in CMR now allow measurement of diffuse myocardial fibrosis using a T1 mapping technique and calculating the extracellular volume fraction (Ve) [53–55]. Because myocardial fibrosis has a larger Ve than normal myocardium, Ve correlates with the degree of diffuse myocardial fibrosis [62•]. Although this technique has not yet been reported in SSc, it is likely to be an important future assessment tool in SSc both for detection of subclinical disease and response to novel SSc therapies. Figure 2 displays an example of increased Ve in a patient with SSc compared with normal.
Figure 2. Diffuse myocardial fibrosis quantification by cardiac magnetic resonance.
Late gadolinium enhanced (LGE), mid-left ventricular short axis images and corresponding postcontrast T1 mapping of the blood pool and myocardium in a normal control (left panel) and a patient with diffuse cutaneous SSc (right panel). Although LGE images demonstrate no focal areas of myocardial fibrosis, postcontrast myocardial T1 mapping demonstrates faster recovery (shorter T1) in the SSc patient. Precontrast and postcontrast T1 values in the blood and myocardium are used to calculate the extracellular volume fraction (Ve). In the example shown, there is substantially greater diffuse myocardial fibrosis (higher Ve) in the SSc patient compared with normal despite similar appearance of the left ventricle.
Cardiac magnetic resonance absolute perfusion
Like the detection of myocardial scar, traditional methods for detecting abnormalities in myocardial perfusion (i.e. ischemia) also rely on relative differences between adjacent myocardial territories. Thus, currently available perfusion techniques are most helpful for focal epicardial coronary disease and less helpful for diffuse microvascular ischemia such as that seen in SSc. Using models similar to those used for thermodilution estimation of cardiac output, absolute myocardial blood flow can now be calculated by first-pass perfusion CMR [56]. Perfusion quantification may improve the detection of diffuse limitations in coronary vasodilator reserve in SSc and may provide a novel way to quantify its response to therapy.
Specific cardiac manifestations of systemic sclerosis
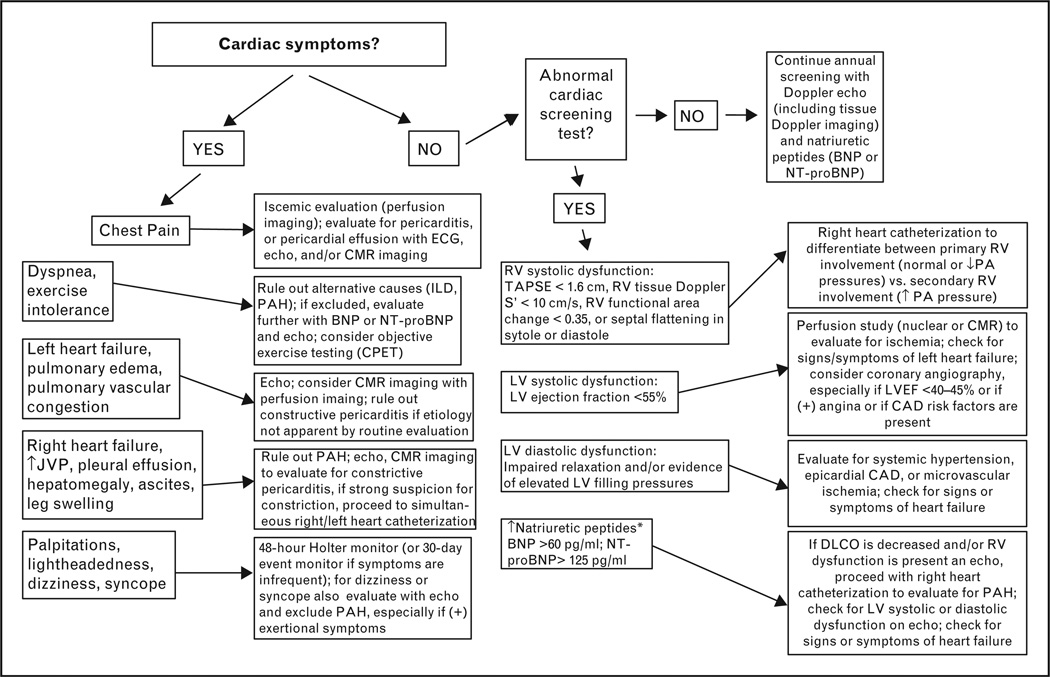
Given the many types of cardiac involvement in SSc, it is important for all those involved in the care of SSc patients to understand the screening, diagnosis, and treatment of cardiac manifestations. Figure 3 displays an algorithm for the diagnosis of cardiac manifestations in SSc when cardiac symptoms are present or when screening tests are abnormal. Table 1 lists the various types of cardiac manifestations of SSc, their prevalence, diagnostic techniques, and recommended treatments.
Figure 3. Clinical algorithm for screening and diagnosis of cardiac involvement in systemic sclerosis.
BNP, B-type natriuretic peptide; CAD, coronary artery disease; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise testing; DLCO, carbon monoxide diffusing capacity; ILD, interstitial lung disease; JVP, jugular venous pulsations; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PA, pulmonary artery; PAH, pulmonary arterial hypertension; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion. *Differential diagnosis also includes noncardiac causes of natriuretic peptide evaluation including renal insufficiency, sepsis/critical illness, glucocorticoids, malignancy, and weight loss/cachexia.
Table 1.
Prevalence, diagnosis, and treatment of cardiac manifestations in systemic sclerosis
| Cardiac manifestation | Prevalence | Diagnostic tests | Treatment |
|---|---|---|---|
| LV systolic dysfunction | 5.4% | Echocardiography, CMR imaging, natriuretic peptides | Beta-blockera, ACE-inhibitor/ARB; calcium channel blocker if microvascular coronary disease is present; referral to heart failure specialist |
| LV diastolic dysfunction | 30–35% | Echocardiography, tissue Doppler imaging, natriuretic peptides | Treatment of heart failure symptoms if they exist; evaluate for microvascular ischemia and treat with calcium channel blocker if present |
| Primary RV dysfunction | 38% | Echocardiography, tissue Doppler imaging, CMR imaging, natriuretic peptides | Digoxin, diuretics for signs, symptoms of right heart failure; rule out pulmonary hypertension with invasive hemodynamic testing |
| Microvascular coronary artery disease | > 60% | Myocardial perfusion imaging (either nuclear imaging or CMR imaging) | Calcium channel blockers, ACE-inhibitors/ARB; consider ranolazine if angina is present; statins |
| Macrovascular (epicardial) coronary artery disease | 25%b | Coronary angiography; calcium score and coronary CT may be useful for screening | Coronary stenting or standard medical management of coronary artery disease; statins |
| Myocarditis | Rare | Echocardiography, cardiac MRI with gadolinium contrast, endomyocardial biopsy | Cyclophosphamide, intravenous pulse steroids; if symptomatic systolic heart failure is present, treat according to guidelines; co-management with heart failure specialist |
| Pericardial effusion | 5–16% | Echocardiography with Doppler imaging to look for echocardiographic signs of cardiac tamponade | No treatment indicated unless symptomatic; rule out renal crisis; cautious diuretics in patients with symptoms of dyspnea or right heart failure; reserve pericardiocentesis for severe symptoms/cardiac tamponade, and avoid pericardiocentesis in patients with significant PAH/RV dysfunction |
| Constrictive pericarditis | Rare | CMR imaging, cardiac CT, echo (including Doppler and tissue Doppler); simultaneous right and left heart catheterizationa | Treatment of right heart failure symptoms: diuretics, sodium and fluid restriction; pericardial stripping surgery contraindicated in most cases |
| Bradyarrhythmias | Rare | Holter monitor, event monitor | Pacemaker according to standard guidelines |
| Tachyarrhythmias | 15% | Holter monitor, event monitor | Centrally-acting calcium channel blockers (diltiazem, verapamil); Beta -blockers should be avoided if Raynaud’s is present; consider ablation or defibrillator in select cases |
Vasodilating beta-blockers, such as carvedilol, are preferred, especially in patients with Raynaud’s phenomenon.
Defined as coronary artery calcium score greater than 101.
Intrinsic myocardial disease: myocardial fibrosis, microvascular ischemia, and left ventricular dysfunction
Recurrent vasospasm, poor vasodilator reserve, focal ischemia, recurrent ischemia-reperfusion injury, and inflammation (when present) may all contribute to the development of myocardial fibrosis and the varied clinical manifestations of myocardial disease in SSc, which include asymptomatic LV systolic or diastolic dysfunction and clinically overt heart failure [63•].
Overt heart failure, in turn, can present as acute myocarditis or myopericarditis with a rapid decline in LV ejection fraction; chronic heart failure with reduced ejection fraction (systolic heart failure); or chronic heart failure with preserved ejection fraction (diastolic heart failure), each of which has different treatment options (Table 1).
The beneficial effects of vasodilators, such as calcium channel blockers mostly of dihydropyridine type, angiotensin converting enzyme inhibitors, and endothelin receptor antagonist have been demonstrated in SSc patients [4]. Recently, the potential long term benefit of calcium channel blockers was demonstrated in a large series of 7073 SSc patients: age, male gender, digital ulcerations, myositis, and lung involvement were each independently associated with increased prevalence of LV dysfunction; in contrast, calcium channel blocker use appeared to be protective (odds ratio 0.41; 95% CI 0.22–0.74) [5••]. Although these data are highly promising, it should be noted that the aforementioned study was observational and therefore cannot prove a cause-and-effect relationship between calcium channel blocker use and reduction in incidence of LV dysfunction.
Right ventricular dysfunction
Several studies have demonstrated primary abnormalities of RV function in SSc, independent of pulmonary hypertension [48,64–71]. A study by Meune et al. [71] focused on cardiac function in 42 SSc patients with normal pulmonary arterial pressure and less than 5 years of disease duration compared with matched controls; using radionuclide ventriculography, these investigators found that reduced RV ejection fraction (present in greater than one-third of patients) correlated with both LV ejection fraction and peak filling rate, whereas no correlation was found with either pulmonary function impairment or pulmonary arterial pressure, thereby suggesting intrinsic myocardial involvement in these patients. Others have found abnormalities in RV diastolic function using tissue Doppler imaging [70] and TAPSE [69] in patients with SSc. Despite these findings, data on therapeutics for primary RV dysfunction in SSc are lacking; therefore, treatment is empiric (Table 1).
Pericardial disease
Patients with SSc can develop a range of complications due to pericardial disease, including acute pericarditis, pericardial effusion, cardiac tamponade, and constrictive pericarditis [3]. Pericardial effusion is common in SSc and often asymptomatic. At autopsy, the prevalence of peri- cardial involvement has been reported to be as high as 78%; however, clinically symptomatic pericardial disease is only present in 5–16% [3,47,72]. When significant pericardial disease is present, it can cause considerable morbidity in patients with SSc. The pathophysiology of pericardial disease in SSc is not well known.
Dunne et al. [73•] recently reviewed all cases in the literature of large pericardial effusions and cardiac tamponade in SSc. They found that cardiac tamponade is an infrequent complication of SSc, PAH is an important risk factor, and when a hemodynamically significant pericardial effusion occurs in the patient with SSc-PAH, prognosis is poor. In this situation, the authors advocate stabilization of RV function with pulmonary vasodilators, followed by cautious pericardiocentesis only when absolutely necessary.
Diagnosis of constrictive pericarditis, and its differentiation from restrictive cardiomyopathy, can be challenging, especially in the patient with SSc in whom both pathophysiologic processes can coexist. Abnormal interventricular spetal motion and preserved longitudinal mitral annular early diastolic (E’) tissue velocity are the two echocardiographic signs that favor a diagnosis of constrictive pericarditis [74].
Conduction system disease
Fibrosis of the conduction system disease can occur in SSc, and its relationship to myocardial fibrosis is variable [75]. When conduction system fibrosis occurs, it most commonly involves the sinoatrial node [76], though conduction system abnormalities in SSc are rarely symptomatic [77]. However, patients with SSc can develop palpitations, syncope, and even sudden death. In addition, in the Genetics and Environment in Scleroderma Outcome Study (GENOSIS), clinically significant arrhythmia on ECG was associated with increased mortality [78]. Thus, recognition and identification of bradyarrhythmias and tachyarrhythmias is essential. Several ECG abnormalities have been reported in SSc, as summarized in detail by Lubitz et al. [77]. Electrocardiographic abnormalities are predictive of survival, although it is unclear whether these changes reflect overall disease burden or contribute directly to morbidity and mortality.
As opposed to the relatively low incidence of symptomatic conduction system disease and bradyarrhythmias, ventricular and supraventricular arrhythmias are common [3,77,79•,80,81]. Advanced age and SSc disease burden, and possibly extent of lung involvement, have been associated with arrhythmic burden. Unfortunately, there is limited data on the use of 3SSc-specific therapies, vasodilators, antiarrhythmics, or devices such as pacemakers or defibrillators in patients with SSc. Bernardo et al. [82•] recently reported a case series of 10 patients with SSc, all of whom had evidence of ventricular arrhythmia on 24-h Holter monitoring. All underwent implantable cardioverter-defibrillator (ICD) placement; after 3 years of follow-up, 30% of the patients had ventricular tachyarrhythmias, which were appropriately terminated, suggesting potential life-saving benefit of ICD implantation.
Conclusion
Early diagnosis and treatment of direct cardiac involvement in SSc are essential. On the basis of available data, ideal screening includes annual comprehensive echocar-diography (including tissue Doppler imaging) and natriuretic peptide testing (either BNP or NT-proBNP). If present, symptoms and signs of possible cardiac involvement should be thoroughly investigated, thereby ensuring timely intervention in the at-risk patient. For the most common type of cardiac involvement, intrinsic myocardial disease, treatment with vasodilators improves myocardial perfusion and may deter the development of overt LV systolic dysfunction by reducing progression of myocardial fibrosis. Despite advances in our understanding of cardiac involvement in SSc, several questions remain unanswered:
What are the best ways to screen for cardiac involvement? How often? What modalities?
Which abnormalities on cardiac screening tests are associated with functional limitation and/or adverse outcomes?
Does treatment of SSc patients with asymptomatic cardiac involvement (detected on screening tests) improve outcomes?
Fortunately, many centers are actively investigating cardiac manifestations of SSc and hope to answer these questions. Novel diagnostic techniques and multicenter studies should yield important new data, which will hopefully ultimately result in improved outcomes.
Key points.
Cardiac involvement is common in SSc and associated with increased morbidity and mortality.
Diffuse cutaneous SSc, rapid progression of skin thickening, and older age at SSc onset are all risk factors for cardiac involvement in SSc.
Echocardiography, including tissue Doppler imaging, and natriuretic peptides are the key screening modalities for cardiac involvement in SSc; a B-type natriuretic peptide (BNP) greater than 60 pg/ml or N-terminal pro-B-type natriuretic peptide (NT-proBNP) greater than 125 pg/ml are signs of possible cardiac involvement.
Calcium channel blockers are associated with a reduced frequency of LV systolic dysfunction and may be helpful for both prevention and treatment of cardiac involvement in SSc.
Acknowledgements
D.C.L. has received research grant funding from the American Heart Association (#0575041N) and St. Jude Medical. S.J.S. has received research grants from the National Institutes of Health (R01 HL107577), American Heart Association (#0835488N), Actelion (Entelligence Award), and Gilead Sciences.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 621 – 622).
- 1.Meune C, Vignaux O, Kahan A, et al. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis. 2010;103:46–52. doi: 10.1016/j.acvd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii45–iii48. doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- 3.Champion HC. The heart in scleroderma. Rheum Dis Clin North Am. 2008;34:181–190. doi: 10.1016/j.rdc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SJ, Kahan A. Cardiac involvement: evaluation and management. In: Varga J, Denton C, Wigley F, editors. Scleroderma: from pathogenesis to comprehensive management. New York: Springer; 2011. [Google Scholar]
- 5. Allanore Y, Meune C, Vonk MC, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010;69:218–221. doi: 10.1136/ard.2008.103382.This is the largest study thus far of LV systolic dysfunction in SSc. The authors found a prevalence of 5.4% (defined as LV ejection fraction < 55%) and identified several factors associated with LV systolic dysfunction. Importantly, the authors found that calcium channel blocker usewas associated with reduced risk of LV systolic dysfunction.
- 6.Kobayashi H, Yokoe I, Hirano M, et al. Cardiac magnetic resonance imaging with pharmacological stress perfusion and delayed enhancement in asymptomatic patients with systemic sclerosis. J Rheumatol. 2009;36:106–112. doi: 10.3899/jrheum.080377. [DOI] [PubMed] [Google Scholar]
- 7.Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1012 Italian patients. Medicine (Baltimore) 2002;81:139–153. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 8. Domsic RT, Rodriguez-Reyna T, Lucas M, et al. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis. 2011;70:104–109. doi: 10.1136/ard.2009.127621.In this prospective study of 826 patients with SSc, patients with a rapid skin thickness progression rate had more frequent development of cardiac involvement at 1 year (P = 0.03)
- 9.Manno RL, Wigley FM, Gelber AC, Hummers LK. Late-age onset systemic sclerosis. J Rheumatol. 2011;38:1317–1325. doi: 10.3899/jrheum.100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El-Shafie MM, Salem SS, Moghazi AA. Left ventricular myocardial ischemia in collagen disease associated with pulmonary hypertension: an evaluation by rest-stress gated SPECT and coronary angiography. Nucl Med Commun. 2011;32:641–648. doi: 10.1097/MNM.0b013e32834654f8.This study included 20 patients with SSc, of whom 10 had PAH. Myocardial perfusion imaging showed that 3/10 patients with SSc and PAH had LV perfusion defects, demonstrating concomitant PAH and direct cardiac involvement in SSc.
- 11.Vogel-Claussen J, Skrok J, Shehata ML, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology. 2011;258:119–127. doi: 10.1148/radiol.10100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum. 2010;39:269–277. doi: 10.1016/j.semarthrit.2008.06.002.This Canadian cohort study followed 185 patients with SSc for 10 years and found that cardiac involvement, hypertension, and dcSSc were independently associated with worse survival. Of the 42 patients that died during follow up, the most common causes were ILD (n= 10), cardiac involvement (n= 9), and PAH (n= 5)
- 13.Czirjak L, Kumanovics G, Varju C, et al. Survival and causes of death in 366 Hungarian patients with systemic sclerosis. Ann Rheum Dis. 2008;67:59–63. doi: 10.1136/ard.2006.066340. [DOI] [PubMed] [Google Scholar]
- 14.Joven BE, Almodovar R, Carmona L, et al. Survival, causes of death, and risk factors associated with mortality in Spanish systemic sclerosis patients: results from a single university hospital. Semin Arthritis Rheum. 2010;39:285–293. doi: 10.1016/j.semarthrit.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 15. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815. doi: 10.1136/ard.2009.114264.The EULAR investigators followed 5860 patients with SSc, and there were 284 deaths druing follow-up. Of the 234 deaths that were available for review, 55% were attributed directly to SSc. Of the SSc-related causes of death, 35% were attributed to ILD, 26% to PAH, and 26% to cardiac causes, primarily heart failure and arrhythmia.
- 16.Plazak W, Kopec G, Tomkiewicz-Pajak L, et al. Heart structure and function in patients with generalized autoimmune diseases: echocardiography with tissue Doppler study. Acta Cardiol. 2011;66:159–165. doi: 10.1080/ac.66.2.2071246. [DOI] [PubMed] [Google Scholar]
- 17.Rosato E, Maione S, Vitarelli A, et al. Regional diastolic function by tissue Doppler echocardiography in systemic sclerosis: correlation with clinical variables. Rheumatol Int. 2009;29:913–919. doi: 10.1007/s00296-008-0827-x. [DOI] [PubMed] [Google Scholar]
- 18.Plaksej R, Kosmala W, Frantz S, et al. Relation of circulating markers of fibrosis and progression of left and right ventricular dysfunction in hypertensive patients with heart failure. J Hypertens. 2009;27:2483–2491. doi: 10.1097/HJH.0b013e3283316c4d. [DOI] [PubMed] [Google Scholar]
- 19.Can I, Onat AM, Aytemir K, et al. Detecting subclinical biventricular impairment in scleroderma patients by use of pulsed-wave tissue Doppler imaging. Tex Heart Inst J. 2009;36:31–37. [PMC free article] [PubMed] [Google Scholar]
- 20.Allanore Y, Wahbi K, Borderie D, et al. N-terminal pro-brain natriuretic peptide in systemic sclerosis: a new cornerstone of cardiovascular assessment? Ann Rheum Dis. 2009;68:1885–1889. doi: 10.1136/ard.2008.098087. [DOI] [PubMed] [Google Scholar]
- 21.Meune C, Avouac J, Wahbi K, et al. Cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography during routine care: a controlled study of 100 consecutive patients. Arthritis Rheum. 2008;58:1803–1809. doi: 10.1002/art.23463. [DOI] [PubMed] [Google Scholar]
- 22.Kepez A, Akdogan A, Sade LE, et al. Detection of subclinical cardiac involvement in systemic sclerosis by echocardiographic strain imaging. Echocardiography. 2008;25:191–197. doi: 10.1111/j.1540-8175.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 23.Allanore Y, Meune C, Kahan A. Outcome measures for heart involvement in systemic sclerosis. Rheumatology (Oxford) 2008;47(Suppl 5):v51–v53. doi: 10.1093/rheumatology/ken268. [DOI] [PubMed] [Google Scholar]
- 24.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 25.Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Early detection of cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography: relationship with neurohormonal activation and endothelial dysfunction. J Rheumatol. 2010;37:993–999. doi: 10.3899/jrheum.090931. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22:776–792. doi: 10.1016/j.echo.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 28. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010.Key recommendations for evaluation of the right heart by echocardiography. Previously, echocardiographic assessment of the RV had been primarily qualitative; however, the authors recommend a shift to more quantitative approaches particularly given the advent of newer echocardiographic imaging techniques.
- 29.Abbas AE, Fortuin FD, Schiller NB, et al. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 30. Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the rightventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:268–276. doi: 10.1164/rccm.201004-0601OC.A mid-systolic notch in the Doppler echocardiography RV outflow tract flow envelope is a sign of elevated pulmonary vascular resistance and PAH; although not specifically studied by these authors, the presence of this finding in a patient with SSc should raise suspicion for PAH.
- 31.Hesselstrand R, Wildt M, Ekmehag B, et al. Survival in patients with pulmonary arterial hypertension associated with systemic sclerosis from a Swedish single centre: prognosis still poor and prediction difficult. Scand J Rheumatol. 2011;40:127–132. doi: 10.3109/03009742.2010.508751. [DOI] [PubMed] [Google Scholar]
- 32.Mathai SC, Bueso M, Hummers LK, et al. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35:95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 33.Allanore Y, Meune C. N-terminal pro brain natriuretic peptide: the new cornerstone of cardiovascular assessment in systemic sclerosis. Clin Exp Rheumatol. 2009;27:59–63. [PubMed] [Google Scholar]
- 34.Ciurzynski M, Bienias P, Lichodziejewska B, et al. Noninvasive diagnostic and functional evaluation of cardiac involvement in patients with systemic sclerosis. Clin Rheumatol. 2008;27:991–997. doi: 10.1007/s10067-008-0837-9. [DOI] [PubMed] [Google Scholar]
- 35.Choi HJ, Shin YK, Lee HJ, et al. The clinical significance of serum N-terminal pro-brain natriuretic peptide in systemic sclerosis patients. Clin Rheumatol. 2008;27:437–442. doi: 10.1007/s10067-007-0724-9. [DOI] [PubMed] [Google Scholar]
- 36.Giannoni A, Tani C, Clerico A, et al. When the heart is burning: amino-terminal pro-brain natriuretic peptide as an early marker of cardiac involvement in active autoimmune rheumatic disease. Int J Cardiol. 2011;148:161–167. doi: 10.1016/j.ijcard.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Maisel A, Mueller C, Adams K, Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allanore Y, Borderie D, Meune C, et al. N-terminal pro-brain natriuretic peptide as a diagnostic marker of early pulmonary artery hypertension in patients with systemic sclerosis and effects of calcium-channel blockers. Arthritis Rheum. 2003;48:3503–3508. doi: 10.1002/art.11345. [DOI] [PubMed] [Google Scholar]
- 40.Mukerjee D, Yap LB, Holmes AM, et al. Significance of plasma N-terminal pro-brain natriuretic peptide in patients with systemic sclerosis-related pulmonary arterial hypertension. Respir Med. 2003;97:1230–1236. doi: 10.1016/s0954-6111(03)00254-3. [DOI] [PubMed] [Google Scholar]
- 41.Williams MH, Handler CE, Akram R, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27:1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 42.Mathai SC, Hassoun PM. N-terminal brain natriuretic peptide in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2007;28:140–141. doi: 10.1093/eurheartj/ehl425. author reply 141. [DOI] [PubMed] [Google Scholar]
- 43.Allanore Y, Borderie D, Avouac J, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum. 2008;58:284–291. doi: 10.1002/art.23187. [DOI] [PubMed] [Google Scholar]
- 44.Carlo-Stella N, Belloli L, Biondi ML, et al. Serum N-terminal pro-brain natriuretic peptide, a marker of skin thickness in systemic sclerosis? Clin Rheumatol. 2009;28:241–242. doi: 10.1007/s10067-008-1048-0. [DOI] [PubMed] [Google Scholar]
- 45. Cavagna L, Caporali R, Klersy C, et al. Comparison of brain natriuretic peptide (BNP) and NT-proBNP in screening for pulmonary arterial hypertension in patients with systemic sclerosis. J Rheumatol. 2010;37:2064–2070. doi: 10.3899/jrheum.090997.This study included 135 patients with SSc, of whom 20 had PAH. For identification of PAH, the area underthe receiver-operator characteristic curve for BNP was 0.74 (95% CI, 0.59–0.89) and 0.63 (95% CI, 0.46–0.80) for NT-proBNP. BNP was independently predictive of PAH (P= 0.032) whereas NT-proBNP was not (P= 0.50)
- 46. Mok MY, Lau CS, Chiu SS, et al. Systemic sclerosis is an independent risk factor for increased coronary artery calcium deposition. Arthritis Rheum. 2011;63:1387–1395. doi: 10.1002/art.30283.Coronary artery calcium scans were performed in 53 SSc patients and 106 controls. Regression analyses revealed SSc to be an independent predictor of increased coronary calcium score ≥ 101 (OR 10.9, 95% CI 2.2–53.8, P= 0.003). Among SSc-specific factors, only disease duration was associated with the presence of more severe coronary calcification (OR 1.1, 95% CI 1.0–1.3, P= 0.02)
- 47.HachullaA L, Launay D, Gaxotte V, et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis. 2009;68:1878–1884. doi: 10.1136/ard.2008.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bezante GP, Rollando D, Sessarego M, et al. Cardiac magnetic resonance imaging detects subclinical right ventricular impairment in systemic sclerosis. J Rheumatol. 2007;34:2431–2437. [PubMed] [Google Scholar]
- 49.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. quiz 453–455. [DOI] [PubMed] [Google Scholar]
- 50.Blessberger H, Binder T. Non-invasive imaging: two dimensional speckle tracking echocardiography: basic principles. Heart. 2010;96:716–722. doi: 10.1136/hrt.2007.141002. [DOI] [PubMed] [Google Scholar]
- 51.Pavlopoulos H, Nihoyannopoulos P. Strain and strain rate deformation parameters: from tissue Doppler to 2D speckle tracking. Int J Cardiovasc Imaging. 2008;24:479–491. doi: 10.1007/s10554-007-9286-9. [DOI] [PubMed] [Google Scholar]
- 52.Teske AJ, De Boeck BW, Melman PG, et al. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound. 2007;5:27. doi: 10.1186/1476-7120-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 54.Messroghli DR, Greiser A, Frohlich M, et al. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. doi: 10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 55.Schelbert EB, Testa SM, Meier CG, et al. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee DC, Johnson NP. Quantification of absolute myocardial blood flow by magnetic resonance perfusion imaging. JACC Cardiovasc Imaging. 2009;2:761–770. doi: 10.1016/j.jcmg.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 58.Popovic ZB, Kwon DH, Mishra M, et al. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhance-ment magnetic resonance imaging. J Am Soc Echocardiogr. 2008;21:1299–1305. doi: 10.1016/j.echo.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Matias C, Isla LP, Vasconcelos M, et al. Speckle-tracking-derived strain and strain-rate analysis: a technique for the evaluation of early alterations in right ventricle systolic function in patients with systemic sclerosis and normal pulmonary artery pressure. J Cardiovasc Med (Hagerstown) 2009;10:129–134. doi: 10.2459/JCM.0b013e32831af028. [DOI] [PubMed] [Google Scholar]
- 60.Schattke S, Knebel F, Grohmann A, et al. Early right ventricular systolic dysfunction in patients with systemic sclerosis without pulmonary hypertension: a Doppler tissue and speckle tracking echocardiography study. Cardiovasc Ultrasound. 2010;8:3. doi: 10.1186/1476-7120-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vohringer M, Mahrholdt H, Yilmaz A, et al. Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR) Herz. 2007;32:129–137. doi: 10.1007/s00059-007-2972-5. [DOI] [PubMed] [Google Scholar]
- 62. Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovas- cular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636.This study validates CMR imaging as a technique for the evaluation of diffuse myocardial fibrosis by comparing CMR results to histologic fibrosis on endomyocardial biopsy.
- 63. Mizuno R, Fujimoto S, Saito Y, et al. Cardiac Raynaud’s phenomenon induced by cold provocation as a predictor of long-term left ventricular dysfunction and remodelling in systemic sclerosis: 7-year follow-up study. Eur J Heart Fail. 2010;12:268–275. doi: 10.1093/eurjhf/hfp198.Myocardial contrast echocardiography demonstrated cardiac Raynaud’s phenomenon in 15 out of 51 SSc patients in this cohort. After mean follow up of 7.1 years, five patients developed LV systolic dysfunction, and cardiac Raynaud’s phenomenon was a strong independent predictor for the development of systolic dysfunction.
- 64.George BJ, Kwan MD, Morris MJ. Isolated right ventricular failure in scleroderma heart disease. Cardiol Rev. 2004;12:279–281. doi: 10.1097/01.crd.0000134912.95852.db. [DOI] [PubMed] [Google Scholar]
- 65.Giunta A, Tirri E, Maione S, et al. Right ventricular diastolic abnormalities in systemic sclerosis: relation to left ventricular involvement and pulmonary hypertension. Ann Rheum Dis. 2000;59:94–98. doi: 10.1136/ard.59.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez A, Seres L, Ferrer E, et al. Isolated right ventricular systolic dysfunction in scleroderma. Rev Esp Cardiol. 2008;61:990–991. [PubMed] [Google Scholar]
- 67.Hsiao SH, Lee CY, Chang SM, et al. Right heart function in scleroderma: insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr. 2006;19:507–514. doi: 10.1016/j.echo.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Huez S, Roufosse F, Vachiery JL, et al. Isolated rightventricular dysfunction in systemic sclerosis: latent pulmonary hypertension? Eur Respir J. 2007;30:928–936. doi: 10.1183/09031936.00025607. [DOI] [PubMed] [Google Scholar]
- 69.Lee CY, Chang SM, Hsiao SH, et al. Right heart function and scleroderma: insights from tricuspid annular plane systolic excursion. Echocardiography. 2007;24:118–125. doi: 10.1111/j.1540-8175.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 70.Lindqvist P, Caidahl K, Neuman-Andersen G, et al. Disturbed right ventricular diastolic function in patients with systemic sclerosis: a Doppler tissue imaging study. Chest. 2005;128:755–763. doi: 10.1378/chest.128.2.755. [DOI] [PubMed] [Google Scholar]
- 71.Meune C, Allanore Y, Devaux JY, et al. High prevalence of right ventricular systolic dysfunction in early systemic sclerosis. J Rheumatol. 2004;31:1941–1945. [PubMed] [Google Scholar]
- 72.Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis. 1997;56:393–394. doi: 10.1136/ard.56.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dunne JV, Chou JP, Viswanathan M, et al. Cardiac tamponade and large pericardial effusions in systemic sclerosis: a report of four cases and a review of the literature. Clin Rheumatol. 2011;30:433–438. doi: 10.1007/s10067-010-1667-0.Cardiac tamponade is an infrequent complication of SSc, and 26 cases of large pericardial effusions have been reported in the literature. PAH was associated with seven of the cases, and the authors suggest that management of large pericardial effusions in SSc-PAH patients should begin with stabilization of right ventricular function, followed by cautious pericardiocentesis.
- 74.Dal-Bianco JP, Sengupta PP, Mookadam F, et al. Role of echocardiography in the diagnosis of constrictive pericarditis. J Am Soc Echocardiogr. 2009;22:24–33. doi: 10.1016/j.echo.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 75.D’Angelo WA, Fries JF, Masi AT, et al. Pathologic observations in systemic sclerosis (scleroderma): a study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46:428–440. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- 76.Ridolfi RL, Bulkley BH, Hutchins GM. The cardiac conduction system in progressive systemic sclerosis: clinical and pathologic features of 35 patients. Am J Med. 1976;61:361–366. doi: 10.1016/0002-9343(76)90373-9. [DOI] [PubMed] [Google Scholar]
- 77.Lubitz SA, Goldbarg SH, Mehta D. Sudden cardiac death in infiltrative cardiomyopathies: sarcoidosis, scleroderma, amyloidosis, hemachromatosis. Prog Cardiovasc Dis. 2008;51:58–73. doi: 10.1016/j.pcad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Assassi S, Del Junco D, Sutter K, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum. 2009;61:1403–1411. doi: 10.1002/art.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010;49:355–360. doi: 10.1093/rheumatology/kep394.This prospective study enrolled 68 patients with SSc who all underwent 24-h Holter monitoring. Compared with controls, the SSc patients had higher median heart rate turbulence onset (P= 0.001) and lower median turbulence slope (P= 0.003), indicating autonomic nervous system impairment in SSc.
- 80.Wozniak J, Dabrowski R, Luczak D, et al. Evaluation of heart rhythm variability and arrhythmia in children with systemic and localized scleroderma. J Rheumatol. 2009;36:191–196. doi: 10.3899/jrheum.080021. [DOI] [PubMed] [Google Scholar]
- 81.Bielous-Wilk A, Poreba M, Staniszewska-Marszalek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol. 2009;14:251–257. doi: 10.1111/j.1542-474X.2009.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bernardo P, Conforti ML, Bellando-Randone S, et al. Implantable cardioverter defibrillator prevents sudden cardiac death in systemic sclerosis. J Rheumatol. 2011;38:1617–1621. doi: 10.3899/jrheum.100480.The authors of this study enrolled 10 SSc patients with ventricular arrhythmia on 24-h Holter monitoring, and placed an ICD in all patients. ICD analysis after 36 months showed that 3 of the 10 patients had ventricular tachyarrhythmias that were appropriately cardioverted.