Abstract
Importance
Growing evidence of cell-to-cell transmission of neurodegenerative disease (ND)–associated proteins (NDAPs) (ie, tau, Aβ, and α-synuclein) suggests possible similarities in the infectious prion protein (PrPsc) in spongiform encephalopathies. There are limited data on the potential human-to-human transmission of NDAPs associated with Alzheimer disease (AD) and other non-PrPsc ND.
Objective
To examine evidence for human-to-human transmission of AD, Parkinson disease (PD), and related NDAPs in cadaveric human growth hormone (c-hGH) recipients.
Design
We conducted a detailed immunohistochemical analysis of pathological NDAPs other than PrPsc in human pituitary glands. We also searched for ND in recipients of pituitary-derived c-hGH by reviewing the National Hormone and Pituitary Program (NHPP) cohort database and medical literature.
Setting
University-based academic center and agencies of the US Department of Health and Human Services.
Participants
Thirty-four routine autopsy subjects (10 non-ND controls and 24 patients with ND) and a US cohort of c-hGH recipients in the NHPP.
Main Outcome Measures
Detectable NDAPs in human pituitary sections and death certificate reports of non-PrPsc ND in the NHPP database.
Results
We found mild amounts of pathological tau, Aβ, and α-synuclein deposits in the adeno/neurohypophysis of patients with ND and control patients. No cases of AD or PD were identified, and 3 deaths attributed to amyotrophic lateral sclerosis (ALS) were found among US NHPP c-hGH recipients, including 2 of the 796 decedents in the originally confirmed NHPP c-hGH cohort database.
Conclusions and Relevance
Despite the likely frequent exposure of c-hGH recipients to NDAPs, and their markedly elevated risk of PrPsc-related disease, this population of NHPP c-hGH recipients does not appear to be at increased risk of AD or PD. We discovered 3 ALS cases of unclear significance among US c-hGH recipients despite the absence of pathological deposits of ALS-associated proteins (TDP-43, FUS, and ubiquilin) in human pituitary glands. In this unique in vivo model of human-to-human transmission, we found no evidence to support concerns that NDAPs underlying AD and PD transmit disease in humans despite evidence of their cell-to-cell transmission in model systems of these disorders. Further monitoring is required to confirm these conclusions.
Current evidence implicates the cell-to-cell transmission of the major pathogenic proteins of Alzheimer disease (AD), Parkinson disease (PD), frontotemporal lobar degeneration (FTLD), amyotrophic lateral sclerosis (ALS), and related neurodegenerative diseases (NDs) in the progression of these disorders, as demonstrated by studies of the ND-associated proteins (NDAPs) in animal1–7 and cell culture model experiments.8–10 This compelling evidence of cell-to-cell transmissibility of AD, PD, and several other NDAPs is reminiscent of the prion protein11–17 (PrPsc), which is defined as a proteinaceous infectious particle18 that causes human and other mammalian spongiform encephalopathies. However, despite these similarities in disease protein spreading, non-PrPsc NDAPs fulfill some, but not all, of the revised Koch postulates adapted for proof of disease transmission by PrPsc and other NDAPs19; therefore, it is unclear if any NDAP other than PrPsc is truly a proteinaceous infectious particle that may transmit human disease. Indeed, some question the safety of organ transplants from ALS donors20 and NDAPs have been referred to as “prionoids”14 and even as “prions”7 because of these emerging transmission data.
Notably, a worldwide outbreak of iatrogenic Creutzfeldt-Jakob disease (CJD) occurred beginning in the mid-1980s that affected individuals treated with human growth hormone (hGH) extracted from cadaveric pituitary glands (c-hGH).21 Since international cohorts of c-hGH recipients, including patients in the United States who received c-hGH through the National Hormone and Pituitary Program (NHPP), continue to be studied, they provide a unique opportunity to explore possible human-to-human transmission of non-PrPsc NDs. More than 200 patients worldwide22,23 developed CJD from peripheral administration of c-hGH preparations contaminated with PrPsc from affected donors. Thus, an analysis of the rate of ND development in these patients might provide insight as to whether non-PrPsc NDAPs transmit disease in humans. To examine this issue, we first determined the potential exposure of c-hGH recipients to non-PrPsc NDAPs by evaluating sections of anterior (adenohypophysis) and posterior (neurohypophysis) human pituitary glands for the presence of pathological deposits of these disease proteins. Next, we examined the frequency of NDs in c-hGH recipients worldwide in the published literature as well as in the US NHPP database. We focused on AD, PD, FTLD, and ALS since these are the most common NDs associated with motor and cognitive impairments in the general population.
METHODS
IMMUNOHISTOCHEMICAL ANALYSIS
Thirty-four autopsy patients (10 non-ND controls and 24 patients with ND) at the Center for Neurodegenerative Disease Research with available pituitary tissue were analyzed (eTable 1, http://www.jamaneuro.com). All neuropathological procedures were performed in accordance with the institutional review board at the University of Pennsylvania and with an approved informed written consent. Pathological assessment was performed using immunohistochemical methods as previously described24 to detect pathological tau, Aβ, α-synuclein, TDP-43, FUS, and ubiquilin. To identify fibrillar species of NDAPs that formed amyloid deposits, sections were also stained with the amyloid-binding dye Thioflavin S.
Semiquantitative scores of pathological deposits of NDAPs were correlated with age at death using a nonparametric Spearman correlation (SPSS version 19.0; SPSS).
NHPP DATABASE EVALUATION
Data were collected on recipients of c-hGH through the NHPP, and the description of this cohort as well as the NHPP data gathering methods have been published.25 All patients originally identified as NHPP c-hGH recipients with known date of birth and vital status as of January 1, 1979 (the start of National Death Index records), were included in the study (n = 6190). Death certificate data were obtained for deceased patients through 2008 and searched for mention of AD, PD, or ALS. The observed number of deaths within the cohort attributed to each of these 3 diseases was compared with an expected number, which was calculated by using US mortality data for disease rates broken down by sex, race, 5-year age group, and the International Classification of Diseases. Statistical probabilities were calculated using a Poisson distribution with a Bonferroni multiple comparisons correction to set the significance level at .05/3 = .0167.
RESULTS
PATHOGENIC NDAPs IN THE ADENO/NEUROHYPOPHYSIS OF PATIENTS WITH ND AND CONTROLS
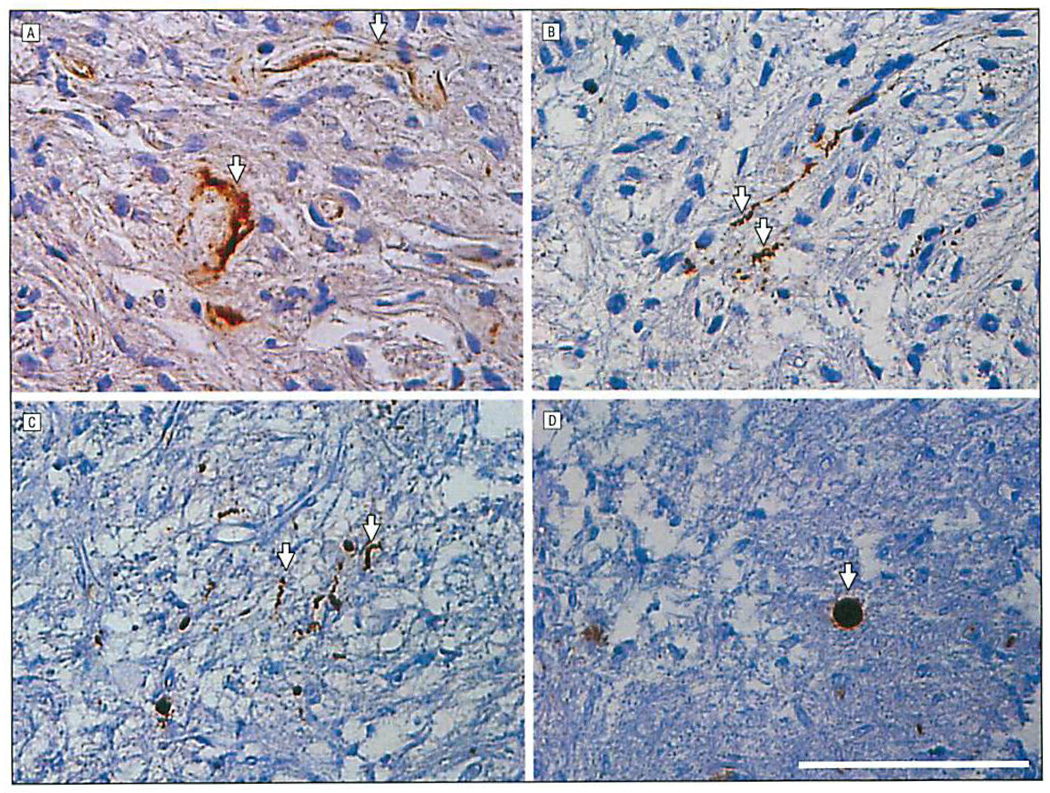
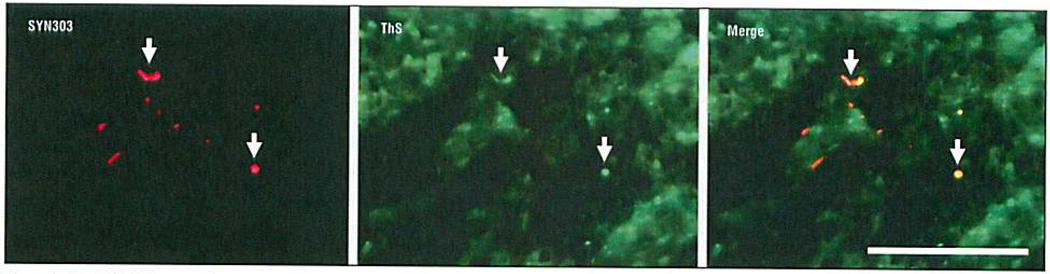
Examination of pituitary samples using the methods described earlier revealed a low number of pathological deposits of tau, Aβ, and α-synuclein in both ND and control cases (Figure 1 and Table). These deposits were defined as pathological because they deviated from the known normal distribution of these proteins in the brain, were detected with antibodies to abnormal posttranslational modifications or pathological conformers of these disease proteins, or, in some cases, resembled lesions seen in the brains or olfactory epithelium of patients with AD and PD.26 Diffuse perivascular Aβ deposits and amyloid angiopathy in blood vessel walls were visualized by Aβ immunohistochemical analysis (Figure 1A) in both the adenohypophysis and neurohypophysis of all patients with AD. Similar to diffuse Aβ plaques in the cortex, the Aβ deposits in the pituitary glands studied herein were not stained by the amyloid-binding dye Thioflavin S. Mild amounts of tau-positive dystrophic neurites and axonal swellings were detected (Figure 1B) in the neurohypophysis of most tauopathy patients (ie, subjects with AD or progressive supranuclear palsy), and a subset of these pathological tau deposits were weakly Thioflavin S positive. Inclusions composed of pathological α-synuclein appeared as dystrophic neurites (Figure 1C) while others resembled cortical Lewy bodies (Figure 1D). Lewy body–like inclusions and a subset of dystrophic Lewy neurites also were Thioflavin S positive, similar to Lewy bodies and Lewy neurites in PD brains (Figure 2). These α-synuclein–stained inclusions were seen mostly in patients with PD; however, they also occurred in some of the non-ND controls and patients with AD. Alzheimer disease–related Aβ and tau deposits were also detected in patients with PD and non-ND control cases, especially in older individuals. Indeed, a positive correlation was found with age and severity of tau (r = 0.56; P = .001) and Aβ (r = 0.45; P = .008) inclusions in all cases. Amyotrophic lateral sclerosis–associated deposits labeled by TDP-43, FUS, or ubiquilin were not detected in any of the sections reviewed.
Figure 1.
Immunohistochemical analysis of human Alzheimer disease pituitary sections showing perivascular diffuse Aβ deposits (arrows) (A) and tau-reactive dystrophic neurites (arrows) (B),and human Parkinson disease neurohypophysis displaying α-synuclein–reactive dystrophic neurites (arrows) (C) and a Lewy body–like inclusion (arrow) (D). Scale bar = 100 µm.
Table.
Neuropathological Assessment of the Adeno/Neurohypophysis for NDAP Immunoreactive Pathologiesa
| Patient No./ Age at Death, y |
Neuropathological Diagnosis |
Tau | Aβ | α-Synuclein |
|---|---|---|---|---|
| 1/67 | Normal | 0 | + + | 0 |
| 2/72 | Normal | + + | + | + + |
| 3/56 | Normal | + + + | + | 0 |
| 4/38 | Normal | 0 | 0 | 0 |
| 5/49 | Normal | 0 | 0 | 0 |
| 6/42 | Normal | 0 | 0 | 0 |
| 7/76 | Normal | 0 | 0 | 0 |
| 8/72 | Normal | + + | 0 | + + |
| 9/60 | Normal | + + | 0 | 0 |
| 10/82 | Normal | + + + | 0 | 0 |
| 11/87 | AD | + + + | + + + | 0 |
| 12/89 | AD | + + | + | 0 |
| 13/78 | AD | + + | + + | 0 |
| 14/83 | AD | + + | + | 0 |
| 15/84 | AD | + + | + + + | 0 |
| 16/81 | AD | 0 | + | 0 |
| 17/82 | AD | + | + | 0 |
| 18/72 | AD | 0 | + | 0 |
| 19/88 | AD | + + + | + + + | 0 |
| 20/79 | PD | + + | + | + + + |
| 21/24 | PD | 0 | 0 | + |
| 22/30 | PD/ADb | + + + | + | + |
| 23/86 | PD/ADb | + | + + + | + |
| 24/74 | PD | 0 | 0 | + |
| 25/74 | PD/ADb | + + | + | 0 |
| 26/77 | PD/ADb | + | 0 | 0 |
| 27/78 | PSP | + + | 0 | 0 |
| 28/82 | PSP | + | 0 | 0 |
| 29/87 | ALS | + + | 0 | 0 |
| 30/40 | ALS | 0 | 0 | 0 |
| 31/73 | ALS | 0 | + + | 0 |
| 32/62 | ALS | 0 | + | 0 |
| 33/73 | ALS-FTLD | + | 0 | 0 |
| 34/69 | FTLD-TDP | 0 | 0 | 0 |
Abbreviations: AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; FTLD, frontotemporal lobar degeneration; FTLD-TDP, frontotemporal lobar degeneration characterized by TDP-43 inclusions; NDAP, neurodegenerative disease–associated protein; PD, Parkinson disease; PSP, progressive supranuclear palsy; 0, absent; +, rare; + +, mild; + + +, moderate.
Because no FUS, TDP-43, or ubiquilin-positive pathology was observed in the pituitary glands studied herein, this Table does not include these negative findings.
These cases had a primary diagnosis of Lewy body PD and, because of sufficient comorbid plaque and tangle pathology, a secondary diagnosis of AD.
Figure 2.
Double-label immunofluorescence of neurohypophysis in a patient with Parkinson disease showing α-synuclein (SYN303)–reactive dystrophic Lewy neurites colocalized with Thioflavin S (ThS) amyloid-binding dye (arrows), indicating amyloid-like properties of these inclusions. Scale bar = 100 µm.
NHPP DATABASE REVIEW
Overall, about 7700 patients are estimated to have been treated through the NHPP from 1963 to 1985. In the latter 1980s, most of these patients were identified, and their treatment through the NHPP with c-hGH containing extracts of cadaveric pooled pituitary glands was confirmed. For the present study, we had sufficient data for analysis of 6190 subjects of whom 796 were deceased. The mean age at death was 27.2 years (range, 0–77 years) and mean duration from first treatment to death was 16.3 years (range, 0–45 years) (eTable 2). No mention of AD or PD appeared on the death certificate of any of the 796 subjects, but the cause of death for 2 patients was listed as ALS. The first patient was a 30-year-old man who received c-hGH between September 1976 and April 1985 for idiopathic isolated growth hormone deficiency. He died in February 2000 of “respiratory failure” due to “ALS,” but no autopsy was performed. The second patient was a 30-year-old woman who received c-hGH between 1978 and 1984 for idiopathic multiple pituitary hormone deficiency. The cause of her death in February 1997 was “respiratory failure, ALS, and amyotrophic partial sclerosis.” An autopsy was performed on this patient and medical records were reviewed by 2 physicians who separately reported the diagnosis as “probable ALS” and “confirmed ALS,” but studies to identify TDP-43 inclusions seen in nearly all sporadic ALS cases were not reported, nor were FUS or ubiquilin examined. Based on US mortality data available through 2008, a total of 0.31 deaths attributed to ALS were expected in this cohort through that year; the probability of 2 or more cases was P = .04, which did not meet our criteria for an elevated rate of disease (P ≤ .02). We found an additional US c-hGH recipient in the published literature who developed ALS at age 18 years, about 140 months after treatment for idiopathic panhypopituitarism with c-hGH (from 1966–1979).27 This additional ALS decedent identified among the NHPP recipients had not been originally identified and included among the 6190 confirmed NHPP c-hGH recipients; thus, he was not included in the earlier statistical analysis. Inclusion of this patient in this analysis with the entire estimated NHPP cohort, assuming the remainder of the approximately 7700 patients had a similar demographic profile to the known 6190 patients, indicates that the probability of observing 3 or more decedents with ALS was 0.006, which would suggest a higher rate of death attributed to ALS in this cohort than in the general population. Systematic review of the literature found no reports of AD, PD, or any additional ALS cases in worldwide C-hGH recipients.
COMMENT
Our findings herein indicate that pathological species of tau, Aβ, and α-synuclein are found in the adeno/neurohypophysis of normal individuals and those with ND, but, this notwithstanding, these NDAPs are unlikely to propagate between individuals as a disease-causing infectious agent based on our review of the NHPP database for the following reasons.
First, it is highly likely that c-hGH recipients were exposed to the pathogenic proteins (ie, tau, Aβ, and α-synuclein) of AD, PD, and FTLD-tau during the frequent administration of c-hGH that patients received over periods of several years. This assumption is based on the fact that low levels of pathological deposits of these NDAPs were present in both affected and unaffected subjects in our immunohistochemical analysis. Indeed, a similar burden of PrPsc inclusions has been demonstrated in the neurohypophysis of sporadic CJD cases, thereby establishing pituitary gland extracts as the likely source of PrPsc for c-hGH recipients.28 Compared with CJD with an incidence of about 1 case per million, the incidence of AD is at least 3 orders of magnitude higher and the incidence of PD at least 2 orders of magnitude higher.29 Thus, provided the pathogenic species of the NDAPs linked to AD, FTLD-tau, and PD as well as PrPsc were similarly affected by the c-hGH purification process, c-hGH recipients would most likely have had a much higher probability of exposure to pathological tau, Aβ, and α-synuclein than to PrPsc. In addition, our observations of abnormal deposits of NDAPs in aged control pituitary tissue further increase the likelihood of potential exposure to these proteins.
Second, although more than 200 cases of iatrogenic CJD have been identified to date among the estimated 30 000 c-hGH recipients treated between 1959 and 1985 worldwide30 (7700 in the United States, 1880 in France, and 1800 in the United Kingdom alone23), we found no reports of AD, FTLD, or PD, suggesting that these diseases may not be transmissible between humans.
Several caveats should be noted regarding the interpretation of these findings. First, it is currently unclear which species of NDAPs (monomers, oligomers, or fibrillar forms) is responsible for transmission seen in published models of disease, although the reports by Luk et al2,3 used preformed α-synuclein fibrils to transmit lethal Lewy body disease in an animal model. We demonstrate varying degrees of both amyloid-like and diffuse deposits for Aβ, tau, and α-synuclein in the neurohypophysis (Figure 1 and Figure 2). Despite the relative stability of PrPsc, it is still probable that most forms of these non-PrPsc NDAPs observed herein could also survive the relatively crude sequential extraction process used to purify c-hGH prior to 1977 in the United States,31 because the pathological species of NDAPs in AD and related proteinopathies are known to remain insoluble in harsher detergents used in experimental sequential extraction techniques.32,33
Our retrospective analysis is limited to reports in the literature and interrogation of a death certificate database that may not be comprehensive enough to detect all clinically manifest NDs. Indeed, neurologic diseases (ie, neoplasms, head trauma, and radiation necrosis) that occur in some c-hGH recipients may be difficult to distinguish from an emerging ND. However, the NHPP database did enable recognition of an increase in CJD in the US cohort of NHPP c-hGH recipients.
Another uncertainty is the potential incubation period for transmitted NDAPs. The reported mean incubation time for prion disease from midpoint of treatment in c-hGH recipients worldwide was 17 years but ranged from 5 to as long as 42 years.23 Endocrine failure or the underlying etiology of hormone deficiency contributed substantially to the young mean age at death of the patients in our cohort (27.2 years) (eTable 2).34 Despite this, more than half the deceased patients survived 15 years or more after the midpoint of c-hGH treatment, and 19% survived 25 years or more (eTable 2). Additionally, the large number of living patients (about 4600 of the cohort) also have not died of an ND after a long follow-up period of 25 years or more from the initial treatment (eTable 2).
The time required for the underlying neuropathology to cause clinical disease in non-PrPsc ND is not clear, but most likely it varies widely among different individuals and commonly spans several decades. There is evidence of neuropathological changes long before the onset of clinical disease. Early preneurofibrillary tangle pathology has been found in asymptomatic patients as young as the first decade of life.35 Furthermore, biomarker studies of AD suggest amyloidosis may be evident decades before clinical symptoms in AD.36,37 Indeed, new criteria to identify asymptomatic “preclinical” AD highlight the importance of AD neuropathological change as an abnormal prodrome to clinical AD.38 As such, it is possible that susceptible c-hGH patients could be in an early asymptomatic phase of transmitted ND that may not have become clinically manifested yet and thus not detected by our study. The lack of autopsy data for the NHPP cohort limits our ability to examine for evidence of a potential subclinical NDAP transmission and thus provide a more definitive conclusion on the subclinical human-to-human transmission of NDAPs; however, we found no evidence to support clinical transmission of AD or PD in this unique cohort after a relatively long incubation period (as compared with our experience with CJD). Continued follow-up of recipients of c-hGH, with reviews of the clinical and autopsy records of those who may die in the future with an ND listed as a cause of death, will be important to confirm these findings.
The discovery of 2 deaths attributed to ALS among the initially confirmed cohort of NHPP c-hGH recipients and 1 additional case identified in the literature, especially at such young ages, is disquieting. However, the identification of ALS cases among c-hGH recipients does not definitively indicate transmission of pathogenic TDP-43, ubiquilin, or FUS since we found no evidence of these proteins in the adeno/neurohypophysis of any of the cases studied herein. Notably, unlike tau, Aβ, and α-synuclein pathology, no abnormal TDP-43 deposits occur in the olfactory epithelium as well.26 These data suggest it is very unlikely c-hGH recipients were exposed to ALS-associated pathogenic proteins (ie, TDP-43, FUS, and ubiquilin). Furthermore, autopsy was not performed in 1 case and the others lacked state-of-the-art techniques for modern diagnosis; thus, the molecular etiology of the clinical syndrome in these cases remains uncertain. Indeed, 1 case was described to have degeneration of sensory tracts,27 which is atypical for ALS. Although the earlier-mentioned data suggest infectivity to be an unlikely etiology, surveillance of the c-hGH cohort for ALS and related NDs is necessary to monitor the occurrence of additional cases.
In our follow-up of the unusually young ALS case-patient identified in our literature review,27 we learned that no transmission of ALS per se occurred in a capuchin monkey that in September 1986 had received an intracerebral inoculation of 0.1 mL of a 20% suspension of this patient’s frozen cervical cord tissue. The inoculated monkey died in August 1997 without having developed signs of a neurological disease; an autopsy report, however, was unavailable (P. Brown, MD, and D. M. Asher, MD, oral and written communication, June 18 and 25, 2012). In addition, since the early 1970s, investigators at the National Institutes of Health conducted primate transmission studies with tissues from 58 other cases of ALS, 105 cases of AD, and 24 cases of PD with dementia; in none of these studies did the inoculated primates develop lower motor neuron signs, behavioral changes, or a movement disorder consistent with a non-PrPsc ND, nor did neuropathologists find postmortem evidence for the transmission of these diseases39 (P. Brown, MD, and D. M. Asher, MD, oral and written communication, June 18 and 25, 2012). In contrast, there were at least 300 cases of experimentally transmitted prion diseases during this same period.39 Despite this substantial negative body of evidence for non-PrPsc ND transmission in nonhuman primates, 2 studies in the early 1990s reported that subclinical AD-like plaques were induced in marmosets following central nervous system inoculation with human brain lysates (0.3 mL of 10% saline suspensions).40,41 The majority of human brain lysates shown to induce Aβ pathology in these studies were derived from CJD cases and not AD and there was also no evidence of transmission of clinical disease or tau pathology in any of these inoculated primates.
To our knowledge, only 1 other group of human subjects can provide some additional insights into the transmissibility of NDAPs and that is those patients with PD who received striatal fetal mesencephalic grafts as experimental therapy. Neurons within these grafts showed evidence of PD-like α-synuclein Lewy body pathology, but the number of patients whose grafts showed this pathology was small and only rare grafted neurons developed α-synuclein Lewy body pathology at or beyond 10 years postgrafting.42–45 However, while this α-synuclein pathology could reflect the transmission of pathological α-synuclein from the host striatum to the grafted neurons, other explanations are possible, such as the effects of the hosts’ PD neurodegenerative condition on the grafts aside from the α-synuclein pathology.12,46
In summary, despite the limitations of this study discussed earlier, to our knowledge, we provide the most compelling human in vivo evidence currently available to suggest that while there are some similarities between the cell-to-cell spread of PrPsc and non-PrPsc NDAPs in experimental models there is currently no documentation that AD, FTLD-tau, or PD-associated proteins (ie, tau, Aβ, or α-synuclein) transmit disease in human or nonhuman primates like PrPsc. Prospective monitoring of all c-hGH recipients for CJD and non-PrPsc NDs should be continued.
Acknowledgments
Drs Lee and Trojanowski report single consulting services to Pfizer, Johnson & Johnson, MetLife, and Bristol-Myers Squibb; royalty payments through Penn licenses; and research support from AstraZeneca and Bristol-Myers Squibb.
Funding/Support: This study was supported by grant P30 AG010124-20, an AD Core Centre grant, and grant T32-AG000255 from the National Institute on Aging and Intramural Research Program and the National Institute of Child Health and Development, National Institutes of Health.
Additional Contributions: We thank P. Brown, MD, and D. M. Asher, MD, for insightful comments and communication on previous National Institutes of Health primate studies and Kurt Brunden, PhD, Kelvin Luk, PhD, Adam Walker, PhD, and Jiali Li, PhD, and Jing Gou and Kaylan Tripathy for thoughtful critiques of the manuscript. We also thank Melanie Cedrone, Theresa Schuck, John Robinson, Kevin Raible, and Jonathan Bekisz for technical assistance.
Footnotes
Author Contributions: Study concept and design: Irwin, Schonberger, Lee, and Trojanowski. Acquisition of data: Irwin, Abrams, Schonberger, Leschek, Mills, Lee, and Trojanowski. Analysis and interpretation of data: Irwin, Abrams, Schonberger, Leschek, Mills, Lee, and Trojanowski. Drafting of the manuscript: Irwin, Abrams, Schonberger, and Trojanowski. Critical revision of the manuscript for important intellectual content: Irwin, Abrams, Schonberger, Leschek, Mills, Lee, and Trojanowski. Statistical analysis: Irwin, Abrams, and Schonberger. Obtained funding: Schonberger, Mills, Lee, and Trojanowski. Administrative, technical, and material support: Leschek. Study supervision: Trojanowski.
Conflict of Interest Disclosures: The authors report no financial disclosures related to the current study.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Online-Only Material: The eTables are available at http://www.jamaneuro.com.
REFERENCES
- 1.Eisele YS, Obermüller U, Heilbronner G, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330(6006):980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luk KC, Kehm V, Carroll J, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kane MD, Lipinski WJ, Callahan MJ, et al. Evidence for seeding of beta-amyloid by intracerebral infusion of Alzheimer brain extracts in beta-amyloid precursor protein-transgenic mice. J Neurosci. 2000;20(10):3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. De novo induction of amyloid-β deposition in vivo. Mol Psychiatry. 2O12;17(12):1347–1353. doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- 7.Stöhr J, Watts JC, Mensinger ZL, et al. Purified and synthetic Alzheimer’s amyloid beta(Aβ) prions. Proc Natl Acad Sci U S A. 2012;109(27):11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luk KC, Song C, O’Brien P, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J Biol Chem. 2011;286(21):18664–18672. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Lim HS, Masliah E, Lee HJ. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res. 2011;70(4):339–348. doi: 10.1016/j.neures.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of α-synuclein transfer. Cell Death Differ. 2011;18(9):1425–1433. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamaguchi T, Eisele YS, Varvel NH, Lamb BT, Walker LC, Jucker M. The presence of Aβ seeds, and not age per se, is critical to the initiation of Aβ deposition in the brain. Acta Neuropathol. 2012;123(1):31–37. doi: 10.1007/s00401-011-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64(6):783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 2012;209(5):889–893. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prusiner SB. Cell biology: a unifying role for prions in neurodegenerative diseases. Science. 2012;336(6088):1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy J, Revesz T. The spread of neurodegenerative disease. N Engl J Med. 2012;366(22):2126–2128. doi: 10.1056/NEJMcibr1202401. [DOI] [PubMed] [Google Scholar]
- 18.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 19.Walker L, Levine H, Jucker M. Koch's postulates and infectious proteins. Acta Neuropathol. 2006;112(1):1–4. doi: 10.1007/s00401-006-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes BB, Diamond MI. Amyotrophic lateral sclerosis and organ donation: is there risk of disease transmission? Ann Neurol. doi: 10.1002/ana.23684. [published online July 12, 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown P, Gajdusek DC, Gibbs CJ, Jr, Asher DM. Potential epidemic of Creutzfeldt-Jakob disease from human growth hormone therapy. N Engl J Med. 1985;313(12):728–731. doi: 10.1056/NEJM198509193131205. [DOI] [PubMed] [Google Scholar]
- 22.Brown P, Brandel JP, Preece M, Sato T. Iatrogenic Creutzfeldt-Jakob disease: the waning of an era. Neurology. 2006;67(3):389–393. doi: 10.1212/01.wnl.0000231528.65069.3f. [published correction appears in Neurology. 2006;67(8):1528]. [DOI] [PubMed] [Google Scholar]
- 23.Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis. 2012;18(6):901–907. doi: 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52(2):205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 25.Mills JL, Fradkin J, Schonberger L, et al. Status report on the US human growth hormone recipient follow-up study. Horm Res. 1990;33(2–4):116–119. doi: 10.1159/000181494. discussion 120. [DOI] [PubMed] [Google Scholar]
- 26.Arnold SE, Lee EB, Moberg PJ, et al. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67(4):462–469. doi: 10.1002/ana.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rappaport EB, Graham DJ. Pituitary growth hormone from human cadavers: neurologic disease in ten recipients. Neurology. 1987;37(7):1211–1213. doi: 10.1212/wnl.37.7.1211. [DOI] [PubMed] [Google Scholar]
- 28.Peden AH, Ritchie DL, Uddin HP, et al. Abnormal prion protein in the pituitary in sporadic and variant Creutzfeldt-Jakob disease. J Gen Virol. 2007;88(pt 3):1068–1072. doi: 10.1099/vir.0.81913-0. [DOI] [PubMed] [Google Scholar]
- 29.Prusiner SB. Shattuck lecture: neurodegenerative diseases and prions. N Engl J Med. 2001;344(20):1516–1526. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 30.Will RG. Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br Med Bull. 2003;66:255–265. doi: 10.1093/bmb/66.1.255. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelmi AE. Fractionation of human pituitary glands. Can J Biochem Physiol. 1961;39:1659–1668. doi: 10.1139/o61-183. [DOI] [PubMed] [Google Scholar]
- 32.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 33.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 34.Mills JL, Schonberger LB, Wysowski DK, et al. Long-term mortality in the United States cohort of pituitary-derived growth hormone recipients. J Pediatr. 2004;144(4):430–436. doi: 10.1016/j.jpeds.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jack CR, Jr, Vemuri P, Wiste HJ, et al. Alzheimer's Disease Neuroimaging Initiative. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol. 2011;68(12):1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown P, Gibbs CJ, Jr, Rodgers-Johnson P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35(5):513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- 40.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Evidence for the experimental transmission of cerebral beta-amyloidosis to primates. Int J Exp Pathol. 1993;74(5):441–454. [PMC free article] [PubMed] [Google Scholar]
- 41.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate: comparison with transmission of spongiform encephalopathy. Mol Neurobiol. 1994;8(1):25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- 42.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 43.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 44.Mendez I, Vifluela A, Astradsson A, et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;14(5):507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008;23(16):2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 46.Kordower JH, Brundin P. Propagation of host disease to grafted neurons: accumulating evidence. Exp Neurol. 2009;220(2):224–225. doi: 10.1016/j.expneurol.2009.09.016. [DOI] [PubMed] [Google Scholar]