Abstract
Type 2 diabetes is a result of chronic insulin resistance and loss of functional pancreatic β-cell mass. Strategies to preserve β-cell mass and a greater understanding of the mechanisms underlying β-cell turnover are needed to prevent and treat this devastating disease. Genistein, a naturally-occurring soy isoflavone, is reported to have numerous health benefits attributed to multiple biological functions. Over the past 10 years, numerous studies have demonstrated that genistein has anti-diabetic effects, in particular, direct effects on β-cell proliferation, glucose-stimulated insulin secretion and protection against apoptosis, independent of its functions as an estrogen receptor agonist, antioxidant, or tyrosine kinase inhibitor. Effects are structure-specific and not common to all flavonoids. While there are limited data on the effects of genistein consumption in humans with diabetes, there are a plethora of animal and cell-culture studies that demonstrate, at physiologically-relevant concentrations (<10 µM), a direct effect of genistein on β-cells. The effects appear to involve cAMP/PKA signaling and there are some studies that suggest an effect on epigenetic regulation of gene expression. This review focuses on the anti-diabetic effects of genistein in both in-vitro and in-vivo models and potential mechanisms underlying its direct effects on β-cells.
1. Introduction
Diabetes mellitus is a chronic disease of epidemic proportion, currently afflicting approximately 26 million people in the US (8 % of the US population), with an additional 79 million classified as “pre-diabetic” 1. While the availability of novel drugs, techniques, and surgical intervention has improved the survival rate of individuals with diabetes, the prevalence of diabetes is still rising in Americans, with the number of people with diabetes projected to more than double in the next 15 years 2. In the United States, patients with diabetes each spend an average of $6,000 annually on medical costs for treating this disease. As such, there is an imperative need for developing strategies such as discovery of effective low-cost natural products to prevent and treat this chronic disease.
Type 2 diabetes (T2D) is a result of chronic insulin resistance and loss of pancreatic islet β-cell mass and function. In humans, islets represent approximately 1–2% of total pancreas tissue 3, and up to 80% of cells in islets are insulin-secreting β-cells 4. The mass of β-cells is controlled by the balance between neogenesis (differentiation of precursor cells into β-cells), transdifferentiation (differentiation of other cell types into β-cells), proliferation of pre-existing β-cells, hypertrophy and apoptosis 5, 6. While peripheral insulin resistance (IR) is common during obesity in experimental animals and people, most obese individuals don’t develop diabetes because of increased β-cell mass and insulin secretion in response to peripheral IR. However, a small portion of individuals with IR eventually progress to T2D, which is largely due to insulin secretory dysfunction and significant apoptosis of functional β-cells 4, 7–10, leading to an inability to compensate for IR. Indeed, those with T2D always manifest increased β-cell apoptosis and reduced β-cell mass 8, 9, 11, 12. Thus, loss of functional β-cell mass (leading to a reduction in insulin secretion) plays a central role in the development of T2D 4. Little is known about the mechanisms controlling β-cell proliferation, function and apoptosis in a model of IR and diabetes, which is a major obstacle for designing more effective strategies to prevent and treat this disease. Nevertheless, the search for novel and cost-effective agents that can enhance or preserve islet β-cell mass and function, thereby providing a strategy to prevent or treat diabetes is extremely important to decrease the burden of morbidity from diabetes and related complications.
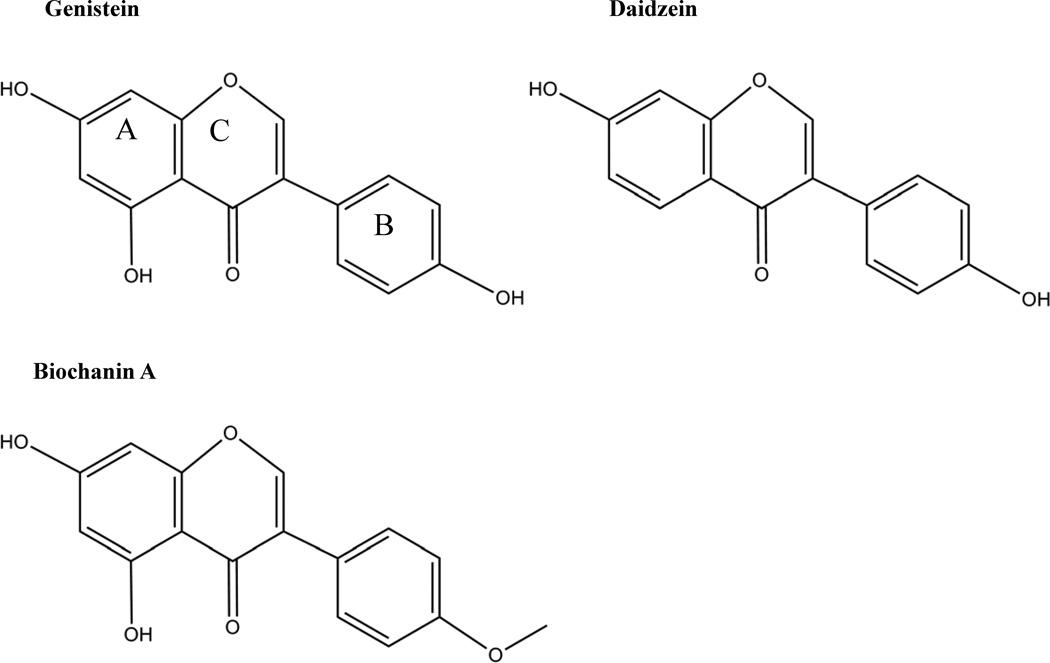
Soy isoflavones are widely used as a dietary supplement in the U.S. for various presumed health benefits 13–15, although the research evidence supporting the beneficial effects of genistein consumption on human health is not well established. Genistein is the most abundant isoflavone in soy followed by daidzein, which lacks only the hydroxyl group at C5 compared to genistein. Isoflavones in soy are conjugated to glucose as glycosides. Following consumption of a soy-rich meal, the glycoside derivatives are cleaved to aglycones by bacterial β-glucosidases in the gut 16. The main structural characteristics of isoflavone aglycones are two aromatic rings, A and B, linked by a heterocyclic pyrane ring, C (Figure 1). Most of the research discussed in this review focuses on the aglycones. The aglycones can be absorbed into the bloodstream and further modified in the liver by glucuronidation. The glucuronidated phase II conjugated compounds are either excreted in bile and reabsorbed in the gut, or excreted in urine 16. These glucuronidated derivatives show peak concentrations in the circulation at 1–2 h post-consumption of soy and again at up to 10 h later 17, 18, with the second peak associated with microbial metabolism in the large intestine 19. In other words, there is a time lag between small intestinal absorption and appearance in the circulation, and colonic microbial metabolism leading to further absorption at up to 10 h later. About 20–30 % of circulating genistein is in the unconjugated form 20. In Japanese women at delivery, fetal cord blood displayed almost double the concentration of genistein glucuronides and sulfoglucuronides as did plasma, and amniotic fluid slightly lower than plasma 21. These results suggest that maternal supplementation of genistein has the potential to influence fetal metabolism and growth, and that genistein metabolism may be unique in the fetus. In the colon, genistein can be further metabolized to dihydrogenistein (DHG) or 6’-hydroxy-O-desmethylangolensin (6-OH-O-Dma), while daidzein can be reduced to dihydrodaidzein (DHD) and converted to O-desmethylangolensin (O-Dma) or equol 22. These metabolites can be absorbed or be further metabolized to phenols in the colonic lumen 18. Clover and alfalfa are sources of biochanin A and formononetin, which are methylated versions of genistein and daidzein, respectively. Demethylation of these compounds occurs in the intestine by acetogenic bacteria and in the liver 23.
Figure 1.
Chemical structure of isoflavones
Genistein intake is considered safe as no toxic effects were observed in rats 24–26, mice 27, monkeys 28, and humans 29, 30 following pharmacological administration. Genistein has been previously investigated for its potential beneficial effects on cancer treatment, cognitive function, and cardiovascular and skeletal health, with a primary focus on exploring its potential hypolipidemic, anti-oxidative and estrogenic effects 13–15. While various biological effects of genistein will be briefly discussed, this review will mainly focus on the potential anti-diabetic effects of genistein with an emphasis on β-cell function and diabetes.
2. Estrogenic effects of genistein
The three main endogenous estrogens in humans are estrone, estradiol and estriol. 17β-estradiol is the primary reproductive hormone before menopause in non-pregnant females and is produced primarily by ovaries. It has an array of biological functions in various tissues, which include mitogenic stimulation in breast and uterus, cardioprotective action, anti-apoptosis, and beneficial effect on cognitive function. The endogenous estrogen primarily acts through its receptors in humans and animals. There are two well recognized estrogen receptors (ERs), ERα and ERβ, which differ in the C-terminal ligand-binding domain 31. Recent in-vitro studies provide evidence that plasma membrane-associated G-protein coupled receptor GPR30 is a novel membrane ER that mediates the membrane-initiated estrogen signaling in cancer cells 32–34. At concentrations of at least 10−8 M, genistein displayed estrogenic properties such as ER-dependent transcriptional activation of a transfected reporter gene construct containing copies of a consensus estrogen response element in embryonic kidney cells, increased proliferation rate of MCF-7 cells, and inhibited osteoclast formation (Table 1) 35. These assays were performed in parallel using estradiol, and the effective concentration of estradiol needed to mediate these effects were on the order of 10 to 1,000-fold lower than genistein, however, it should be pointed out that the effective concentrations of genistein are physiologically achievable following consumption of a soy-based product 35. Isoflavones can reach the lower micromolar range (approximately 5 µM) following ingestion of soy foods, peaking at 1–2 and 4–10 h post-consumption of soy foods 17, 36, 37. At the highest concentration used (10−4 M) MCF-7 cell proliferation decreased. In a cell-free system with recombinant human ERα or ERβ, it was shown that genistein displayed a relative binding affinity (RBA) for ERα and β of roughly 3 and 18 %, respectively 35. In that study, the RBA represented the ratio of the amount of genistein required to reduce the radiolabeled-E2 binding by 50%, as compared to unlabeled-E2. The RBA of the genistein metabolic intermediate, dihydrogenistein was approximately 10-fold lower 35. Daidzein and other genistein metabolites were also less effective at mediating estrogen-dependent effects. However, in an earlier study using insect cells over-expressing ERα or ERβ, genistein showed higher affinity to ERα (4% of estradiol) and particularly ERβ (87% of estradiol) 31. Interestingly, it was demonstrated in these studies that, unlike estradiol that binds to both ERα and ERβ with nearly equal affinity, genistein has much higher affinity for ERβ than ERα, which may be due to slight differences in steroid binding sites of the two receptors. Recent studies show that genistein also binds to GPR30 in cancer cells 38. The biological consequences of this interaction remain to be investigated.
Table 1.
In vitro studies of genistein function in non-β-cell type models
| Model | Genistein (µM) |
Duration | Effect | Mechanism | Ref |
|---|---|---|---|---|---|
| 293 embryonic human kidney | 0.00010 | 24 h | Gene transactivation | ERα and ERβ activation | 35 |
| RAW 264.7 cell | 0.010 | 120 h | Osteoclast formation inhibitor | ERα and ERβ activation | |
| RAW 264.7 cell | >10 | 120 h | apoptosis | ||
| MCF-7 cell | 0.0010 | 48 h | proliferation | ERα and ERβ activation | |
| MCF-7 cell | 1 | 48 h | proliferation inhibition | ||
| In vitro | 0.0000015 | n/a | Concentration to displace labeled-E2 from ERα | IC50 to determine relative binding affinity (RBA) for ERs | |
| In vitro | 0.000014 | n/a | Concentration to displace labeled-E2 from ERβ | IC50 to determine RBA for ERs | |
| In vitro | 15–50 | n/a | Inhibit lipid peroxidation | 53 | |
| In vitro | 200 | 7 h | Inhibit peroxy radical oxidation of LDL |
54 |
|
| 5 | 107 min | inhibit copper-mediated oxidation of LDL | |||
| In vitro | 100 | n/a | scavenge at least 20% of hydroxyl radicals, 30% of DPPH radicals and 22% superoxide anion radicals | 55 | |
| In vitro | 180 | inhibition of peroxidation of deoxyribose by hydroxyl radicals | IC50 | ||
| In vitro | >13 | n/a | Reduce LDL oxidation | 140 |
3. The effects of genistein on enzymes
Genistein is also an enzyme inhibitor, and it can directly inhibit the activities of protein tyrosine kinase (~≥25 µM) 39, α-glucosidase (Ki = 5.7×10−5 µM) 40, DNA topoisomerase II (≥ 20 µM) 41, 42, and S6 kinase (≥15 µM) 43. Insulin and IGF receptors are tyrosine kinase receptors, and tyrosine phosphorylation is central to intracellular signaling in β-cells 44. Tyrosine kinase inhibitors can augment glucose-stimulated insulin secretion, most likely due to the negative feedback effect of insulin on insulin secretion 45. Studies attributing effects of genistein in β-cells to its tyrosine kinase inhibitory activity should be interpreted with caution. Specific tyrosine kinase inhibitors at similar concentrations did not enhance glucose-stimulated insulin secretion or up-regulate insulin mRNA abundance in INS-1 cells 44. The effective concentrations of genistein needed for tyrosine kinase inhibition (higher micromolar range) are physiologically unrealistic.
4. The antioxidant effect of genistein
Genistein has been reported to exhibit antioxidant activity in aqueous phase systems 46, 47. However, the antioxidant effect of genistein was achieved only at concentrations ranging from 25–100 µM, suggesting that genistein is not a physiologically effective antioxidant since the achievable levels of genistein through dietary supplementation in the human circulation is no more than 7 µM 48, 49. Indeed, at physiologically achievable concentrations (approximately 5 µM), genistein was shown to be very weak in its ability to scavenge reactive oxygen species (ROS) 50–52. Consistently, Patel et al 53 demonstrated that genistein inhibited lipid peroxidation at concentrations between 15 and 50 µM, further demonstrating that it was a relatively poor antioxidant. However, one study showed that genistein at 5 µM inhibited copper-mediated oxidation of LDL inhibited peroxy radical oxidation of LDL at 200 µM concentration 54. In another study, at concentrations of ≥ 1 µM, genistein showed a greater oxygen radical absorbance capacity (i.e., antioxidant behavior towards peroxyl radicals) than an α-tocopherol analogue, Trolox 55. In the same study however, concentrations of at least 100 µM were used to demonstrate that genistein could scavenge 20% of hydroxyl radicals, 30% of DPPH radicals and 22% superoxide anion radicals 55. The IC50 for inhibition of peroxidation of deoxyribose by hydroxyl radicals was 180 µM, also a physiologically unrealistic concentration 55. Genistein was unable to form stable free radicals (i.e., stable semiquinone free radical) and did not chelate transition metals that generate ROS 55. Although genistein, at physiologically achievable concentrations, is capable of reducing oxidative stress, in vivo, dietary intake of genistein has no significant anti-oxidant activity in oxidative stress agent streptozotocin-induced diabetic animals 56 and in in healthy postmenopausal women 57.
5. The epigenetic effects of genistein
More recently, studies have appeared that link genistein to epigenetic regulation of gene expression. There are data to suggest that genistein indirectly influences histone acetylation and methylation and DNA methylation at specific gene loci in cancer studies (reviewed by 58). These effects led to reactivation of tumor suppressor genes in various cell lines through promoter demethylation and increased histone acetylation 59. Genistein is a relatively weak DNA methyltransferase and histone deacetylase (HDAC) inhibitor, but effects on both enzymes may act synergistically, leading to transcriptional activation 59. For example, treatment of KYSE 510 cells with 5 µM genistein for 5 d, in conjunction with 0.5 µM trichostatin (TSA), a HDAC inhibitor, led to an increase in mRNA abundance of RARβ, p16 and MGMT as compared with genistein treatment alone 60. This effect of treatment with both compounds was not associated with increased histone acetylation, suggesting that other mechanisms were responsible for transcriptional activation 60. An important question in epigenetics is how maternal nutrition (epigenetic programming is most dynamic during the fetal period) affects growth, development, and longevity of the offspring. Genistein is detected in human fetal cord blood and amniotic fluid in various structural forms, thus having the potential to affect fetal metabolism 21. There are a number of studies involving maternal supplementation of genistein to pregnant rodents. Offspring of rats that received genistein (supplemented in the diet at 300 mg/kg feed) during pregnancy displayed reduced thymus mass, altered lymphocyte populations, and reduced testosterone, suggesting that genistein supplementation during gestation may have unfavorable effects on reproductive physiology 61. On the other hand, there are several animal studies showing that maternal supplementation of genistein has favorable long-term metabolic consequences in offspring that are associated with lower body weight and fat mass 62, 63, which may be due to modification of fetal epigenetic patterns by geneistein in the maternal diet 63, 64. Indeed, maternal supplementation of genistein altered coat color distribution and reduced obesity in mice carrying the agouti Avy allele, with hypermethylation of the retrotransposon upstream of the Agouti gene transcription start site 63. These results suggest a direct effect on DNA methylation as methylation at this retrotransposon is directly influenced by dietary supplementation of methyl donors. There are limited data to indicate that genistein directly influences DNA methyltransferase activity (i.e., cell-free system) and no reports, to our knowledge, of genistein-mediated chromatin-remodeling and transcriptional reactivation in a diabetic model.
6. The anti-diabetic actions of genistein
A number of health benefits are attributed to isoflavones and recent evidence indicates a potential for genistein as a preventative and therapeutic treatment for patients with IR and diabetes. Following dietary supplementation, concentrations of circulating genistein may reach as high as 7 µM in humans 17 and rodents 65, suggesting that many of the studies conducted with genistein in both cell culture (Table 2) and animal models (Table 3) have physiological relevance. In humans, soy consumption has been linked to positive outcomes on glycemic control in postmenopausal women 66, 67 and diabetic rodents 68, 69. Although there are many studies that support the benefits of soy consumption in health, this review will focus on the anti-diabetic properties of genistein, with an assessment of both in vitro and in vivo studies, and its potential to influence pancreatic β-cell function. For ease of comparison among studies, particularly to comment upon differences in model system, concentrations of genistein, duration of treatment and effects observed, results from different reports are summarized in Table 2 (cell culture studies) and Table 3 (animal studies).
Table 2.
Comparison of studies that evaluate the effect of genistein function in β-cells
| Model | Genistein (µM) |
Duration | Effect | Mechanism | Ref |
|---|---|---|---|---|---|
| INS-1 cells | 100 | 1 h | ↑ GSIS | Tyrphostin 25, a tyrosine kinase inhibitor, also ↑ insulin release | 44 |
| Rat islets | 100 | 1 h-4d | ↑ GSIS & ↓ proliferation | 99 | |
| MIN6 cells | 100 | ↑ GSIS | ↑ cAMP accum., calcium dependent | 98 | |
| RIN cells and rat islets | 5–40 | 48 h | No effect on GSIS Prevented IL1-β and IFNγ mediated ↓ in GSIS, cell viability, proliferation and prevented ↑ in iNOS and NO production |
107 | |
| INS-1, MIN6 cells and mouse islets | 0.01–10 | 30 min | ↑ GSIS, cAMP and PKA activation, with maximal effect at 5 µM | Not dependent on ER or tyrosine kinase inhibition. | 103 |
| INS-1 cells and human islets | >0.1 | 24h | Cell proliferation | Increased cAMP/PKA and ERK1/2 activity | 56 |
| RINm5F cells, human and rat islets | 25 &100 | 24 h | 25 µM ↓ apoptosis & 100 µM ↑ apoptosis | 110 | |
| Mouse islets | 10–100 | 1 h | ↑ GSIS. No ↑ in cAMP. ↓ Ca2+ influx. Daidzein showed less potent effects. | Effect abolished by adrenaline or removing extracellular Ca2+. No effect of blocking ATP-sensitive K+ channels. | 100 |
| Rat islets | 50–500 | 1 h | 50 µM: ↑ GSIS >100 µM: ↓ GSIS |
97 | |
| Human islets | 100 | 24 h | Prevented high-glucose induced cell damage | Reversed by anti-estrogen | 109 |
| INS-1 cells and rat islets | 50 | 2 h | ↑ Leu/Gln, glucose and pyruvate stimulated insulin secretion | CaMK II activation, but not PKA activation or tyrosine kinase inhibition | 106 |
| INS-1 cells | 100 | 90 min &12 h | ↑ GSIS after 90 min. ↑ insulin mRNA after 8 h | Other tyrosine kinase inhibitors did not ↑ GSIS. Genistin & daidzein ↑ insulin mRNA. | 44 |
| INS-1 cells, mouse and human islets | 1–5 | 48 h | ↑ GSIS and pyruvate SIS, ↑ Ca2+ in cells | PKA inhibitor blocked the effect | 141 |
Table 3.
Effects of genistein on β-cell function in animal diabetic models
| Model | Genistein (µM) |
Duration | Effect | Mechanism | Ref |
|---|---|---|---|---|---|
| C57Bl/6J, 1 mo. old, STZ at 40mg/kg BW×5 d. | 250 mg/kg of diet | 2 wk | ↓ FBG, ↑ serum insulin, improved glucose tolerance, ↑ β cell mass & ↓ apoptosis in diabetics | Preservation of functional β cell mass | 56 |
| C57BL/6 10-mo. old, STZ, 90 mg/kg BW | 250 mg/kg diet | 1 mo. in high-fat diet | ↓ FBG, ↑ serum insulin, & ↓ β cell apoptosis in diabetics | 84 | |
| C57BL/6 8 wk old, STZ, 45 mg/kg BW×5d | 10 mg/kg BW via I.P. injection (3/wk) | 10 wk | ↓ FBG, ↓ proteinuria, albumin, nephrin & collagen excretions, ↓ oxidative stress | Increased renal phospho-Tyr and phospho- ERK/ERK | 81 |
| ♀ NOD mice, 8 wk old | 200 mg/kg diet | 9 wk | ↓ FBG, ↑ plasma insulin & C-peptide, ↓BUN, ↑ insulin in β-cells, ↓ gluconeogenis in liver, ↓ plasma FFA & triglyceride, & ↑ HDL | 89 | |
| ♂ Sprague Dawley, 7–8 wk old, alloxan-220 mg/kg BW | 30 mg/kg BW oral gavage | 4 wk post-alloxan injection | ↓ FBG, improved glucose tolerance, ↑ serum insulin, preserved islet mass | 80 | |
| Isolated islets | 6.25, 12.5 and 25 µM | 24 h | 12.5 & 25 µM prevented apoptosis | 80 | |
| ♂ Sprague Dawley, (80–90 g), STZ, 50 mg/kg BW | 600 mg/kg diet (after STZ) | 3 wk | ↓ FBG and ↑ plasma insulin, ↓ HbA1C, ↓ hepatic antioxidant enzyme activities | 79 |
6. 1. Human studies
Data on human soy isoflavone intake and diabetes risk are limited, particularly studies that use purified genistein as a treatment. In a cross-sectional study involving post-menopausal women, daily soy intake was associated with lower body mass index and fasting circulating insulin levels 70. There are a number of epidemiological studies from Asian populations, in which soy has long been a staple of the diet. Among overweight but not lean Japanese women, soy intake was associated with a reduced risk of T2D 71. In a group of middle-aged Chinese women with no history of diabetes, cancer or cardiovascular disease, intake of legumes, including soy, was associated with reduced T2D risk 72. In post-menopausal Chinese women with pre-diabetes or early stage untreated diabetes, 3 or 6 month treatment with soy protein (15 g), either alone, or supplemented with isoflavones (100 mg), did not affect measures of glycemic control and insulin resistance 73. Other intervention trials show that isoflavone consumption after the onset of T2D was associated with positive health outcomes. In post-menopausal women being treated for T2D, daily isoflavone intake (100 mg of aglycones) for one year led to an improvement in insulin sensitivity and blood lipid parameters, reducing the risk for cardiovascular disease 74. Similar results were observed in a more short-term study with post-menopausal women being treated for T2D, where 12-week supplementation of soy (30 g isolated soy protein containing 132 mg of glucoside conjugated isoflavones) lowered fasting insulin, insulin resistance, glycated hemoglobin (A1C) , and total and LDL cholesterol 66. In contrast, a later study showed that daily consumption of isoflavones (132 mg) for 3 months had no effect on body mass index, plasma A1C, glucose, insulin or lipids in postmenopausal women with T2D, suggesting that other components of the soy could be responsible for the health benefits observed in the previous study 75. Six weeks of consuming a soy product that provided at least 165 mg of isoflavones per day led to improved blood lipid profiles but not glucose homeostasis in a group of T2D patients 76. Clearly, there are conflicting results on the effects of isoflavone consumption on cholesterol levels and measures of glucose control, with most of the studies using a soy-based product containing a mixture of different components. A recent meta-analysis of 24 human studies revealed that although soy consumption did not affect measures of glycemic control (e.g., fasting insulin, glucose and A1C), studies with intact soy protein rather than purified isoflavones or protein extract were associated with reductions in fasting blood glucose 77. This suggests components of soy other than isoflavones or interactions of isoflavones with other soy components were attributable to the anti-diabetic effects of soy in human studies. A limitation to comparing across different human trials is the range of isoflavone content in different soy products, other matrix components and methods for soy processing, all of which influence bioavailability and efficacy of the compounds 77. The metabolism and biological effect of genistein could be altered when consumed as a soy isoflavone mixture. For example, there is possibility that daidzein, which is considered an inactive analog of genistein in various biological actions and always mixed with genistein in isoflavone products used in human studies, may interfere with the effect of genistein. Indeed, data from a recent human study examining the effect of genistein administration on metabolic parameters show that genistein administration at 54 mg/day decreased fasting glucose and increased glucose tolerance and insulin sensitivity in postmenopausal women 78. Moreover, the genetic background of the human population, age and underlying health condition also influence the response to soy or isoflavone consumption. The studies described herein encompass lean, obese, pre-diabetic, diabetic and untreated, diabetic and treated, from a variety of ethnic backgrounds. The geographic backgrounds of patients are not always reported, nor are other demographic factors that could be useful in determining associations between isoflavone intake and health indicators. Combined with the effects of different lifestyle factors, other health interventions, and duration of treatments, it is challenging to find a strong association between soy intake and diabetes risk. It was suggested that there may be differences in metabolism and hence bioavailabilty of flavonoids in human compared with other mammals 73. Another observation is that the majority of human trials involved women and the gender-specific effects of isoflavones are unknown. Regardless, human studies using genistein alone to test its anti-diabetic efficacy are scarce, and therefore, whether this isoflavone can be used to prevent or ameliorate T2D in humans is largely unknown. As discussed in the remainder of this review, there are many data from animal models and in vitro to show that genistein may be a novel anti-diabetic compound, an effect that is independent of previously known biological functions as a phytoestrogen, tyrosine kinase inhibitor, or antioxidant.
6. 2. Animal studies
In animal models, there are multiple studies that show an anti-diabetic effect of genistein when it is administered pharmacologically by oral gavage, or when included in the diet (Table 3). Dietary supplementation of genistein at 600 mg/kg diet in streptozotocin (STZ, a toxic alkylating agent exclusively transported into cells by GLUT2)-induced diabetic rats for 3 weeks led to significant reductions in fasting blood glucose and a concomitant increase in plasma insulin levels, suggestive of a protective effect on β-cell function 79, given that STZ induces diabetes by selectively causing pancreatic β-cell destruction. Male Sprague Dawley rats that were 7–8 weeks of age, subjected to i.p. injection of alloxan (220 mg/kg BW), a toxic glucose analogue that destroys β-cells, developed diabetes and were used as a similar model to study the anti-diabetic effects of genistein 80. In this study rats were orally gavaged with 8, 18 or 30 mg/kg BW of genistein daily for 4 weeks after receiving alloxan injections. The 8 mg dose was intended to represent daily intake of a soy rich diet in humans and was not effective in offsetting the effects of alloxan. The 30 mg dose was the most effective in mitigating the effects of alloxan, with reduced fasting blood glucose, increased serum insulin and enhanced islet mass after 4 weeks, compared with rats that received alloxan but not genistein 80. Consistently, in diabetic mice that consumed a genistein-supplemented diet (250 mg/kg diet) prior to induction of diabetes by STZ there was a preservation of islet mass due to enhanced proliferation and reduced apoptosis relative to the control mice 56. Accordingly, dietary intake of genistein prevented STZ-induced rises in fasting blood glucose, and improved glucose tolerance and circulating insulin levels. 56. Interestingly, genistein had no effect on blood lipid profiles or hepatic antioxidant activities in diabetic mice 56, suggesting that the beneficial effect of genistein on pancreatic islets is not due to a secondary action whereby genistein modulates these variables. Elmarkby et al 81 induced diabetes in 8-week old C57BL/6 mice by injecting STZ at 45 mg/kg body weight for 5 days, and they then administered genistein at 10 mg/kg body weight for 3 times a week for 10 weeks. They observed that diabetic mice treated with genistein displayed significant reductions in fasting blood glucose as well as reductions in protein, albumin, nephron and collagen excretions, and a decrease in urinary monocyte chemoattractant protein-1 excretion and renal intercellular adhesion molecule-1 expression.
Recent studies indicated that the capacity of β-cell regeneration is very limited in middle-aged and older animals 82, 83, demonstrating that animal models for T2D should take into account that human T2D occurs mostly in middle-aged and older individuals with manifestation of reduced β-cell mass 11. We recently reported the results of a study where we fed a high-fat diet (60 % of calories from lard) supplemented with 250 mg/kg genistein to 10-month old C57BL/6 mice for 4 weeks and induced diabetes with a 90 mg/kg dose of STZ 84, which does not cause diabetes in chow-fed mice 85–87. This mouse model shares the metabolic characteristics of human T2D manifested with insulin resistance and reduced β-cell mass and function 85–87. At 1 week post-STZ injection, mice that consumed genistein-supplemented diets displayed 30% lower fasting blood glucose values than those that consumed the high fat diet alone. Genistein supplementation also protected against STZ-induced destruction of β-cell mass, with mice that ingested genistein having more than twice the β-cell mass of those that did not consume genistein 84.
The non-obese diabetic (NOD) mouse is a commonly used model for studying autoimmune-mediated T1D, and typically develop hyperglycemia at around 12 weeks of age 88. Nine-week old females provided with genistein-supplemented (200 mg/kg) diets for 9 weeks displayed reductions in fasting blood glucose, increases in plasma insulin and C-peptide, and enhanced insulin staining in the islets of pancreas sections 89, consistent with the observations in STZ-induced diabetic mice 56, 84. Genistein treatment also decreased activity of gluconeogenic enzymes in the liver and improved the blood lipid profiles in NOD mice. For the entire duration of the study, blood glucose levels of mice consuming the genistein-supplemented diet were similar to that of “healthy” levels observed at younger ages 89.
Genistein was also shown to exert an anti-diabetic action in obese diabetic (db/db) mice, a genetic T2D animal model, which shares the metabolic characteristics of human T2D with peripheral insulin resistance and reduced β-cell mass and function 90, 91. Specifically, diabetic mice fed genistein at 200 mg/kg diet displayed significant lower blood glucose and A1C levels and improved glucose tolerance and plasma insulin/glucagon ratio, which were associated with higher hepatic glucokinase activity but significantly lower activities of glucose-6-phosphatase, phosphoenolpyruvate carboxykinase , and fatty acid synthase in the livers as compared to those in the control db/db mice 92. These results suggest that genistein may exert anti-diabetic effects in T2D mice by modulating hepatic glucose and lipid metabolism. In a more recent study in which 6 week old db/db mice were given a diet containing 1,000 mg/kg genistein for 8 weeks, it was demonstrated that genistein significantly reduced plasma glucose levels 93.
Taken together, these results from several animal models with different ages consistently show that genistein is effective at preventing diabetes as well as treating the condition. The data further suggest that genistein exerts anti-diabetic effects at least partially through enhancing β-cell proliferation and reducing apoptosis, thereby preserving functional β-cell mass. Thus, genistein may be a viable option for treating both T1D and T2D, which needs to be investigated in humans.
6. 3. Genistein has insulinotropic effects
Insulin is critical for carbohydrate and fat metabolism. In healthy subjects, insulin is released in exquisitely exact amounts to meet the metabolic demand. Specifically, β-cells sense changes in plasma glucose concentration and response by releasing corresponding amounts of insulin 94. Decrease in both sensing and secreting capacity of β-cells results in abnormal glucose homeostasis. While no pharmacological agent can restore the exact kinetics of insulin secretion in response to glucose 95, insulinotropic agents are still very important for effective glycemic control in diabetic patients. A preponderance of evidence supports a role for genistein in β-cell function, namely through potentiation of glucose-stimulated insulin secretion (GSIS) and β-cell regeneration and protection against apoptosis. In general, although several early studies demonstrated that genistein at very high concentrations (≥100 µM) inhibits insulin secretion in β-cells 96, 97, most studies that evaluated the effects of genistein on β-cell function reported a dose-dependent increase in GSIS from both clonal pancreatic β-cells 98 and cultured islets 99, 100, robust across a variety of cell models, exposure times and genistein concentrations (Table 2). However, the doses used in most of these studies (> 20 µM) are well above those concentrations physiologically achievable through dietary means. The total plasma genistein levels are typically in the range of 1–7 µM in both rodents and humans following dietary ingestion of genistein 15, 48, 49, 65, 101, 102. In contrast with these studies in which pharmacological doses of genistein were used, our group recently observed that treatment of MIN6, INS-1 or mouse islets with genistein as low as 10 nM for 30 min enhanced GSIS, with a maximal effect at 5 µM genistein 103. This effect of genistein is as potent as that of incretin hormone glucagon-like peptide-1, the most potent insulinotropic hormone found to date 104, 105. The effect of genistein on GSIS was not dependent on estrogen receptor (ER) and also not related to an inhibition of PTK. Consistently, a study by others also showed that daidzein, a genistein analog without inhibitory action on PTK 41, enhanced GSIS 100. Further analysis demonstrated that genistein activates the cAMP/PKA signaling cascade to exert such an insulinotropic effect. These finding for the first time suggest a novel role of genistein in the regulation of insulin secretion. These results clearly demonstrate differences in the biological activity of genistein at physiological and pharmacological doses.
In another study, 50 µM genistein stimulated Leu/Gln stimulated insulin secretion by roughly 2-fold, whereas genistein had no effect on insulin secretion under low glucose conditions 106. Activation of IRS-1 or AKT was influenced by genistein under conditions of at least 200 µM, suggesting that genistein’s actions on β-cells were not mediated through inhibition of the insulin tyrosine kinase receptor 106. Calcium levels and phospho-Ca2+/calmodulin kinase II (CaMK II) were increased by genistein potentiation of Leu/Gln-insulin secretion, and insulin secretion was further enhanced in CaMK II-overexpressing cells, suggesting a role of Ca2+ signaling in genistein’s effect on β-cells 106. Consistent with this, others reported that genistein-mediated increases in GSIS were blunted by removal of extracellular Ca2+ calcium or through addition of Ca2+ antagonists 98. As hinted at earlier, calcineurin signaling pathways may be a direct target of genistein in β-cells. As aforementioned however, the physiological relevance of this observation is questionable, given that genistein doses used in these studies are far beyond those physiologically achievable in humans or experimental animals.
6. 4. The effects of genistein on β-cell survival
Others reported that genistein at concentrations ranging from 5 to 40 µM mitigated the effect of cytokine (IL-1β + IFNγ)-mediated reductions in GSIS 107. In the same study, concentrations of at least 5 µM genistein (lowest concentration tested) were associated with a prevention of cytokine-induced reductions in rat insulinoma (RIN) cell viability, proliferation, and reduced the cytokine-mediated increase in iNOS expression and activity and nitric oxide production 107. Nitric oxide inhibits iron-containing enzymes, thus those involved in glucose oxidation (TCA cycle, electron transport chain) are particularly vulnerable, leading to reduced rates of ATP and subsequent insulin release 108. Interestingly, the effects of genistein on mitigating cytokine-induced changes in β-cell function were attributed to suppression of NFkB, ERK-1/2 and JAK/STAT pathways 107.
In human islets, 100 µM genistein prevented high-glucose (24 h incubation) mediated cell damage by off-setting reductions in cell growth and DNA fragmentation 109. These effects were reversed when cells were treated with the estrogen antagonist ICI182780, suggesting that genistein’s effects were mediated by ERs 109. In another study, 100 µM genistein increased apoptosis in RINm5F β-cells and rat and human islets after 24 h, while 25 µM genistein reduced apoptosis 110. Similarly, others observed that 100 µM genistein reduced islet proliferation while enhancing GSIS 99. Although these data from in-vitro studies suggest a protective action of genistein in β-cells, the biological relevance of this finding is unclear.
6. 5. The effect of genistein on pancreatic β-cell proliferation
6. 5. 1. Pancreatic β-cell growth factors
Nutrients
A number of factors, including nutrients and growth factors, influence β-cell mass. The effects of nutrients, hormones, transcription factors and signaling pathways in β-cell function and turnover has been reviewed extensively 5. This discussion will be limited to those most relevant to the effects of genistein on β-cells.
Glucose is a known inducer of insulin secretion and β-cell proliferation. Growth/survival effects of glucose in β-cells are thought to be mediated via activation of the insulin receptor substrate-2 (IRS-2) protein in the insulin/insulin-like growth factor-1 (IGF-1) signaling pathway 111. Inhibiting glucose phosphorylation (first step in glycolysis) with mannoheptulose dampens the effect of glucose-stimulated β-cell proliferation, suggesting that metabolism of glucose is essential to its effects as a stimulator of growth 112. In non-diabetic and mildly diabetic rats, following a 24 h glucose infusion, β-cell mass was enhanced due to increased neogenesis and a repression of apoptosis 113. Chronic exposure to elevated levels of glucose, a condition known as glucotoxicity, on the other hand, induces β-cell apoptosis 114. Similarly, there are reports that short term exposure to free fatty acids stimulates insulin secretion while chronic exposure leads to reductions in insulin secretion and apoptosis, particularly in the presence of excess glucose (reviewed by 113).
Amino acids were demonstrated to have a trophic effect and also stimulate insulin secretion 115. Under in-vitro conditions, individual amino acids are not effective stimulators of insulin secretion; however, in combination, certain amino acids can synergistically enhance insulin secretion 116. Arginine, because of its positive charge at neutral pH, and amino acids transported into the cell by Na+-dependent transporters, are able to enhance insulin granule exocytosis through depolarization of the cell membrane 116. Amino acids that feed into the TCA cycle, such as glutamine and alanine, may indirectly enhance insulin secretion through increases in ATP, similar to oxidation of glucose 116.
6.5.2.Hormones
There are a number of hormonal growth factors that act directly on β-cells, including IGF-1 and insulin. Insulin and IGF-1 signaling occur through phosphorylation and subsequent activation of IRS proteins, which then activate the phosphatidylinositol 3-kinase (PI3K)/AKT and extracellular signal-regulated kinases (ERK 1/2) signaling cascade, leading to β-cell proliferation 56. The incretin hormone, glucagon-like peptide-1 (GLP-1), potentiates glucose-stimulated insulin secretion. It is released from L-cells of the distal small intestine after a meal 117. Binding to its receptor on the membrane of β-cells leads to a dramatic increase in intracellular cAMP levels. The activation of PI3K seems to be essential, as treatment of cells with PI3K inhibitor LY294002 or wortmannin blocked the effect of GLP-1 on β-cell proliferation 118. While there has been success in developing GLP-agonist drugs with longer half-lives (e.g., Exendin-4) as anti- diabetic agents, there is no proof that these agents do indeed induce cell proliferation in diabetic patients, and GLP-induced cell proliferation was shown to be substantially reduced in old, obese animals 4. Thus, there is a tremendous challenge in identifying a growth factor that induces cell proliferation through a novel mechanism.
6.5.3.Physiological stimuli
One of the best known examples of a physiological condition that stimulates islet mass expansion is pregnancy. In rodents, islet mass increases up to 4-fold during pregnancy and returns to normal size after birth 119, 120. During pregnancy, Men1, a gene that is mutated in multiple endocrine neoplasia type 1 (MEN1), is down-regulated in pancreatic islets, leading to an increase in β-cell proliferation 120. Infusion of prolactin, a pregnancy hormone, down-regulated Men1 expression and enhanced proliferation of β-cells. Glucose infusion led to down-regulation of Men1, providing a clue as to how glucose is able to stimulate β-cell proliferation 121. In another study, prolactin enhanced INS-1 cell proliferation via the JAK2/STAT5 pathway and PI3K activation 122. Placental lactogen also stimulates β-cell proliferation via a similar pathway. In addition to activating the JAK/STAT pathway, growth hormone and prolactin signaling lead to ERK1/2, IRS-1 and -2, PI3K, and PKC activation, and increased intracellular [Ca2+] 123, 124. It was also suggested that serotonin (5-HT) plays a role in regulating β-cell mass during pregnancy, with β-cells expressing and secreting 5-HT as well as expressing the receptors 5-HTR2B and 5-HTR1D 125.
Pancreatic damage also induces β-cell proliferation. Transgenic mouse models for chemically-induced conditional destruction of β-cells revealed that after induction of apoptosis and subsequent cessation of the apoptosis, mice were able to recover their islet mass and did so through an increase in β-cell proliferation 126. These findings demonstrate the plasticity of islet mass under a range of physiological conditions. Understanding the pathways underlying these adaptations can provide useful information for targeting β-cells in diabetic patients.
6.5.4. Signaling pathways that lead to β-cell proliferation
A greater understanding of β-cell function requires a complete knowledge of the cellular signaling pathways that regulate insulin secretion and β-cell proliferation. As alluded to above, PI3K/AKT signaling pathway is important for β-cell proliferation and is activated by numerous factors, such as insulin, IGFs, GLP-1 and nutrients 113. The PI3K is a target of IRS, which is activated after activation of the receptor tyrosine kinase. The PI3K then activates PDK1 via phosphatidylinositol-3,4,5-triphosphate, resulting in AKT activation. The AKT, also known as protein kinase B, contains a pleckstrin homology domain that recognizes phosphoinositides, such as phosphatidylinositol (3,4)-bisphosphate, binding to which leads to positioning of AKT at the plasma membrane and phosphorylation and subsequent activation by PDK1. Activated AKT then mediates phosphorylation of amino acids on proteins such as GSK3 and FOXO1 113. Expression of pancreatic and duodenal homeobox 1(Pdx1), which regulates transcription of a number of genes essential for normal islet function, is enhanced by FOXA2 but is repressed by FOXO1, with both transcription factors sharing a common binding site on the Pdx1 gene 5, 127. Nuclear localization of Pdx1 and FOXO1 are mutually exclusive, with the nuclear presence of one causing cytosolic translocation of the other 127. In addition to the IRS/PI3K/AKT pathway, insulin and IGF signaling also activates the Ras-Raf1-MEK-ERK pathway, which also leads to β-cell proliferation 5.
Cyclic AMP is a secondary signaling messenger that plays a role in mediating insulin secretion and β-cell growth. The cAMP binds to the regulatory subunit of PKA, leading to a release of the active subunit. Elevation of cAMP also activates the cAMP-regulated guanine nucleotide exchange factors, which contribute to insulin excytosis. It is now recognized that cAMP enhances GSIS, insulin synthesis, and β-cell proliferation and neogenesis through activation of PKA 128. PKA can regulate gene expression by phosphorylating the nuclear cAMP response element (CRE)-binding protein (CREB). Phosphorylated CREB subsequently binds to the CRE site and recruits the coactivator CREB-binding protein and thereby activates gene transcription. Depletion of CREB in mice leads to β-cell apoptosis and diabetes 128. Notably, cAMP/PKA signaling in β-cells leads to an increase in the cell cycle regulatory protein, Cyclin D1. Cyclin D2 and cyclin dependent kinase 4 (CDK4) are targets of c-Myc, an oncogene predicted to play a role in β-cell replication 5.
The Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway is activated by several β-cell mitogens, such as growth hormone and prolactin. Phosphorylation of STAT proteins (e.g., STAT-5) by phosphorylated JAKs leads to dimerization, nuclear translocation and binding to target gene elements to regulate transcription 129.
The Ca2+/calmodulin-dependent protein phosphatase 2-b (calcineurin-b) and nuclear factor of activated T-cells (NFAT) is a new addition to the list of pathways purported to play a role in β-cell proliferation 5. This pathway is activated by increases in cytosolic Ca2+ and is of particular relevance to this review as genistein function in β-cells is linked to changes in cellular Ca2+ flux.
6.6. Genistein may be a novel growth factor for pancreatic β-cells
Genistein treatment increased β-cell proliferation in cell culture models as well as in the pancreas of genistein-treated mice 56. Our group has been actively investigating the role of genistein in β-cell physiology and the mechanism through which it exerts a protective effect in both in-vitro and in-vivo experiments. In INS-1 cells, as little as 0.1 µM genistein is enough to enhance cell replication and increase protein abundance of cyclin D1, a major cell cycle regulator for β-cell proliferation, with maximal effects observed at 5 µM 56. At 1 µM, genistein as well as genistin and biochanin A exerted a similar effect on INS-1 cell proliferation, but other isoflavone metabolic intermediates or flavonoids from different structural families had no such an effect, indicating that the effect of genistein is structure-specific. The effect is also cell-type specific as genistein showed no effect on proliferation of pancreatic ductal cells, fibroblasts, human aortic endothelial cells and rat vascular smooth muscle cells. In the presence of the ER antagonist, ICI182,780, there was no change in genistein-mediated increases in β-cell proliferation. In addition, 17β-estradiol itself had no stimulatory effect on β-cell proliferation. These observations further suggest that the classical estrogen signaling machinery mediated by ERα and ERβ is not involved in this genistein effect 56. Genistein treatment was also associated with increases in intracellular cAMP, PKA activity, and active ERK1/2, suggesting that the cAMP/PKA and ERK1/2 pathways are stimulated by genistein treatment 56. Identical effects were observed in human islet β-cells that were exposed to genistein, suggesting a non-species-specific and human-relevant effects. Others reported a similar accumulation of intracellular cAMP following genistein treatment of β-cells 98. While this finding is very interesting and important given the critical role for cAMP signaling in maintaining normal β-cell insulin secretion, proliferation, and viability, how genistein triggers cAMP signaling is still unknown. Interestingly, a recent study shows that genistein can bind and activate GPR30 in cancer cells 38. GPR30 is coupled to GTP-binding protein Gαs to stimulate adenylate cyclase, thereby triggering cAMP signaling in cancer cells 32. While the biological role of this orphan receptor is still largely unknown, deletion of this receptor in mice leads to hyperglycemia and impaired glucose tolerance 130, suggesting that it may play a role in maintaining blood glucose homeostasis. More recent studies using GPR30 knockout, insulin-deficient diabetic mice demonstrated that GPR30 plays a role in protection of islet survival and maintenance of glucose homeostasis 130, 131. Based on these data, it is tempting to speculate that genistein effects on islet β-cells could be mediated via GPR30 on β-cells, an aspect that remains to be determined.
7. Structural specificity
What are the structural features of genistein that confer various biological effects at certain doses? It was suggested that the C2-C3 double bond in conjugation with 4-oxo in the C ring, and 3- and 5-hydroxy groups and the 4-oxo atom in the A and C rings are responsible for genistein’s antioxidant activity (Figure 1) 55. Flavonoids with more than one hydroxyl group may be more effective antioxidants against peroxyl radicals 55. While it is not completely clear how genistein interacts with the ERs, it is speculated that genistein binds to the ERs through its hydroxyl group at C4’ which interacts with the Glu-Arg-water triad in the ERs and also through flavone hydroxyl group at C7 which may interact with the distal histidine residue at the end of the ERs cavity 132. The effect of genistein on β-cell proliferation was also structure-specific, and suggested to be due to the hydroxyl group at the 5C position on the A ring, since equol and 17β-estradiol, which lack this hydroxyl group, were unable to stimulate β-cell proliferation 56. Interestingly, when the hydroxyl group at position 7C was replaced with glucose (genistin) or at the 4C position with a methyl group (biochanin A), there was no difference in cell proliferation, suggesting that these structural features are not critical for mediating the effects of genistein on β-cell proliferation 56. This is important as we consider biological availability of genistein and the observation that isoflavones are modified by the intestinal microflora to various metabolites through addition of sugar groups or methyl groups.
8. Comparing studies
The genistein effect on β-cells is in contrast to its effects observed in cancer-related studies, where genistein treatment inhibited proliferation and induced apoptosis of cancer cell lines 133–139. It is important to point out that effects are very much dependent on genistein concentration and model system used. In one study, for example, genistein inhibited EGF-induced proliferation of colon cancer cells, but was only effective when administered at a concentration of 150 µM, roughly 100-fold times the concentration of physiologically achievable circulating concentrations of genistein and concentrations effective for enhancing proliferation of β-cells 137.
The mode of genistein action may depend on many factors, including concentration, mode of administration (oral gavage vs. dietary supplementation vs. i.p. injection), genetic background of the animal, age of the animal, duration of treatment, health of the animal and diabetic model used. The diabetic model further complicates the scenario as researchers use a variety of STZ dosage conditions in animals of various ages and genetic backgrounds in order to induce diabetes. Furthermore, genistein has pleiotropic effects and the biological effect of genistein is dose-dependent. As we have discussed, at certain concentrations genistein can act as a free radical scavenger, ER agonist, PTK inhibitor and possibly act through a novel mechanism yet to be discovered (e.g., GPR30 agonist). Therefore, it is very controversial to uncover the mechanism underlying the various physiological effects of genistein.
9. Conclusions and implications
In conclusion, flavonoids, which are naturally-occurring, cost-effective compounds, exert multiple biological functions and have numerous health benefits. Isoflavone genistein may represent a promising candidate for alternative or complementary approach to prevent and treat diabetes. Numerous cell-culture and animal-based studies demonstrate positive effects of genistein on β-cell function. A caveat to this is that due to pleiotropic effects of isoflavones (e.g., PTK inhibitors, ER agonists, etc.), differences in model system, concentrations, duration of treatment and other environmental factors, it is very difficult to compare results across studies. In cell culture studies, the cell culture media composition, support matrix, method for islet isolation, and other factors may influence the response to treatment with genistein. In animal studies, the genetic background of the animal, species, age, diet, gender, route and dose of genistein treatment and other factors may influence the physiological response.
A greater understanding of the exact mechanisms by which genistein protects β-cells from damage and enhances proliferation, and the structural basis for these effects, will facilitate design of more effective nutraceutical or pharmaceutical compounds that target a specific effect with enhanced bioavailability and minimal side effects.
Acknowledgments
This work was supported by grants from National Center for Complementary and Alternative Medicine of National Institute of Health (1R01AT007077-01 to DL) and the American Diabetes Association research award (7-11-BS-84 to DL).
References
- 1.ADA. 2008 www.diabetes.org/diabetes-basics/diabetes-statistics.
- 2.Shaw JE, Sicree RA, Zimmet PZ. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Sever CE, Demetris AJ, Zeng J, Carroll P, Tzakis A, Fung JJ, Starzl TE, Ricordi C. Acta Diabetol. 1992;28:233–238. doi: 10.1007/BF00779005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoffers DA. Horm Metab Res. 2004;36:811–821. doi: 10.1055/s-2004-826168. [DOI] [PubMed] [Google Scholar]
- 5.Tarabra E, Pelengaris S, Khan M. Int J Endocrinol. 2012;2012:516718. doi: 10.1155/2012/516718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner-Weir S, Weir GC. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 7.Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Diabetes. 2002;51:1443–1452. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 8.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 9.Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S. J Clin Endocrinol Metab. 2004;89:5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- 10.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF. Endocr Rev. 2006;27:356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 11.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 12.Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes. 2004;53:1509–1516. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- 13.Erdman JW., Jr Circulation. 2000;102:2555–2559. doi: 10.1161/01.cir.102.20.2555. [DOI] [PubMed] [Google Scholar]
- 14.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 15.Si H, Liu D. J Nutr. 2008;138:297–304. doi: 10.1093/jn/138.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhathena SJ, Velasquez MT. The American journal of clinical nutrition. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 17.King RA, Bursill DB. The American journal of clinical nutrition. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 18.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. The Journal of nutrition. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. The Journal of nutrition. 1995;125:2307–2315. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Hendrich S, Murphy PA. The Journal of nutrition. 2003;133:399–404. doi: 10.1093/jn/133.2.399. [DOI] [PubMed] [Google Scholar]
- 21.Adlercreutz H, Yamada T, Wahala K, Watanabe S. Am J Obstet Gynecol. 1999;180:737–743. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- 22.Kelly GE, Nelson C, Waring MA, Joannou GE, Reeder AY. Clin Chim Acta. 1993;223:9–22. doi: 10.1016/0009-8981(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 23.Tolleson WH, Doerge DR, Churchwell MI, Marques MM, Roberts DW. Journal of agricultural and food chemistry. 2002;50:4783–4790. doi: 10.1021/jf025549r. [DOI] [PubMed] [Google Scholar]
- 24.Roberts D, Veeramachaneni DN, Schlaff WD, Awoniyi CA. Endocrine Journal. 2000;13:281–286. doi: 10.1385/ENDO:13:3:281. [DOI] [PubMed] [Google Scholar]
- 25.Lamartiniere CA, Zhang JX, Cotroneo MS. American Journal of Clinical Nutrition. 1998;68:1400S–1405S. doi: 10.1093/ajcn/68.6.1400S. [DOI] [PubMed] [Google Scholar]
- 26.Makela S, Savolainen H, Aavik E, Myllarniemi M, Strauss L, Taskinen E, Gustafsson JA, Hayry P. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7077–7082. doi: 10.1073/pnas.96.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milligan SR, Balasubramanian AV, Kalita JC. Environmental Health Perspectives. 1998;106:23–26. doi: 10.1289/ehp.9810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthony MS, Clarkson TB, Hughes CL, Morgan TM, Burke GL. Journal of Nutrition. 1996;126:43–50. doi: 10.1093/jn/126.1.43. [DOI] [PubMed] [Google Scholar]
- 29.Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ, Thomas BF, Albright C, Busby MG, Crowell JA, Zeisel SH. American Journal of Clinical Nutrition. 2002;76:1126–1137. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 30.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, Zeisel SH. American Journal of Clinical Nutrition. 2002;75:126–136. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 31.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 32.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 33.Thomas P, Pang Y, Filardo EJ, Dong J. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 34.Tong JS, Zhang QH, Wang ZB, Li S, Yang CR, Fu XQ, Hou Y, Wang ZY, Sheng J, Sun QY. PLoS One. 2010;5:e15408. doi: 10.1371/journal.pone.0015408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, Han KO. J Steroid Biochem Mol Biol. 2006;101:246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Adlercreutz H, Markkanen H, Watanabe S. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 37.Morton MS, Wilcox G, Wahlqvist ML, Griffiths K. J Endocrinol. 1994;142:251–259. doi: 10.1677/joe.0.1420251. [DOI] [PubMed] [Google Scholar]
- 38.Thomas P, Dong J. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 40.Lee DS, Lee SH. FEBS Lett. 2001;501:84–86. doi: 10.1016/s0014-5793(01)02631-x. [DOI] [PubMed] [Google Scholar]
- 41.Akiyama T, Ogawara H. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- 42.Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Cancer research. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 43.Linassier C, Pierre M, Le Pecq JB, Pierre J. Biochem Pharmacol. 1990;39:187–193. doi: 10.1016/0006-2952(90)90664-7. [DOI] [PubMed] [Google Scholar]
- 44.Neye H, Verspohl EJ. Exp Clin Endocrinol Diabetes. 1998;106:292–298. doi: 10.1055/s-0029-1211988. [DOI] [PubMed] [Google Scholar]
- 45.Verspohl EJ, Tollkuhn B, Kloss H. Cell Signal. 1995;7:505–512. doi: 10.1016/0898-6568(95)00020-p. [DOI] [PubMed] [Google Scholar]
- 46.Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Nutrition & Cancer. 1993;20:1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- 47.Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Free Radical Research. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 48.Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Journal of Nutrition. 1995;125:2307–2315. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- 49.King RA, Bursill DB. American Journal of Clinical Nutrition. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 50.Patel RP, Moellering D, Murphy-Ullrich J, Jo H, Beckman JS, Darley-Usmar VM. Free Radic Biol Med. 2000;28:1780–1794. doi: 10.1016/s0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 51.Patel RP, Boersma BJ, Crawford JH, Hogg N, Kirk M, Kalyanaraman B, Parks DA, Barnes S, Darley-Usmar V. Free Radical Biology & Medicine. 2001;31:1570–1581. doi: 10.1016/s0891-5849(01)00737-7. [DOI] [PubMed] [Google Scholar]
- 52.Chacko BK, Chandler RT, Mundhekar A, Khoo N, Pruitt HM, Kucik DF, Parks DA, Kevil CG, Barnes S, Patel RP. Am J Physiol Heart Circ Physiol. 2005;289:H908–H915. doi: 10.1152/ajpheart.00781.2004. [DOI] [PubMed] [Google Scholar]
- 53.Patel RP, Boersma BJ, Crawford JH, Hogg N, Kirk M, Kalyanaraman B, Parks DA, Barnes S, Darley-Usmar V. Free Radic Biol Med. 2001;31:1570–1581. doi: 10.1016/s0891-5849(01)00737-7. [DOI] [PubMed] [Google Scholar]
- 54.Kerry N, Abbey M. Atherosclerosis. 1998;140:341–347. doi: 10.1016/s0021-9150(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 55.Kruk I, Aboul-Enein HY, Michalska T, Lichszteld K, Kladna A. Luminescence. 2005;20:81–89. doi: 10.1002/bio.808. [DOI] [PubMed] [Google Scholar]
- 56.Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D. Endocrinology. 2010;151:3026–3037. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ. American Journal of Clinical Nutrition. 2003;78:123–130. doi: 10.1093/ajcn/78.1.123. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Chen H. Epigenetics. 2011;6:888–891. doi: 10.4161/epi.6.7.16315. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert ER, Liu D. Curr Med Chem. 2010;17:1756–1768. doi: 10.2174/092986710791111161. [DOI] [PubMed] [Google Scholar]
- 60.Fang M, Chen D, Yang CS. The Journal of nutrition. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 61.Klein SL, Wisniewski AB, Marson AL, Glass GE, Gearhart JP. Mol Med. 2002;8:742–749. [PMC free article] [PubMed] [Google Scholar]
- 62.Su Y, Shankar K, Simmen RC. The Journal of nutrition. 2009;139:945–951. doi: 10.3945/jn.108.103820. [DOI] [PubMed] [Google Scholar]
- 63.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Environmental health perspectives. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dolinoy DC, Huang D, Jirtle RL. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naaz A, Yellayi S, Zakroczymski MA, Bunick D, Doerge DR, Lubahn DB, Helferich WG, Cooke PS. Endocrinology. 2003;144:3315–3320. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- 66.Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 67.Cheng SY, Shaw NS, Tsai KS, Chen CY. J Womens Health (Larchmt) 2004;13:1080–1086. doi: 10.1089/jwh.2004.13.1080. [DOI] [PubMed] [Google Scholar]
- 68.Lu MP, Wang R, Song X, Chibbar R, Wang X, Wu L, Meng QH. Nutr Res. 2008;28:464–471. doi: 10.1016/j.nutres.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. The Journal of nutrition. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 70.Goodman-Gruen D, Kritz-Silverstein D. The Journal of nutrition. 2001;131:1202–1206. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 71.Nanri A, Mizoue T, Takahashi Y, Kirii K, Inoue M, Noda M, Tsugane S. J Nutr. 2010;140:580–586. doi: 10.3945/jn.109.116020. [DOI] [PubMed] [Google Scholar]
- 72.Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, Shu XO. The American journal of clinical nutrition. 2008;87:162–167. doi: 10.1093/ajcn/87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu ZM, Chen YM, Ho SC, Ho YP, Woo J. The American journal of clinical nutrition. 2010;91:1394–1401. doi: 10.3945/ajcn.2009.28813. [DOI] [PubMed] [Google Scholar]
- 74.Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Diabetes care. 2012;35:226–232. doi: 10.2337/dc11-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez S, Jayagopal V, Kilpatrick ES, Chapman T, Atkin SL. Diabetes care. 2007;30:1871–1873. doi: 10.2337/dc06-1814. [DOI] [PubMed] [Google Scholar]
- 76.Hermansen K, Sondergaard M, Hoie L, Carstensen M, Brock B. Diabetes care. 2001;24:228–233. doi: 10.2337/diacare.24.2.228. [DOI] [PubMed] [Google Scholar]
- 77.Liu ZM, Chen YM, Ho SC. The American journal of clinical nutrition. 2011;93:1092–1101. doi: 10.3945/ajcn.110.007187. [DOI] [PubMed] [Google Scholar]
- 78.Villa P, Costantini B, Suriano R, Perri C, Macri F, Ricciardi L, Panunzi S, Lanzone A. The Journal of clinical endocrinology and metabolism. 2009;94:552–558. doi: 10.1210/jc.2008-0735. [DOI] [PubMed] [Google Scholar]
- 79.Lee JS. Life sciences. 2006;79:1578–1584. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 80.Yang W, Wang S, Li L, Liang Z, Wang L. Pancreas. 2011;40:396–402. doi: 10.1097/MPA.0b013e318204e74d. [DOI] [PubMed] [Google Scholar]
- 81.Elmarakby AA, Ibrahim AS, Faulkner J, Mozaffari MS, Liou GI, Abdelsayed R. Vascul Pharmacol. 2011;55:149–156. doi: 10.1016/j.vph.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Rankin MM, Kushner JA. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Diabetes. 2009;58:1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu Z, Gilbert ER, Pfeiffer L, Zhang Y, Fu Y, Liu D. Appl Physiol Nutr Metab. 2012;37:480–488. doi: 10.1139/h2012-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo J, Quan J, Tsai J, Hobensack CK, Sullivan C, Hector R, Reaven GM. Metabolism. 1998;47:663–668. doi: 10.1016/s0026-0495(98)90027-0. [DOI] [PubMed] [Google Scholar]
- 86.Strowski MZ, Li Z, Szalkowski D, Shen X, Guan XM, Juttner S, Moller DE, Zhang BB. Endocrinology. 2004;145:5259–5268. doi: 10.1210/en.2004-0610. [DOI] [PubMed] [Google Scholar]
- 87.Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB. Diabetes. 2006;55:1695–1704. doi: 10.2337/db05-1602. [DOI] [PubMed] [Google Scholar]
- 88.Serreze DV, Gaedeke JW, Leiter EH. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9625–9629. doi: 10.1073/pnas.90.20.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi MS, Jung UJ, Yeo J, Kim MJ, Lee MK. Diabetes Metab Res Rev. 2008;24:74–81. doi: 10.1002/dmrr.780. [DOI] [PubMed] [Google Scholar]
- 90.Wang Q, Brubaker PL. Diabetologia. 2002;45:1263–1273. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- 91.Cavaghan MK, Ehrmann DA, Polonsky KS. J Clin Invest. 2000;106:329–333. doi: 10.1172/JCI10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ae Park S, Choi MS, Cho SY, Seo JS, Jung UJ, Kim MJ, Sung MK, Park YB, Lee MK. Life sciences. 2006;79:1207–1213. doi: 10.1016/j.lfs.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 93.Babu PV, Si H, Fu Z, Zhen W, Liu D. The Journal of nutrition. 2012;142:724–730. doi: 10.3945/jn.111.152322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rutter GA, Parton LE. Front Horm Res. 2008;36:118–134. doi: 10.1159/000115360. [DOI] [PubMed] [Google Scholar]
- 95.Doyle ME, Egan JM. Pharmacol Rev. 2003;55:105–131. doi: 10.1124/pr.55.1.7. [DOI] [PubMed] [Google Scholar]
- 96.Jones PM, Persaud SJ. Biochem Soc Trans. 1994;22:209S. doi: 10.1042/bst022209s. [DOI] [PubMed] [Google Scholar]
- 97.Persaud SJ, Harris TE, Burns CJ, Jones PM. J Mol Endocrinol. 1999;22:19–28. doi: 10.1677/jme.0.0220019. [DOI] [PubMed] [Google Scholar]
- 98.Ohno T, Kato N, Ishii C, Shimizu M, Ito Y, Tomono S, Kawazu S. Endocr Res. 1993;19:273–285. doi: 10.1080/07435809309026682. [DOI] [PubMed] [Google Scholar]
- 99.Sorenson RL, Brelje TC, Roth C. Endocrinology. 1994;134:1975–1978. doi: 10.1210/endo.134.4.8137766. [DOI] [PubMed] [Google Scholar]
- 100.Jonas JC, Plant TD, Gilon P, Detimary P, Nenquin M, Henquin JC. Br J Pharmacol. 1995;114:872–880. doi: 10.1111/j.1476-5381.1995.tb13285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santell RC, Chang YC, Nair MG, Helferich WG. Journal of Nutrition. 1997;127:263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- 102.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 103.Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Diabetes. 2006;55:1043–1050. doi: 10.2337/diabetes.55.04.06.db05-1089. [DOI] [PubMed] [Google Scholar]
- 104.Thorens B. Proc Natl Acad Sci U S A. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holst JJ. Gastroenterology. 1994;107:1848–1855. doi: 10.1016/0016-5085(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 106.Lee SJ, Kim HE, Choi SE, Shin HC, Kwag WJ, Lee BK, Cho KW, Kang Y. Mol Cells. 2009;28:167–174. doi: 10.1007/s10059-009-0119-7. [DOI] [PubMed] [Google Scholar]
- 107.Kim EK, Kwon KB, Song MY, Seo SW, Park SJ, Ka SO, Na L, Kim KA, Ryu DG, So HS, Park R, Park JW, Park BH. Mol Cell Endocrinol. 2007;278:18–28. doi: 10.1016/j.mce.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Corbett JA, McDaniel ML. Diabetes. 1992;41:897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- 109.Zhong WW, Liu Y, Li CL. Intern Med. 2011;50:2129–2134. doi: 10.2169/internalmedicine.50.5320. [DOI] [PubMed] [Google Scholar]
- 110.Elliott J, Scarpello JH, Morgan NG. J Endocrinol. 2002;172:137–143. doi: 10.1677/joe.0.1720137. [DOI] [PubMed] [Google Scholar]
- 111.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Mol Cell Biol. 2009;29:3219–3228. doi: 10.1128/MCB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.King DL, Kitchen KC, Chick WL. Endocrinology. 1978;103:1321–1327. doi: 10.1210/endo-103-4-1321. [DOI] [PubMed] [Google Scholar]
- 113.Bernard C, Berthault MF, Saulnier C, Ktorza A. FASEB J. 1999;13:1195–1205. doi: 10.1096/fasebj.13.10.1195. [DOI] [PubMed] [Google Scholar]
- 114.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M. Diabetes. 2005;54(Suppl 2):S108–S113. doi: 10.2337/diabetes.54.suppl_2.s108. [DOI] [PubMed] [Google Scholar]
- 115.Newsholme P, Brennan L, Rubi B, Maechler P. Clin Sci (Lond) 2005;108:185–194. doi: 10.1042/CS20040290. [DOI] [PubMed] [Google Scholar]
- 116.Newsholme P, Bender K, Kiely A, Brennan L. Biochem Soc Trans. 2007;35:1180–1186. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- 117.Drucker DJ. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 118.Buteau J, Roduit R, Susini S, Prentki M. Diabetologia. 1999;42:856–864. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- 119.Rieck S, Kaestner KH. Trends Endocrinol Metab. 2010;21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H, Li W, Wang Q, Wang X, Li F, Zhangr C, Wu L, Long H, Liu Y, Li X, Luo M, Li G, Ning G. Endocrinology. 2011 [Google Scholar]
- 122.Hugl SR, Merger M. JOP. 2007;8:739–752. [PubMed] [Google Scholar]
- 123.Sorenson RL, Brelje TC. Horm Metab Res. 1997;29:301–307. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 124.Swenne I, Hill DJ, Strain AJ, Milner RD. J Endocrinol. 1987;113:297–303. doi: 10.1677/joe.0.1130297. [DOI] [PubMed] [Google Scholar]
- 125.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khalaileh A, Gonen-Gross T, Magenheim J, Nir T, Porat S, Salpeter S, Stolovich-Rain M, Swisa A, Weinberg N, Dor Y. Diabetes Obes Metab. 2008;10(Suppl 4):128–135. doi: 10.1111/j.1463-1326.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 127.Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, 3rd, Wright CV, White MF, Arden KC, Accili D. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galsgaard ED, Friedrichsen BN, Nielsen JH, Moldrup A. Diabetes. 2001;50(Suppl 1):S40–S41. doi: 10.2337/diabetes.50.2007.s40. [DOI] [PubMed] [Google Scholar]
- 130.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 131.Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. Diabetes. 2009;58:2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ljunggren J, Gustafsson JA, Carlquist M. Embo J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McCall JL, Burich RA, Mack PC. Leuk Res. 2010;34:69–76. doi: 10.1016/j.leukres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 134.Singletary K, Ellington A. Anticancer Res. 2006;26:1039–1048. [PubMed] [Google Scholar]
- 135.Shao ZM, Alpaugh ML, Fontana JA, Barsky SH. J Cell Biochem. 1998;69:44–54. [PubMed] [Google Scholar]
- 136.Santibanez JF, Navarro A, Martinez J. Anticancer Res. 1997;17:1199–1204. [PubMed] [Google Scholar]
- 137.Qi W, Weber CR, Wasland K, Savkovic SD. BMC Cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rajah TT, Du N, Drews N, Cohn R. Pharmacology. 2009;84:68–73. doi: 10.1159/000226123. [DOI] [PubMed] [Google Scholar]
- 139.Peterson G, Barnes S. Cell Growth Differ. 1996;7:1345–1351. [PubMed] [Google Scholar]
- 140.Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Free Radic Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 141.Fu Z, Liu D. Eur J Pharmacol. 2009;616:321–327. doi: 10.1016/j.ejphar.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]