Abstract
Evolutionary imperatives bred a vigorous and highly orchestrated behavioral and immune response to the microbial world that served to promote species survival and propagation. The resultant legacy is an inflammatory bias which goes largely unchecked in the modern world and is provoked not only by pathogens but also now by people. In this commentary, the authors’ contributions to the Special Issue on Inflammation and Mental Health are described, beginning with the origins of the inflammatory bias, its roots in genetic predispositions to behavioral adaptations and ultimately maladaptations, and its consequences on the developing brain. In addition, the mechanisms by which the immune system engages behavior are described including a central role for the inflammasome which may serve to link psychological stress with inflammatory and behavioral responses. Neurotransmitter systems that mediate effects of the immune system on behavior are also described along with interactions of the inflammatory bias with depression and their convergent impact on the response to stress and medical illness. Finally, translational implications are discussed including data from a clinical trial using a cytokine antagonist in depressed patients, which suggests an interaction of the inflammatory bias with other evolutionary legacies including those related to food consumption and their modern consequences of obesity and the metabolic syndrome. Taken together, the articles offer a sampling of the rich literature that has evolved regarding the role of the immune system in behavioral disorders. The grounding of this relationship in our evolutionary past may serve to inform future research both theoretically and therapeutically.
Keywords: evolution; inflammation; cytokines; schizophrenia; autism; depression; genetics; stress; T cells; indoleamine 2,3 dioxygenase; oxidative stress; neurotransmitters
1. Introduction
As we have learned more about interactions between the brain and the immune system, it is increasingly apparent that cytokines and other immune molecules and cells play a Janus-faced role in central nervous system (CNS) function. Indeed, immune system molecules and cells are an essential component of numerous processes that are fundamental to the maintenance of neuronal integrity including neurogenesis, synaptic remodeling, and neurotransmission (Yirmiya and Goshen 2011). For example, inhibition of cytokines through the use of antagonists or gene targeting is associated with significant impairments in learning and memory in conjunction with deficits in the elemental processes that support these functions including long-term potentiation (Yirmiya and Goshen 2011). Similar results have been found following removal of cellular components of the immune system including T cells and microglia (Kipnis et al. 2004; Sierra et al. 2013; Ziv et al. 2006). Recent data suggest that even the healing effects of antidepressants may be in part dependent upon the induction of an immune response (Warner-Schmidt et al. 2011).
Side-by-side with these sustaining influences of the immune system on neuronal function is the specter of a destructive force driven by an overactive immune response or inflammation that in its attempt to contain and control a perceived assault can wreak havoc on the body and the brain, ultimately affecting behavior (Dantzer et al. 2008; Miller et al. 2009). While the short-term goal to enact protective responses at the cellular and organismic level is essential to survival, in the long run, chronic immune activation and inflammation, comes at a high cost, contributing to the immense personal and economic burden of neuropsychiatric disorders as well as other illnesses in our society. Much attention has appropriately been paid to the mechanisms of the effects of inflammation on the pathways to pathology in mental illnesses. However, it is important to recognize that there is a method to the madness, beginning with the need to survive in a hostile microbial environment in ancestral times, and ultimately resulting in the legacy of an inflammatory bias which when triggered or fostered by environmental conditions can lead to a host of maladies that are overrepresented in the modern world including allergic disorders, cardiovascular disease, diabetes, cancer and neuropsychiatric disorders (Couzin-Frankel 2010). In this special issue of Brain Behavior Immunity, we will begin with human evolution and through a series of papers will expand upon the basis of the inflammatory bias, its genetic representations, the dire consequences on a developing brain, the mechanisms impacting CNS function, the role of environmental triggers and ultimately translational relevance (Figure 1).
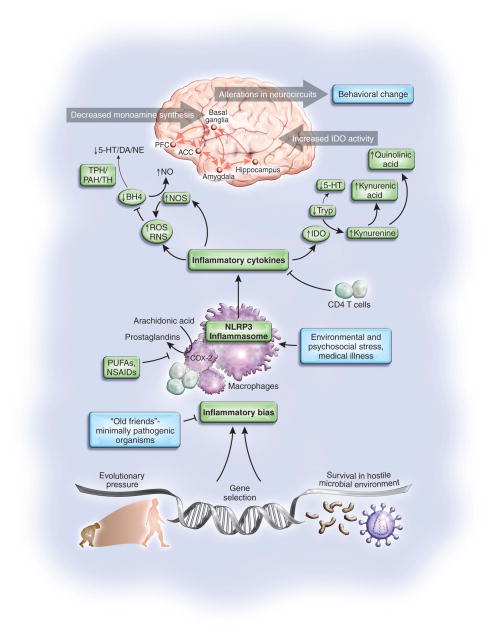
Figure 1. Mechanisms and Consequences of the Evolutionary Legacy of an Inflammatory Bias.
Survival in the ancestral world was contingent upon successful negotiation of a hostile microbial environment which ultimately contributed to a genetically-based inflammatory bias. This inflammatory bias, while essential for survival against pathogens and predators, has in the modern world (in the absence of temperance by exposure to minimally pathogenic organisms and the elaboration of immunomodulatory T cells) been expanded to include the response to psychosocial challenge. This capacity is achieved through immune mechanisms such as the inflammasome, which can respond to a variety of environmental stressors beyond pathogens including psychosocial stress. Once activated, the inflammasome can trigger the release of inflammatory cytokines which in turn stimulate enzyme pathways such as indoleamine 2,3 dioxygenase (IDO) as well as the production of reactive nitrogen and oxygen species (RNS and ROS). Activation of these pathways can then lead to the release of neurotoxic metabolites of kynurenine including quinolinic acid, while also disrupting the synthesis of monoamine neurotransmitters including serotonin (5-HT), dopamine (DA) and norepinephrine (NE) through effects on the availability of monoamine precursors such as tryptophan (Tryp) and tyrosine as well as tetrahydrobiopterin (BH4), which is an essential enzyme co-factor for phenylalanine hydroxylase (PAH), tryptophan hydroxylase (TPH) and tyrosine hydroxylase (TH). These actions of inflammatory cytokines ultimately contribute to alterations in neurocircuits in the brain including the anterior cingulate cortex (ACC) and the prefrontal cortex (PFC) which elaborate a host of behavioral changes that are an essential complement to the integrated immune and behavioral response to pathogens and predators which ultimately aid survival. However, in the context of chronic or overwhelming psychosocial challenge, these same responses can contribute to the malaise, melancholy and madness which are the inflammatory legacy of our evolutionary past. NLRP3: NACHT domain-, leucine-rich repeat-, and pyrin domain-containing protein 3; NO-nitric oxide; NOS-nitric oxide synthase; NSAIDS-non-steroidal anti-inflammatory drugs; PUFAs-polyunsaturated fatty acids.
2. Man meets microbe: the evolutionary imperative
Natural selection favors those individuals who can survive to reproductive age. In the ancestral world, infant mortality secondary to infectious diseases was one of the primary sources of evolutionary pressure applied to the human race (Volk In Press). As a result, individuals with a more vigorous or targeted immune response to prevailing pathogens were the ones most likely to survive and propagate the species (Raison and Miller 2013). So important was the need to fend off these microbial assaults, data suggest that European and Asian peoples were the beneficiaries of a critical immunologic boost through interbreeding with Neanderthals and other now extinct human sub-species (Abi-Rached et al. 2011). Indeed, analysis of modern human DNA reveals the presence of genes derived from Neanderthals that cluster in the human major histocompatibility locus and are associated with a more aggressive immune response to pathogens including viruses (Abi-Rached et al. 2011). Taken together, this evolutionary mandate to mount a vigorous immune response to early encounters with the microbial world has left humankind with an inflammatory legacy that is as important to health today as it was then, albeit with dramatically contrasting benefits and costs.
Given the intimate relationship between the brain and the immune system, it is important to recognize that successful defense against pathogens involves the activation of a complex interconnected suite of both immunologic and behavioral responses (Raison and Miller 2013). From the behavioral side, reorganization of priorities to fighting infection and wound healing requires reduced exploratory behavior (which may be achieved by cytokine effects on the basal ganglia) as well as hypervigilence against future attack (which may be achieved by effects on the anterior cingulate cortex and the amygdala)(Miller 2009). Thus, as the immune and nervous systems developed hand-in-hand through evolutionary time, it should not be surprising that one of the consequences of our bias to inflammatory responses is a vulnerability to behavioral disorders that are coupled to inflammation including reduced exploratory behavior in the form of depression and hypervigilence in the form of anxiety. In support of this notion are recent data indicating that depression risk alleles identified by both candidate gene and genome-wide association study methodologies are regularly associated with immune responses to infection that were likely to enhance survival in the ancestral environment (Raison and Miller 2013).
In the paper by Anders et al. in this issue, the hypothesis that susceptibility genes for depression persist in the human population because of their relevance to protective behavioral and immune responses to microbial challenges is further developed and expanded (Anders et al. 2012). The proposed infection-defense hypothesis not only incorporates the above noted advantages of energy conservation through the depressive symptoms of anhedonia, social withdrawal, fatigue and psychomotor retardation but also invokes the impact of depressive behaviors on reducing exposure to further challenge from infectious or other environmental stressors as well as decreasing the spread of infection to social conspecifics. These conjectures place the impact of evolutionary selection pressures into a larger social context that may ultimately involve the interaction of inflammatory pathways with molecular mechanisms that underlie prosocial behavior. Indeed, data have shown that depression is associated with decreased concentrations of oxytocin (Scantamburlo et al. 2007), a molecule believed to be intimately involved in social relationships, thereby reducing the spread of infection through inhibitory effects on molecular pathways that mediate social attachment.
In the paper by McDade et al. in this issue, an interesting twist on the inflammatory liability imposed by natural selection is proposed and tested by data from a large cohort of young adults residing in the Philippines, an ecological and epidemiological setting that differs substantially from that of the United States (McDade et al. 2012). Borrowing from the “hygiene hypothesis’” that suggests that the inflammatory bias may be tempered by exposure to “old friends” in the form of minimally pathogenic bacteria, viruses and parasites (Raison et al. 2010), McDade and colleagues show that the relationship between perceived stress and inflammation [as measured by c-reactive protein (CRP)] is only significant in individuals with low levels of microbial exposure in infancy. For individuals with increased early exposure to infectious agents (e.g. exposure to animal feces in infancy and birth in the dry season when post-natal infectious exposures tend to be high), perceived stress in adulthood was not associated with elevated CRP. These data suggest that while an inflammatory bias is an evolutionary legacy which has served mankind well, over evolutionary time, checks and balances may have developed within the immune system that are dependent on exposure to some level of pathogenicty. Such exposure has been shown to be commensurate with immune tolerance, responsible in part by elaboration of anti-inflammatory T regulatory cells (Tregs) and their production of the anti-inflammatory cytokines interleukin (IL)-10 and transforming growth factor (TGF)-beta (Raison et al. 2010). Indeed, clinical studies have shown for example that exposure of pregnant women to certain types of bacteria can reduce the likelihood of atopy in their offspring (Kalliomaki et al. 2001). Unfortunately, in more developed countries like the United States, where sanitation is held in high regard, not only is there an inflammatory bias, but there are few “friendly” pathogens to engender a more tolerant immune response, thus potentially contributing to the higher rates of allergic and other disorders related to inflammation in more developed countries (Raison et al. 2010).
3. Genetic legacy of the inflammatory bias
Given the interconnection between the inflammatory and behavioral response to immune challenge, in conjunction with the rich literature indicating that inflammatory markers are increased in patients with a number of neuropsychiatric disorders including anxiety disorders and major depression (Miller et al. 2009), there has been considerable interest in whether a relationship exists between genes that regulate the inflammatory response and the development of depressive disorders. Studies have focused on genes for a variety of inflammatory cytokines as well as genetic variants in pathways related to inflammatory signaling such as the enzymes involved in arachidonic acid (AA) metabolism. In the paper by Bufalino et al. in this issue, the literature in this area is reviewed with the conclusion that single nucleotide polymorphisms in a number of cytokine genes as well as genetic variants influencing T cell function are associated not only with an increased risk for depression but also reduced responsiveness to antidepressant therapy (Bufalino et al. 2012). Similar data are reported for genetic variants in cyclooxygenase 2 and phospholipase 2, both enzymes involved in AA metabolism. These data provide further evidence for a connection between a genetic predisposition to an inflammatory bias and an increased vulnerability to behavioral change including depression. In addition, these data complement findings that genetic variants in IL-6 and phospholipase 2 can predict the behavioral response to cytokine challenge. Indeed, in patients receiving treatment with interferon (IFN)-alpha, those with the low IL-6 synthesizing genotype exhibited significantly fewer depressive symptoms (Bull et al. 2009), while the “at risk” PLA2 polymorphism is associated with lower concentrations of the long-chain omega-3 fatty acid eicosapentaenoic acid (EPA) and more somatic symptoms of depression in both patients treated with IFN-alpha as well as in patients with idiopathic major depression (Su et al. 2010). Of note, EPA and its omega-3 counterpart docosahexaenoic acid (DHA) have been shown to inhibit inflammatory responses in vitro (Li et al. 2005), and decreased plasma EPA and DHA concentrations have been associated with major depression in a meta-analysis of the literature in this area (Lin et al. 2010). Consistent with these data, in a related study in this issue, Lotrich et al. show that lower plasma concentrations of DHA and an elevated AA/EPA+DHA ratio are associated with the development of depression during IFN-alpha therapy (Lotrich et al. 2012).
4. Inflammation and the developing brain
During development, the brain is extremely vulnerable to the impact of physiologic assaults. As indicated above, cytokines play an essential role in the modeling of synapses not only in adulthood but especially during development. Immune cells such as microglia also play an essential role in the developing brain, for example via regulation of neural precursor cell numbers in the cerebral cortex through phagocytosis (Cunningham et al. 2013). Therefore, any disruption in the relative balance or expression of cytokines and/or immune cell (e.g. microglial) activation during this critical period may have dire consequences for the developing brain. An elegant series of papers involving early maternal infection and immune stimulation has paved the way to an increasing appreciation of how infection early during development may have lifelong behavioral consequences. Indeed, administration of poly I:C to pregnant mice has been shown to lead to IL-6 dependent changes in behaviors in the offspring consistent with both autism spectrum disorders (ASD) and schizophrenia (Smith et al. 2007).
In the paper by Garay et al. in this issue, the consequences of maternal immune activation using poly I:C on cytokine expression are explored in multiple brain regions over time (Garay et al. 2012). The results indicate that long-lasting region and age-specific changes occur that persist until at least post-natal day 60. Interestingly, changes in cytokine expression were not associated with immune cell infiltration into the brain or evidence of gross changes in blood brain barrier permeability or microglial number. Moreover, somewhat unexpectedly, significant decreases in a number of cytokines were found during peak periods of synaptogenesis and synaptic plasticity. These data suggest that although maternal immune activation leads to a variety of behavioral changes consistent with ASD and schizophrenia, the mechanism of these alterations may be a consequence of more nuanced changes in cytokine effects on neural plasticity during development as opposed to a monolithic impact of increased inflammation leading to widespread neuronal endangerment.
Consistent with the notion that long lasting change in immune regulation may be associated with ASD, Breece et al. in this issue report significant increases in the frequencies of peripheral blood dendritic cells in patients with ASD (Breece et al. 2012). Dendritic cells play a fundamental role in innate immunity as well as activation of T cells, the induction of tolerance and cytokine and chemokine production. Interestingly, increases in dendritic cells were associated with increased volumes of the left and right amygdala as well as repetitive behaviors and gastrointestinal symptoms. Increased amygdala volumes have been previously reported in children with ASD (Kim et al. 2010), and the link with immune dysregulation is intriguing in light of studies indicating that the amygdala may be a target of peripheral immune activation in studies from healthy individuals administered typhoid vaccination and endotoxin (Harrison et al. 2009; Inagaki et al. 2012). The connection between altered amygdala function and specific behavioral and bodily (gastrointestinal) responses in the context of immune activation warrants further investigation, especially as it relates to the potentially adaptive value of these reactions when viewed in an evolutionary context (e.g. hypervigilance, social withdrawal, and anorexia as protective in response to infection – see above). The paper of Ross et al. in this issue provides further support that a chronic inflammatory response may be related to the development of altered behaviors consistent with ASD (Ross et al. 2013). The study focuses on patients with the 22q11.2 deletion syndrome (also known as DiGeorge syndrome), a genetic disorder that is associated with an increased risk for ASD and schizophrenia as well as altered T cell function including decreased Treg expression. In patients with 22q11.2 deletion, significant correlations were found between increased inflammatory and decreased anti-inflammatory cytokines and ASD symptoms including altered social behavior and increased repetitive behaviors. Given that a number of 22q11.2 subjects do not exhibit ASD-like symptoms, this continuum between increasing behavioral dysfunction and increased inflammation is especially intriguing and provides even greater support for the link between ASD symptoms and immune dysregulation.
Schizophrenia is another developmental disorder that is believed to be linked to immune alterations (Fineberg and Ellman 2013). However studies of the immune response in schizophrenic patients can be complicated by antipsychotic treatment, given that antipsychotic medications significantly impact not only neurotransmitter function but also bodily metabolism in ways that promote weight gain and obesity, which in themselves are associated with increased inflammation. Indeed, in the paper in this issue by Miller et al., patients with schizophrenia and the metabolic syndrome were found to exhibit significantly higher total white blood cell counts, monocyte counts and c-reactive protein (CRP) concentrations in the peripheral blood compared to patients without the metabolic syndrome (Miller et al. 2012). In order to obviate some of the confounds introduced by chronic antipsychotic treatment of the disorder, in the paper by Di Nicola et al. in this issue, serum concentrations and levels of leukocyte gene expression of inflammatory cytokines in first-episode schizophrenia patients were explored along with their association with psychosocial stressors and duration of antipsychotic treatment (Di Nicola et al. 2012). Significantly higher serum and gene expression levels of a variety of inflammatory cytokines were found in first-episode schizophrenia patients compared to healthy controls. Interestingly, a history of childhood trauma and recent stressful life events, which have been associated with exacerbation of schizophrenia, were also associated with higher levels of inflammatory cytokines in the serum and peripheral blood leukocytes of schizophrenia patients. These findings of an inflammatory diathesis in schizophrenia patients relatively early in the course of the disease support the notion that an inflammatory process may contribute to both the development as well as the maintenance of the disorder.
The link between immune dysregulation and developmental disorders like ASD and schizophrenia may have special translational relevance, given recent work suggesting that early immune intervention may be able to reverse developmentally-driven disorders with an immune component. For example, in a mouse model of Rett syndrome involving a genetic mutation of the MECP2 gene, which encodes a methyl CpG-binding protein that affects neuronal and glial function, the behavioral pathology was reversed by replacing genetically-mutated microglia with wild-type (normally functioning) microglia, indicating the therapeutic potential of immune-targeted therapies for developmental disorders (Derecki et al. 2012).
5. Mechanisms of malaise and melancholy
Probably the most studied aspect of interactions between the brain and the immune system are the mechanisms by which immune activation and inflammation can influence behavior leading to symptoms including depression, fatigue, psychomotor retardation, and sleep disturbances. The majority of studies in this area have focused on humans and laboratory animals exposed to a variety of inflammatory stimuli. Mechanisms that have received the most attention are the immune pathways that are involved and their impact on neurotransmitter metabolism and neurocircuitry relevant to mood regulation (Dantzer et al. 2008; Miller In Press). Of note, these studies are highly dependent on animal models that reflect the consequences of chronic inflammation. Although few such animal models exist, in the paper by Kubera et al. in this issue, a novel strategy of chronic lipopolysaccharide (LPS) administration to mice is presented which exhibits fidelity (to chronic inflammation in humans) in terms of chronic inflammatory immune changes and response to antidepressants which are apparent up to 2 months after the last LPS injection (Kubera et al. 2013).
5a. Immune Pathways and the Inflammasome
In a paper in this issue, Iwata et al. propose the provocative hypothesis that the recently characterized inflammasome may serve as a critical link between psychological stress and depression, as well as other illnesses related to inflammation (Iwata et al. 2012). The inflammasome is a protein complex that can detect diverse danger signals including not only pathogen-associated molecules but also molecules associated with cellular damage such as adenosine triphosphate (ATP). Upon activation, the inflammasome can generate an inflammatory response notably through the production of IL-1-beta by activation of a caspase that cleaves the precursor peptide pro-IL-1-beta. Given the capacity of the inflammasome to react to danger signals generated by stimuli other than pathogens, the authors suggest that the inflammasome may be uniquely poised to serve as the molecular mechanism that transduces psychological responses to stress into an inflammatory response in the absence of pathogen challenge. Thus, the inflammasome may represent an evolutionary adaptation that extends the immune and behavioral response to pathogens and the microbial world to include challenges emanating from predators, people and the social world. Although of significant value in detecting and responding to tissue damage and destruction, by virtue of the inflammasome, the inflammatory bias may have been given an entrée into the modern world where people, not pathogens or predators represent the primary challenges.
5b. Neurotransmitter Pathways
Much of the attention in terms of the impact of inflammation on neurotransmitter systems has focused on the effects of inflammatory cytokines on serotonin and dopamine pathways. In the paper by Hayley at al. in this issue, special attention is paid to the impact of the inflammatory cytokine IFN-alpha on serotonin and behavior in mice (Hayley et al. 2012). Acute administration of IFN-alpha into the brain of mice was associated with a significant induction of central cytokines along with significant decreases in the expression of serotonin (5-HT) receptors as well as the induction of anhedonia-like behavior. Decreases in serotonin receptor expression have been reported in other studies in laboratory animals and human cell lines treated with IFN-alpha (Cai et al. 2005; Ping et al. 2012). Moreover, reduced serotonin receptor mRNA has been described in patients with major depression who died by suicide (Anisman et al. 2008). These alterations in serotonin receptor expression as a function of cytokine exposure complement studies indicating that stimulation of cytokine signaling pathways including p38 mitogen activated protein kinase may also reduce serotonin signaling through activation of the expression and function of the serotonin transporter (Haroon et al. 2012; Miller et al. 2009). Data also suggest that cytokine-induced stimulation of the enzyme indoleamine 2,3 dioxygenase (IDO) can decrease the availability of tryptophan, the primary amino acid precursor of serotonin, thereby further reducing serotonin neurotransmission, while also increasing the production of kynurenine and its neuroactive metabolites (see below) (Haroon et al. 2012; Miller et al. 2009). The salutary effects of serotonin reuptake inhibitors on mood symptoms in IFN-alpha-treated patients are consistent with the notion that depletion in serotonin activity may be a consequence of the effects of IFN-alpha and other inflammatory cytokines on serotonin function (Musselman et al. 2001). One of the other cytokines that may contribute to tryptophan depletion and kynurenine generation is IFN-gamma. IFN-gamma is a potent activator of IDO, and in the paper by Myint et al. in this issue, patients with depression were shown to have increased kynurenine compared to controls in association with a polymorphism in the IFN-gamma gene known to be associated with increased IFN-gamma production in vitro (Myint In Press). These data further support the interaction of a genetic bias to inflammation that in turn is associated with metabolic pathways that influence behavior.
In this issue, Corona et al. further explore the role of IDO and its impact on serotonin metabolism using fractalkine-receptor deficient mice treated with lipopolysaccharide (LPS)(Corona et al. 2012). Fractalkines are molecules released by neurons that have been shown to reduce microglial activation during immune challenges with stimuli such as LPS. Fractalkine-receptor deficient mice exhibit an exaggerated inflammatory and behavioral response to LPS. Inhibition of IDO in fractalkine-receptor deficient mice was found to prevent the LPS-induced decrease in the ratio of serotonin to its metabolites as well as block LPS-induced depressive-like behavior. Interestingly, IDO inhibition also reversed LPS-induced activation of microglia. These data suggest that in addition to effects on serotonin metabolism, IDO generates kynurenine metabolites including 3-hydroxykynurenine and quinolinic acid, both of which promote oxidative stress that may chronically activate microglia. Inhibition of IDO appears to block this effect.
The potential role of oxidative stress in the relationship between inflammation and behavior is also explored in patients with major depression in this issue by Rawdin et al. (Rawdin et al. 2012). These investigators report that increased peripheral blood markers of oxidative stress as measured by F2-isoprostanes were positively correlated with peripheral blood IL-6 and negatively correlated with IL-10 in patients with major depression before treatment. After treatment with the antidepressant sertraline, markers of oxidative stress and inflammatory cytokines no longer correlated in these subjects, indicating that reversal of depressive symptoms by targeting the serotonin system may disassociate the connection between oxidative stress and inflammation.
Oxidative stress may also play a role in the metabolism of dopamine. In a paper by Felger et al. in this issue, the authors discuss the vulnerability of tetrahydrobiopterin (BH4) to oxidative degradation (Felger et al. 2012). BH4 is a key cofactor for the enzymes phenylalanine hydroxylase which converts phenylalanine (phen) to tyrosine (tyr) and tyrosine hydroxylase which converts tyrosine to dopamine. In patients administered IFN-alpha, the phen/tyr ratio was significantly increased and negatively correlated with cerebrospinal fluid (CSF) concentrations of both dopamine and its metabolite homovanillic acid. In addition, the phen/tyr ratio correlated positively with fatigue. Of note, CSF concentrations of BH4 were negatively correlated with CSF IL-6 in this study. Taken together, these data indicate that inflammatory cytokines can reduce BH4, possibly through the induction of oxidative stress. Reduction in BH4 in turn can decrease the conversion of phen to tyr and tyr to dopamine leading to fatigue. Although previous work has demonstrated an association between the phen/tyr ratio and depressive symptoms in older adults (Capuron et al. 2011), these data are the first to link peripheral amino acid biomarkers of BH4 activity to both CNS monoamine synthesis as well as CNS concentrations of BH4 and behavior.
5c. Neurocircuitry
There has been considerable interest in the neurocircuits that are engaged by inflammation to lead to changes in behavior. Studies in patients receiving IFN-alpha as well as studies in healthy volunteers administered endotoxin and typhoid vaccination have revealed that in the context of an inflammatory stimuli, multiple brain regions are involved in behavioral change including the basal ganglia and limbic regions including the anterior cingulate cortex, the amygdala and hippocampus (Miller et al. 2013; Miller In Press). In this issue, the paper by Savitz et al. explores the relationship between gene expression in peripheral blood mononuclear cells and brain hemodynamic responses and morphology in unmedicated depressed patients versus healthy controls (Savitz et al. 2012). Interestingly, multiple inflammation-related genes and gene networks were significantly correlated with hemodynamic responses in the amygdala and hippocampus as well as the ventromedial prefrontal cortex. In addition, genes related to nuclear factor-kappa B (NF-kB) and tumor necrosis factor (TNF) were associated with gray matter volume of the caudate, and genes associated with cytokine responses were correlated with the thickness of the left subgenual anterior cingulate cortex. Of note, both the caudate and the subgenual anterior cingulate cortex have been shown to be targets of induced immune responses in previous studies (Miller et al. 2013). Moreover, these brain regions have been shown to be critical in the expression of anhedonia and fatigue as well as depressed mood in the context of inflammation (Miller et al. 2013). Furthermore, the subgenual anterior cingulate cortex is the target of deep brain stimulation therapy in patients with severely treatment resistant depression (Ressler and Mayberg 2007). Taken together, these data are some of the first to support a relationship between peripheral inflammatory markers and activation and morphology of specific brain regions in the context of depression in the absence of exogenous immune stimulation.
6. Environmental Enhancement of the Inflammatory Bias
There are many environmental factors that may interact with the inflammatory legacy to facilitate an exaggerated inflammatory response in ways that ultimately affect our mental and physical health. Probably the most obvious and common challenges in the modern world include psychosocial stress and medical illness. In the paper by Fagundes et al. in this issue, the impact of a laboratory psychosocial stressor on serum IL-6 was examined in healthy adults (Fagundes et al. 2012). Individuals with higher depressive symptoms, potentially indicating chronic stress exposure, exhibited a significantly higher IL-6 response to the laboratory stressor than did individuals with fewer depressive symptoms, suggesting that chronic stress exposure and the resultant depressive symptoms can enhance inflammatory responses. In the paper by Smirnov et al. in this issue, unpredictable chronic mild stress in mice (which induces depressive-like behavior) was found to block the neuroprotective response of Copaxone, a drug widely used to treat multiple sclerosis that is believed to act in part by stimulating a neuroprotective T cell response (Smirnov et al. 2013). The authors suggest that the inhibitory effect of stress on Copaxane-induced neuroprotection may have been related to stress-induced decreases in T cell number and possibly T cell function. Interestingly however, the inhibitory effects of chronic stress on the neuroprotective effects of Copaxone were reversed by treatment with the antidepressant drug fluoxetine. Taken together with the above study, these data suggest that stress-induced depression may lead to immunologic consequences that subvert homeostatic mechanisms that involve T cells and their role in immune regulation. Reversing depression and likely the accompanying neuroendocrine alterations may restore immunoregulatory responses and ultimately foster stress resilience.
In the paper by Steptoe in this issue, the inflammatory response generated during an acute coronary syndrome (ACS, including myocardial infarction or unstable angina) was shown to lead to the development of depression and anxiety. Indeed, white blood cell counts during ACS were associated with depressive symptoms 3 weeks later and with symptoms of anxiety and cognitive symptoms of depression 6 months following ACS (Steptoe et al. 2012). In the paper by Harrison et al. in this issue, an inflammatory stimulus (typhoid vaccination) known to lead to an acute reduction in mood was shown to alter heart rate variability and blood pressure through an impact on brain metabolism in the dorsal anterior and posterior cingulate cortex and the pons (Harrison et al. 2013). These data suggest that a central impact of inflammation on the brain can influence cardiovascular state by contributing to potentially detrimental changes in autonomic cardiovascular control. Thus, while activation of inflammatory pathways may engage neurocircuits relevant to mood, the influence of inflammation on the brain may also affect the cardiovascular system in ways that may contribute to cardiovascular disease.
7. Trials and Tribulations
Although there has been great interest in the hypothesis that the inflammatory bias may contribute to the vulnerability to neuropsychiatric disorders such as depression, the realization of the promise of these theoretical developments has not yet materialized in the clinic. To date, there has been a dearth of studies examining the impact of anti-inflammatory therapies on behavior. A handful of reports suggest that inhibition of inflammation may reduce depressive symptoms in patients with autoimmune and inflammatory disorders, advanced cancer, sleep disorders and depression (Irwin et al. 2009; Monk et al. 2006; Muller et al. 2006; Raison et al. 2013; Tyring et al. 2006). However, few details have been elucidated regarding which patients may be most likely to respond to immune-targeted interventions. Moreover, whether therapies can be successful by targeting the downstream effects of inflammatory cytokines on pathways relevant to depression has yet to be resolved. In the paper by DellaGioia et al. in this issue, 7 days pretreatment of healthy volunteers with the norepinephrine and dopamine reuptake inhibitor bupropion was ineffective in blocking the development of neurovegetative symptoms following LPS administration (Dellagioia et al. 2012). These results were in contrast to the efficacy previously observed with citalopram which blocked fatigue and anhedonia following LPS (Hannestad et al. 2011). The differences between citalopram and bupropion may reflect differences in the pathways targeted by these agents (serotonin versus dopamine and norepinephrine, respectively), or there may be pharmacokinetic differences that favor citalopram over bupropion. For example, citalopram has a significantly longer half-life than bupropion, and the last dose of medication was given the night before LPS administration. Relevant to the identification of which patients may preferentially respond to inflammation-targeted therapies, in the paper by Mehta et al. in this issue, intriguing gene expression data are presented from a randomized clinical trial on the efficacy of the TNF antagonist infliximab in patients with treatment resistant depression (Mehta In Press). Examination of treatment response in the infliximab versus the placebo group revealed a series of gene transcripts which were associated with glucose and lipid metabolism, indicating that alterations in metabolic processes related to obesity and the metabolic syndrome may have special relevance to both inflammation as well as treatment response to anti-inflammatory medications. Of note, in 2014, Brain Behavior and Immunity will initiate a named series devoted to diet, inflammation and the brain. Interestingly, alterations in gene transcripts related to glucose and lipid metabolism were normalized by infliximab treatment in concert with a shift (decrease) in gene transcripts representing inflammatory signaling including decreases in genes related to TNF and NF-kB. Given that there was only one infliximab responder with diabetes and there were no differences between infliximab responders and nonresponders in BMI, these results suggest that incipient processes related to glucose and lipid metabolism driven by inflammation may be some of the earliest indications of which patients may be most likely to benefit from therapies that target the immune system and inflammation to treat depression and other behavioral alterations.
8. Conclusion
This Special Issue of Brain Behavior and Immunity was designed to provide a context within which to understand the complex inter-relationships between the immune system and the brain as they relate to behavioral disorders and mental health. As described, the intimate interconnection between the brain and the immune system exists within the framework of an evolutionary past that has left a legacy of inflammatory bias that has gone unchecked in the modern world with the consequence of excessive inflammation that contributes to multiple diseases including those that affect the brain. The many mechanisms by which the brain can be affected by inflammation are being elucidated, and therapeutic targets to arrest the potential damage are being revealed. Obviously, much more work needs to be done, however through the contributions of the authors of this special issue and the many other scientists committed to understanding the role of the immune system in behavioral disorders, new developments will occur, ultimately ushering in a new era whereby the immune system and the brain can work side-by-side in harmony again.
Highlight.
This article introduces the Special Issue on Inflammation and Mental Health and emphasizes the evolutionary legacy of an inflammatory bias that underlies the contributions of the immune system to behavioral disorders.
Acknowledgments
This work was supported by funds from the National Institute of Mental Health (R21MH0771172 to CLR and R01MH087604 to AHM). In addition, the study was supported by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program and PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources.
Footnotes
Conflict of Interest
The authors have nothing to declare.
Conflict of Interest Statement
All authors declare that there are no conflicts of interest, and all financial disclosures are listed for each author: Charles L. Raison serves on the advisory boards for and receives related travel funds from Pamlab, Lilly, and North American Center for Continuing Education; develops and presents disease state slides for Pamlab, Pfizer and Johnson & Johnson as well as receiving related travel funds for these activities; and develops continuing medical education material for North American Center for Continuing Education and for CME Incite. Andrew H. Miller has served as a consultant for Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Lundbeck Research USA, F. Hoffmann-La Roche Ltd., Schering-Plough Research Institute and Wyeth/Pfizer Inc. and has received research support from Centocor Inc., GlaxoSmithKline, and Schering-Plough Research Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Rached L, Jobin MJ, Kulkarni S, McWhinnie A, Dalva K, Gragert L, Babrzadeh F, Gharizadeh B, Luo M, Plummer FA, Kimani J, Carrington M, Middleton D, Rajalingam R, Beksac M, Marsh SG, Maiers M, Guethlein LA, Tavoularis S, Little AM, Green RE, Norman PJ, Parham P. The shaping of modern human immune systems by multiregional admixture with archaic humans. Science. 2011;334:89–94. doi: 10.1126/science.1209202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Tanaka M, Kinney DK. Depression as an evolutionary strategy for defense against infection. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Breece E, Paciotti B, Nordahl CW, Ozonoff S, Van de Water JA, Rogers SJ, Amaral D, Ashwood P. Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2009;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Khaoustov VI, Xie Q, Pan T, Le W, Yoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Corona AW, Norden DM, Skendelas JP, Huang Y, O’Connor JC, Lawson M, Dantzer R, Kelley KW, Godbout JP. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. Inflammation bares a dark side. Science. 2010;330:1621. doi: 10.1126/science.330.6011.1621. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellagioia N, Devine L, Pittman B, Hannestad J. Bupropion pre-treatment of endotoxin-induced depressive symptoms. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SB, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, Murray RM, Dazzan P, Pariante CM, Mondelli V. Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, Miller AH. Tyrosine metabolism during interferon-alpha administration: Association with fatigue and CSF dopamine concentrations. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory Cytokines and Neurological and Neurocognitive Alterations in the Course of Schizophrenia. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Ortiz N, Pittman B, Bhagwagar Z. Citalopram reduces endotoxin-induced fatigue. Brain Behav Immun. 2011;25:256–259. doi: 10.1016/j.bbi.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cooper E, Voon V, Miles K, Critchley HD. Central autonomic network mediates cardiovascular responses to acute inflammation: Relevance to increased cardiovascular risk in depression? Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Scharf J, Anisman H. Central administration of murine interferon-alpha induces depressive-like behavioral, brain cytokine and neurochemical alterations in mice: A mini-review and original experiments. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66:191–195. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lyoo IK, Estes AM, Renshaw PF, Shaw DW, Friedman SD, Kim DJ, Yoon SJ, Hwang J, Dager SR. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Arch Gen Psychiatry. 2010;67:1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Curzytek K, Duda W, Leskiewicz M, Basta-Kaim A, Budziszewska B, Roman A, Zajicova A, Holan V, Szczesny E, Lason W, Maes M. A new animal model of (chronic) depression induced by repeated and intermittent lipopolysaccharide administration for 4months. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Sears B, McNamara RK. Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: Relationship with interleukin-6. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C. Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Raison CL, Woolwine BJ, Haroon E, Binder EB, Miller AH, Felger JC. Transcriptional Signatures Related to Glucose and Lipid Metabolism Predict Treatment Response to the Tumor Necrosis Factor Antagonist Infliximab in Patients with Treatment-Resistant Depression. Brain Behavior Immunity. doi: 10.1016/j.bbi.2013.04.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AHHE, Raison CL, Felger JC. Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depression and Anxiety. doi: 10.1002/da.22084. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Mellor A, Buckley P. Total and differential white blood cell counts, high-sensitivity C-reactive protein, and the metabolic syndrome in non-affective psychoses. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T, Caligiuri MA, Villalona-Calero MA. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Myint A-M, Bondy B, Baghai TC, Eser D, Nothdurfter C, Schuele C, Zill P, Mueller N, Rupprecht R, Schwarz MJ. Tryptophan metabolism and Immunogenetics in Major Depression: A role for interferon-gamma gene. Brain Behavior Immunity. doi: 10.1016/j.bbi.2013.04.003. In Press. [DOI] [PubMed] [Google Scholar]
- Ping F, Shang J, Zhou J, Zhang H, Zhang L. 5-HT(1A) receptor and apoptosis contribute to interferon-alpha-induced “depressive-like” behavior in mice. Neurosci Lett. 2012;514:173–178. doi: 10.1016/j.neulet.2012.02.087. [DOI] [PubMed] [Google Scholar]
- Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawdin BS, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI, Rosser R, Hamilton SP, Nelson JC, Wolkowitz OM. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Guo Y, Coleman K, Ousley O, Miller AH. Association of IL-12p70 and IL-6:IL-10 ratio with autism-related behaviors in 22q11.2 deletion syndrome: A preliminary report. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, Drevets WC. Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov I, Walsh JT, Kipnis J. Chronic mild stress eliminates the neuroprotective effect of Copaxone after CNS injury. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Wikman A, Molloy GJ, Messerli-Burgy N, Kaski JC. Inflammation and symptoms of depression and anxiety in patients with acute coronary heart disease. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC, Aitchison KJ, Pariante CM. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67:550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Volk AA, Atkinson JA. Infant and child death in the human environment of evolutionary adaptation. Evolution and Human Behavior In Press. [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]