Abstract
Previous studies have revealed white matter abnormalities in the brains of individuals with phenylketonuria (PKU), but the microstructural nature of these abnormalities and their relationship to phenylalanine (Phe) levels and cognitive outcomes is poorly understood. In the current study, the microstructural integrity of white matter in 29 individuals with early-treated PKU and 12 healthy controls was examined using two complementary diffusion tensor imaging (DTI) approaches: region-of-interest (ROI) based analysis and voxel-wise tract based spatial statistics (TBSS) analysis. Relationships among DTI, executive abilities, and Phe level findings were explored. DTI revealed widespread lowering of mean diffusivity (MD) in the white matter of the PKU group in comparison with the control group. Executive abilities were also poorer for individuals with PKU than controls. Within the PKU group, lower MD was associated with higher Phe level and poorer executive abilities. These findings are the first to demonstrate the interplay among microstructural white matter integrity, executive abilities, and Phe control in individuals with PKU.
Keywords: phenylketonuria, white matter, executive, brain, cognition
1. Introduction
Phenylketonuria (PKU) is an autosomal recessive disorder in which metabolism of the amino acid phenylalanine (Phe) is disrupted due to phenylalanine hydroxylase deficiency; as a result, blood Phe levels are elevated in individuals with PKU [1]. The profound sequelae of untreated PKU, such as intellectual disability [1,2] and neurological abnormalities [3], are rarely seen in developed nations today due to newborn screening programs and early implementation of dietary treatment to limit Phe intake. Nonetheless, early-treated PKU is associated with lower than expected intelligence [4] and impairments in specific aspects of cognition, executive abilities in particular [5,6].
For decades it has been hypothesized that the cognitive sequelae of early-treated PKU are related to deficiencies in dopamine, a neurotransmitter of crucial importance in the function of frontal brain regions that subserve executive abilities [7]. There are two key mechanisms underlying dopamine deficiency in PKU. First, the neurochemical cascade by which Phe is converted to tyrosine and tyrosine is converted to dopamine (and other neurotransmitters) is disrupted because Phe is not properly metabolized. Second, available tyrosine must compete with excess Phe for passage across the blood-brain barrier via the large neutral amino acid type 1 (LAT1)-transporter, to which Phe binds more strongly than tyrosine [1].
Of particular relevance to the current investigation, in recent years investigators have turned their attention to white matter pathology as another neural mechanism by which cognition may be compromised. A number of studies have identified observable white matter abnormalities in individuals with PKU [8-17]. These abnormalities are generally characterized by hyperintensities in periventricular brain regions on T2-weighted images and are more pronounced at higher Phe levels. Although these studies based on qualitative examination of the white matter are informative, they are limited in terms of defining the nature of white matter pathology.
An advanced MRI approach, diffusion tensor imaging (DTI), holds promise for enhancing our understanding of white matter pathology. DTI provides detailed information regarding the microstructural integrity of white matter through the measurement of water molecule movement. This imaging approach is sensitive to subtle changes in white matter integrity [18], detecting pathology even when T2-weighted images appear normal [19]. Two DTI measures are most commonly reported: (1) mean diffusivity (MD), which indicates the overall spatial average rate of water molecule movement and (2) fractional anisotropy (FA), which indicates the degree of asymmetry of water molecule movement.
Reductions in MD but normal FA in white matter tracts have been reported in studies of individuals with PKU, and more pronounced decreases in MD have generally been associated with higher blood Phe levels [10-13,15,17,20,21]. These findings indicate that the rate of water molecule movement is restricted in individuals with PKU compared with healthy controls. Most of the studies conducted to date, however, have suffered from limitations such as small sample size or inclusion of both early- and later-treated individuals within the same study. In some studies, DTI was examined only in brain regions having observable white matter abnormalities on T2-weighted images, which may have resulted in failure to identify important changes in other brain regions. Moreover, although changes in MD have been examined in relation to Phe levels, they have not been examined in relation to executive abilities.
In the present study, we conducted a comprehensive examination of the microstructural integrity of the white matter across the brain in a relatively large number of individuals with early-treated PKU. To do so, two complementary DTI approaches were used: (1) region of interest (ROI) analysis and (2) voxel-wise Tract Based Spatial Statistics (TBSS) [22] analysis. In ROI analysis, the brain regions to be examined are established a priori. ROI analysis has several advantages over the voxel-wise analysis, including minimization of type 1 error, minimization of statistical assumptions, and tailoring of ROIs to avoid partial volume effects which reduce the impact of imperfect registration procedures [18,23]. On the other hand, voxel-wise analysis considers all voxels within all major white matter tracts without regard to arbitrary or unreliable boundaries and without a priori assumptions. We employed both approaches to provide a comprehensive overview of the white matter microstructure of individuals with PKU. In addition, Phe level, IQ, and executive abilities were examined in relation to DTI findings. This study is the first to elucidate interrelationships between microstructural white matter integrity, metabolic control, and executive abilities.
2. Material and methods
2.1. Participants
Individuals with PKU (n = 32; 12 female, 20 male) were recruited through metabolic clinics at Washington University (WU; n = 13), University of Missouri (UM; n = 9), University of Florida (n = 4), St. Louis University (n = 3), New York Medical College (n = 2), and University of Nebraska (n = 1). Preliminary analyses revealed no significant differences in cognitive or neuroimaging findings between the two sites (WU and UM) from which the majority of participants with PKU were recruited (p >.05 in all instances). All individuals with PKU were diagnosed soon after birth and were treated early through dietary management to limit Phe intake. Blood Phe obtained closest to the time of cognitive and neuroimaging evaluations (typically the same day) ranged from 115 to 1459 μmol/L (M = 734, SD = 410), which is elevated in comparison with blood Phe in healthy individuals without PKU (i.e., ≤ 120 μmol/L).
Findings from individuals with PKU were compared with those of healthy controls (n = 12; 4 female; 8 male) recruited from the St. Louis community. No participant had a reported history of major medical (e.g., stroke), psychiatric (e.g., depression), or learning (e.g., dyslexia) disorder unrelated to PKU. Age ranged from 6 to 35 years (M = 18.0, SD = 9.0) for the PKU group and 7 to 33 years (M = 17.8, SD = 8.0) for the control group. Education ranged from 0 to 18 years (M = 9.1, SD = 4.6) for the PKU group and 1 to 16 years (M = 10.3, SD = 4.8) for the control group. With regard to race/ethnicity, 3% and 8% of the PKU and control groups, respectively, comprised individuals from minority populations. There were no significant between-group differences in age, education, or race/ethnicity (p >.05 in all instances).
2.2. Procedures
Data from this report are components of a larger study examining the effects of sapropterin dihydrochloride on brain and cognition in individuals with early-treated PKU. Approval to conduct this study was obtained from institutional review boards for the protection of human subjects at WU and UM, the institutions at which neuroimaging and cognitive data for the study were collected. Written informed consent was obtained for all participants and/or their guardians prior to engagement in study procedures. Participants typically completed neuroimaging and cognitive evaluations on the same day in a session lasting approximately four hours. A manuscript involving voxel-wise analyses that included data from a small subset of participants in the current study (n = 9) is under review elsewhere, but neither ROI analyses nor cognitive findings were included in that study.
2.3. Neuroimaging
Structural images were acquired on a Siemens TIM Trio 3.0T imaging system (Erlangen, Germany) with a standard Siemens 12 channel head coil. These images included a T1-weighted (T1W) sagittal, magnetization-prepared rapid gradient echo [MPRAGE; repetition time (TR) = 2000 ms (WU and UM), echo time (TE) = 3.03 ms (WU) and 2.97 (UM), flip angle = 8° (WU and UM), FOV = 256 × 256 pixels (WU) and 256 × 224 (UM), voxel resolution = 0.88 × 0.88 × 0.9 mm (WU and UM) and a T2-weighted (T2W) fast spin echo [TR = 3200 (WU and UM), TE = 475 (WU and UM), flip angle = 120° (WU and UM), FOV = 256 × 256 pixels (WU and UM), voxel resolution = 0.88 × 0.88 × 0.9 mm (WU and UM).
DTI was acquired using an echo planar imaging (EPI) sequence [TR = 12437 (WU) and 9900 (UM), TE = 102 (WU and UM), flip angle = 90° (WU and UM), FOV = 864 × 864 (WU) and 768 × 768 (UM), voxel resolution = 2.0 × 2.0 × 2.0 (WU and UM)]. Diffusion weighted images (DWI) with variable b factor up to 1000 s/mm2 maximum were acquired along 25 non-collinear diffusion gradient orientations. DWIs were registered first to the b=0 unsensitized image, then to the T2W, then to the best T1W (MPRAGE), and finally to an in-house atlas constructed at WU. Parametric maps were then generated for MD and FA.
MD and FA analyses were conducted using two complementary approaches: (1) ROI analysis and (2) voxel-wise analysis using TBSS [24]. ROIs were selected based on a well-established DTI atlas [25] and verified by a neuroradiologist. They were then applied to each participant's MD and FA parametric maps and sampled using Analyze version 8.0 (Mayo Clinic, Rochester). We focused on the following 10 ROIs to provide a sampling across various brain regions: prefrontal cortex, centrum semiovale, posterior parietal-occipital cortex, optic radiation, putamen, corpus callosum (genu, body, splenium), thalamus, and hippocampus. Values from left and right homologous regions were averaged.
As a complementary approach, we used voxel-wise analysis to confirm ROI findings and to permit identification of PKU-related white matter findings in other regions without regard for strict anatomical boundaries. Using each individual's motion-corrected, aligned, and averaged DWI data set, BET (FMRIB Brain Extraction Tool) was used to compute a brain mask, and FDT (FMRIB diffusion toolbox) was used to compute FA and MD images. These images were projected onto a skeleton and set at a threshold of FA= 0.2 for voxel-wise analysis.
2.4. Cognition
General intellectual ability (IQ) and executive abilities (working memory, strategic processing) were assessed for all participants.
2.4.1. General intellectual ability
IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI), which comprises four subtests (i.e., Vocabulary, Similarities, Block Design, Matrix Reasoning). A composite score from these subtests represented IQ. Administration and scoring were in accordance with test manual instructions.
2.4.2. Working memory
Two computerized tasks, 2-back and recognition span, were administered to assess working memory. The 2-back task comprised two conditions: letter and location. In both conditions, on each trial participants observed as one of eight letters (C, F, H, J, N, P, Q, S) was presented at one of eight locations along an imaginary circle. In the letter condition, participants were asked to press a target button when the letter presented was the same as that presented two trials ago and to press a non-target button otherwise. In the location condition, participants were asked to press a target button when any letter appeared in the same location as that occupied two trials ago and to press a non-target button otherwise. Stimuli were visible for 2500 ms, with an intertrial interval of 1000 ms. Participants heard a “beep” following correct responses and a “bloop” following incorrect responses. A total of 96 experimental trials was presented in each condition. Number of errors (false alarms, missed targets) was recorded. Because preliminary analyses revealed no significant interaction between condition (letter, location) and group (PKU, control), number of errors across letter and location conditions was averaged and used in analyses.
The recognition span task comprised two conditions: shape and location. Stimuli for each condition were identical, although instructions differed. Each stimulus comprised an image in which 1 of 12 shapes (e.g., square, triangle, etc.) appeared at 1 of 12 locations within a 3 by 4 grid. Participants watched as series were presented that ranged from 2 to 9 stimuli. Following presentation of the last stimulus of a series, an array appeared comprising a 3 by 4 grid that included all 12 shapes. In the shape condition, participants were asked to point to the shapes that had appeared in the order presented. In the location condition, participants were asked to point to the locations at which shapes had appeared in the order presented. A staircase method was used to determine the number of stimuli presented in each series. Administration always began with a series of 2 stimuli. If participants correctly identified the order in which the stimuli were presented, the next series contained 1 additional stimulus. If participants did not correctly identify the order of presentation, the next series contained 1 less stimulus. Each stimulus in a series remained visible for 1250 ms, with an interstimulus interval of 500 ms. All series within a given condition were administered in a block of 25 series. Working memory scores were recorded as the number of stimuli comprising the longest series correctly recalled. Because preliminary analyses revealed no significant interaction between condition (shape, location) and group (PKU, control), the number of stimuli comprising the longest series correctly recalled across shape and location conditions was averaged and used in analyses.
2.4.3. Strategic processing
Two tasks, word list learning and verbal fluency, were administered to assess strategic processing. In the word list learning task, participants listened as a list of 18 words was read by an examiner at a rate of 1 word/sec. Words comprising the list were drawn from three semantic categories (e.g., clothing, vegetables, components of a house), such that there were 6 words from each category. Following presentation, participants were asked to recall the words in any order. This procedure was repeated for a total of five learning trials, and the number of words correctly recalled on each of the 5 learning trials was recorded. Of particular relevance to strategic processing, a semantic cluster score was also recorded for each trial, which represented the number of words that was sequentially reported from the same semantic category. For example, recall of “spinach-carrot-window-hat-pants-shirt” received a cluster score of 5, because “spinach-carrot” and “hat-pants-shirt” represented sequential recall from vegetable and clothing categories, respectively. The number of words recalled and the number of words semantically clustered on trial 5 were used in analyses.
In the verbal fluency task, participants orally generated items from food and drink categories as rapidly as possible for a period of one minute. The number of correct responses generated was recorded and used in analyses.
3. Results
With the exception of voxel-wise analysis, the primary statistical approach for DTI, cognitive, and Phe level analyses was hierarchical linear regression. Given the relative paucity of comprehensive research exploring the relationships between DTI, cognition, and Phe, the significance level was set at p <.05 and we did not control for multiple comparisons to avoid obscuring promising patterns of results.
3.1. Neuroimaging
Neuroimaging was completed for 29 participants with PKU (3 declined) and all controls. For the ROI analyses, MD and FA for each of the 10 ROIs served as dependent variables in a series of hierarchical linear regression analyses to explore possible differences between the PKU and control groups. Given the broad age range of participants, in all analyses age was entered in the first step as an independent variable. Group was entered in the second step, followed by the interaction between age and group. MD and FA values for each ROI in the PKU and control groups are reported in Table 1, along with statistical findings for each independent variable (interactions between age and group are not reported in Table 1 because only one was statistically significant).
Table 1.
Mean (SD) diffusion tensor imaging (DTI) region of interest (ROI) values for the PKU and control groups, along with statistical findings (R2, F, p) from hierarchical linear regression.
| Mean Diffusivity (MD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Statistical Findings |
||||||||
| Age |
Group |
|||||||
| ROI | PKU | Control | R 2 | F | p | Δ R 2 | Δ F | Δ p |
| Prefrontal | .70 (.05) | .74 (.05) | 0.15 | 6.93 | 0.05 | 0.16 | 8.52 | 0.01 |
| Centrum Semiovale | .64 (.08) | .73 (.03) | 0.28 | 15.42 | 0.001 | 0.23 | 17.66 | 0.001 |
| Parietal Occipital | .70 (.10) | .84 (.05) | 0.22 | 10.88 | 0.005 | 0.34 | 28.80 | 0.001 |
| Optic Radiation | .74 (.09) | .84 (.04) | 0.25 | 12.79 | 0.001 | 0.20 | 14.11 | 0.001 |
| Putamen | .67 (.05) | .71 (.03) | 0.22 | 11.18 | 0.005 | 0.14 | 8.28 | 0.01 |
| Genu of CC | .67 (.09) | .83 (.09) | ns | 0.39 | 29.06 | 0.001 | ||
| Body of CC | .77 (.09) | .85 (.06) | ns | 0.17 | 8.01 | 0.01 | ||
| Splenium of CC | .67 (.08) | .69 (.04) | 0.15 | 6.65 | 0.05 | ns | ||
| Thalamus | .71 (.05) | .73 (.03) | 0.33 | 19.24 | 0.001 | ns | ||
| Hippocampus | .83 (.08) | .84 (.02) | ns | ns | ||||
| Fractional Anisotropy (FA) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Statistical Findings |
||||||||
| Age |
Group |
|||||||
| ROI | PKU | Control | R 2 | F | p | Δ R 2 | Δ F | Δ p |
| Prefrontal | .50 (.07) | .51 (.07) | ns | ns | ||||
| Centrum Semiovale | .45 (.05) | .44 (.04) | ns | ns | ||||
| Parietal Occipital | .42 (.07) | .44 (.08) | ns | ns | ||||
| Optic Radiation | .55 (.05) | .57 (.05) | ns | ns | ||||
| Putamen | .16 (.03) | .14 (.03) | ns | ns | ||||
| Genu of CC | .79 (.06) | .77 (.07) | ns | ns | ||||
| Body of CC | .63 (.07) | .66 (.03) | 0.12 | 5.43 | 0.05 | ns | ||
| Splenium of CC | .82 (.05) | .84 (.03) | ns | ns | ||||
| Thalamus | .34 (.07) | .34 (.06) | 0.20 | 9.59 | 0.005 | ns | ||
| Hippocampus | .18 (.04) | .17 (.03) | ns | ns | ||||
Notes: CC = Corpus Callosum; ns = not significant; df for age (1, 39); df for group (1, 38).
Turning first to findings regarding MD, age accounted for a significant proportion of the variance in MD for the prefrontal cortex, centrum semiovale, posterior parietal occipital cortex, optic radiation, putamen, splenium of the corpus callosum, and thalamus. In all instances, MD decreased as age increased. Of greater interest, after accounting for the variance attributable to age, group accounted for significant variance in MD for the prefrontal cortex, centrum semiovale, posterior parietal occipital cortex, optic radiation, putamen, genu of the corpus callosum, and body of the corpus callosum. In all instances, MD was lower for the PKU than control group. There was a single significant interaction between age and group [ΔR2 =.06, ΔF(1, 37) = 5.28, p <.05], reflecting a decrease in MD for the centrum semiovale as a function of increasing age for the PKU group [R2 =.45, F(1, 27) = 22.35, p <.001] but not the control group.
For FA, there were only two significant findings. Age accounted for significant variance in FA for the body of the corpus callosum and thalamus. No additional variance in FA for any ROI was accounted for by age, group, or the interaction between age and group.
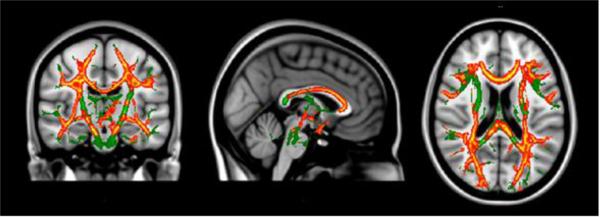
For voxel-wise analyses, DTI parameter images were analyzed using Randomise (FSL, FMRIB, Oxford, UK), a permutation-based multiple comparisons corrected statistical approach [26]. Age was co-varied in all analyses. MD was lower for the PKU than control group across multiple brain regions (see regions highlighted in red and yellow in Figure 1). For example, in agreement with ROI findings, voxel-wise analyses revealed lower MD in the centrum semiovale, posterior parietal occipital cortex, and optic radiation. In addition, an anterior to posterior gradient was evident in the corpus callosum, with the PKU group having lower MD in the genu and body, but not in the splenium, compared with controls.
Figure 1.
Diffusion tensor imaging (DTI) voxel-wise findings comparing PKU and control groups after controlling for age.
Notes: Green = TBSS skeleton; Red = Between-group difference p <.01; Yellow = Between-group difference p <.05.
FA was not significantly different between the PKU and control groups in any brain region. Given that there were no significant between-group findings for FA in either the ROI or voxel-wise analyses, FA was not examined further.
3.2. Cognition
Cognitive scores served as dependent variables in a series of hierarchical linear regression analyses to explore possible differences between the PKU and control groups. Age was entered in the first step as an independent variable in all analyses. Group was entered in the second step, followed by the interaction between age and group. Cognitive scores are reported in Table 2, along with statistical findings (interactions between age and group are not reported because none were statistically significant). The number of participants completing each cognitive task varied slightly due to technical issues and time constraints.
Table 2.
Mean (SD) cognitive scores, along with statistical findings (R2, F, p) from hierarchical linear regression.
| Statistical Findings |
||||||||
|---|---|---|---|---|---|---|---|---|
| Age |
Group |
|||||||
| Cognitive Variable | PKU | Control | R 2 | F | p | Δ R 2 | Δ F | Δ p |
| IQ | 99 (13) | 109 (11) | ns | 0.12 | 5.69 | 0.05 | ||
| 2-Back Errors | 8.7 (5.3) | 3.2 (2.4) | 0.12 | 5.27 | 0.05 | 0.23 | 13.60 | 0.001 |
| Recognition Span | 5.2 (0.7) | 5.7 (1.0) | 0.19 | 9.76 | 0.005 | 0.08 | 4.23 | 0.05 |
| Word List Learning | 12.8 (3.3) | 15.8 (1.7) | 0.13 | 6.00 | 0.05 | 0.19 | 11.21 | 0.005 |
| Word List Clustering | 9.7 (5.1) | 13.7 (3.4) | 0.13 | 6.15 | 0.05 | 0.14 | 7.64 | 0.01 |
| Verbal Fluency | 17.0 (5.5) | 22.1 (7.1) | 0.09 | 4.21 | 0.05 | 0.14 | 7.17 | 0.05 |
Notes: ns = not significant; df for age and group varied because not all participants completed each task.
3.2.1. General intellectual ability
IQ was assessed for all participants with PKU (n = 32) and all controls (n = 12). Group accounted for significant variance in IQ, with lower scores for the PKU than control group. Age did not account for additional variance, as age is already taken into consideration when calculating IQ.
3.2.2. Working memory
Number of errors on the 2-back task was assessed for 30 participants with PKU and all controls. Age accounted for significant variance in number of errors, indicating that number of errors decreased as age increased. Of greater interest, group accounted for additional variance in number of errors, reflecting more errors for the PKU than control group.
Recognition span was assessed for all participants with PKU and 11 controls. Age accounted for significant variance in recognition span, indicating that span increased as age increased. Group accounted for additional variance, reflecting lower span for the PKU than control group.
3.2.3. Strategic processing
Number of words correctly recalled and number of words semantically clustered on trial 5 of the word list learning task were assessed for 31 participants with PKU and all controls. Age accounted for significant variance in both number of words recalled and number of words semantically clustered, with both increasing as a function of increasing age. Group accounted for additional variance in both number of words recalled and number of words semantically clustered, with the PKU group recalling and clustering fewer words than the control group.
Number of words correctly generated on the food/drink verbal fluency task was assessed for 31 participants with PKU and all controls. Age accounted for significant variance in number of words generated, which increased as a function of increasing age. Group accounted for additional variance, reflecting generation of fewer words for the PKU than control group.
3.3. Relationships between Phe level, MD, and cognition within the PKU group
As a starting point, the relationship between age and Phe level at the time of evaluation was examined, because previous research has shown that Phe level generally increases as individuals with PKU age due to relaxation of dietary restrictions on Phe intake [27]. Not surprisingly, hierarchical linear regression showed that age accounted for significant variance in Phe level [R2 = .16, F(1, 30) = 5.87, p <.05], which increased as a function of increasing age. As noted earlier, age was also associated with many of our MD and cognitive findings. Given that age had differential or even conflicting effects on Phe level, MD, and cognition (i.e., Phe level and cognition increased with age, whereas MD in many ROIs decreased with age), in the analyses described below, residuals from linear regression were used in which the contribution of age to Phe level, MD, and cognition was first statistically removed.
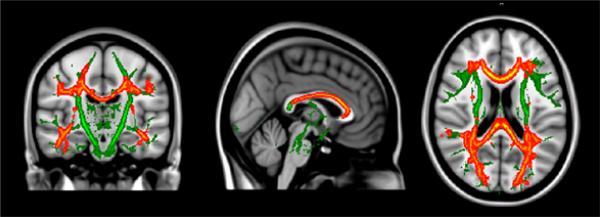
The relationship between Phe level and MD for the PKU group was explored using a series of hierarchical regression analyses. Age-residualized MD for each of the ROIs served as dependent variables, whereas age-residualized Phe level served as the independent variable in all analyses. Statistical findings are reported in Table 3. Results showed that Phe level accounted for significant variance in MD for the centrum semiovale, posterior parietal occipital cortex, optic radiation, putamen, all regions of the corpus callosum (i.e., genu, body, splenium), and hippocampus. In all instances, MD decreased as Phe level increased. In addition, the relationship between Phe level and MD from voxel-wise analysis was examined. Consistent with ROI findings, MD decreased as Phe level increased across multiple brain regions, such as the posterior parietal occipital cortex, optic radiation, and genu and body of the corpus callosum (see Figure 2).
Table 3.
Relationships between phenylalanine (Phe) level and mean diffusivity (MD) region of interest (ROI) values for the PKU group after controlling for age.
| Statistical Findings |
|||
|---|---|---|---|
| ROI | R 2 | F | p |
| Prefrontal | ns | ||
| Centrum Semiovale | 0.52 | 28.76 | 0.001 |
| Parietal Occipital | 0.49 | 25.84 | 0.001 |
| Optic Radiation | 0.40 | 17.93 | 0.001 |
| Putamen | 0.24 | 8.53 | 0.01 |
| Genu of CC | 0.43 | 20.67 | 0.001 |
| Body of CC | 0.53 | 30.02 | 0.001 |
| Splenium of CC | 0.34 | 14.05 | 0.001 |
| Thalamus | ns | ||
| Hippocampus | 0.30 | 11.41 | 0.005 |
Notes: CC = Corpus Callosum; ns = not significant; df for ROI (1, 27).
Figure 2.
Relationship between mean diffusivity (MD) voxel-wise findings and phenylalanine (Phe) level for the PKU group after controlling for age.
Notes: Green = TBSS skeleton; Red = Between-group difference p <.01; Yellow = Between-group difference p <.05.
The relationship between Phe level and cognitive scores for the PKU group was also explored using a series of hierarchical regression analyses. Age-residualized cognitive scores from each task served as dependent variables, whereas age-residualized Phe level served as the independent variable in all analyses. Results showed that Phe level accounted for significant variance in IQ [R2 =.17, F(1, 30) = 6.11, p <.05], number of errors on the 2-back task [R2 =.20, F(1, 28) = 6.87, p <.05], and number of words generated on the verbal fluency task [R2 =.13, F(1, 29) = 4.41, p <.05]. In all instances, cognition was poorer as Phe level increased.
Finally, the relationship between MD and cognitive scores for the PKU group was examined using a series of hierarchical regression analyses. Age-residualized cognitive scores for each task served as dependent variables, whereas age-residualized MD for each ROI served as independent variables. Results showed that MD for the posterior parietal occipital cortex [R2 =.19, F(1, 27) = 6.21, p <.05] and body of the corpus callosum [R2 =.16, F(1, 27) = 5.29, p <.05] accounted for significant variance in IQ. In addition, the genu [R2 =.18, F(1, 26) = 5.61, p <.05] and body [R2 =.15, F(1, 26) = 4.53, p <.05] of the corpus callosum accounted for significant variance in number of words generated on the verbal fluency task. In all instances, cognition was poorer as MD decreased. There were no other statistically significant findings.
4. Discussion
Previous studies have shown that the white matter of the brain [9-15,17,21,28] and executive abilities [5,6] are compromised in individuals with PKU. In addition, compromises in white matter and executive abilities tend to be more pronounced at higher blood Phe levels. That said, there has been no research in which the interrelationships among microstructural white matter integrity, executive abilities, and Phe levels have been examined within a single study, and many of the studies conducted to date have been of limited in size or scope.
In the current study we endeavored to overcome these limitations. To do so, two DTI approaches, ROI and voxel-wise analyses, were used to evaluate the microstructural white matter integrity of individuals with early-treated PKU. Various aspects of executive abilities were assessed using multiple tasks measuring working memory and strategic processing, and IQ was also assessed. Finally, Phe level at the time of DTI and cognitive evaluations was determined. Results from DTI and cognitive evaluations were compared with those of healthy controls, and the relationships among DTI, cognitive, and Phe level findings were explored within the PKU group.
With regard to DTI findings, we identified decreased MD (but comparable FA) in our PKU group compared with our control group across a range of brain regions. This was the case using both ROI and voxel-wise analyses. Previous studies have identified decreased MD in a limited number of brain regions [10-13,15,17,20,21], with some studies examining MD only in regions in which white matter hyperintensities were apparent on T2W images. Our results extend previous findings by identifying widespread microstructural white matter pathology in individuals with PKU using two analytic approaches.
It is interesting to consider possible explanations for decreased MD but normal FA in individuals with PKU. FA reflects the directional asymmetry of water diffusion, and decreases in FA have been reported in association with axonal injury and degeneration following neurologic events such as stroke [29] and traumatic brain injury [18]. In contrast, MD reflects the rate of water diffusion. Prior research suggests that inadequate Phe metabolism may lead to the accumulation of intracellular debris within the white matter of individuals with PKU, which could contribute to decreases in MD [13]. Overall, findings of normal FA but decreased MD suggest relatively intact axons but restricted diffusion of water molecules within axons, possibly due to accumulated debris.
Our finding of decreased MD in anterior (i.e., genu and body) but not posterior (i.e., splenium) regions of the corpus callosum is also of interest. Different proteomic profiles across subregions of the corpus callosum have been reported, with greater similarity between the genu and body compared with the splenium [30]. In addition, the anatomy and neuronal fiber origination of the genu and body are more similar compared with the splenium [31-33]. Although additional research is needed to reach definitive conclusions, it is possible that the proteomic and neural characteristics of corpus callosum subregions make the genu and body particularly susceptible to the metabolic dysregulation associated with PKU.
Turning to executive abilities, we identified poorer working memory and strategic processing in our PKU group compared with our control group across all of the tasks administered (IQ was also poorer for the PKU group). More specifically, the PKU group exhibited increased errors and decreased span on our working memory n-back and recognition span tasks, respectively. Poorer strategic processing in the PKU group was demonstrated by recall and clustering of fewer words on our list-learning task and by generation of fewer words on our verbal fluency task. These findings are consistent with previous reports of impairments in working memory and strategic processing in individuals with early-treated PKU [8,28,34-37].
To understand relationships among metabolic control, microstructural white matter integrity, and executive abilities, we next examined findings within the PKU group. Higher Phe level at the time of neuroimaging and cognitive evaluations was associated with lower MD in 8 of the 10 regions examined using ROI analysis, and voxel-wise analysis verified that higher Phe level was related to widespread white matter compromise. Higher Phe level was also associated with lower IQ, as well as poorer performance on working memory (n-back) and strategic processing (verbal fluency) tasks. These findings are consistent with those of previous studies showing that white matter pathology [8,9,16] and executive impairments [8,28] are more pronounced at higher Phe levels and support the hypothesis that poorer Phe control promotes greater accumulation of intracellular debris and restriction of water diffusion within the white matter.
Examination of relationships between DTI and cognitive findings within the PKU group revealed that lower MD, particularly in anterior regions of the corpus callosum, was related to lower IQ and poorer strategic processing (verbal fluency task). The corpus callosum comprises fibers that interconnect the right and left hemispheres of the brain, and previous research has shown that executive abilities are correlated with corpus callosum integrity in healthy adults [38,39]. It has also been shown that interhemispheric information processing is compromised in children with early-treated PKU [40,41]. As such, it was not surprising that we found relationships between corpus callosum integrity and abilities that require multisensory and multimodal information processing across brain regions (i.e., intelligence and executive abilities).
Our study is the first to demonstrate the interrelationships among microstructural white matter integrity, executive abilities, and Phe level, but there are limitations and future research is needed. For example, because our study was cross-sectional, we were unable to determine how these interrelationships might change as individuals with PKU age and exposure to elevated Phe levels is extended. Longitudinal research is required to address this issue conclusively. Cleary et al. [42] reported that white matter abnormalities were reversible in individuals with PKU following resumption of adequate Phe control. Future research is needed to determine whether similar improvements occur in microstructural white matter integrity following improvements in metabolic control. Finally, although we conducted neuroimaging with a relatively large number of individuals with PKU compared with most other studies, it is possible that additional associations between white matter integrity, cognition, and Phe level would be identified in a larger sample. Despite these limitations and the necessity of future research, our findings greatly strengthen the argument that control of Phe levels is essential for optimizing neural and cognitive outcomes in individuals with PKU.
Highlights.
Widespread brain white matter abnormalities in individuals with phenylketonuria
Impairment in executive abilities in individuals with phenylketonuria
Phenylalanine level related to compromised white matter and executive abilities
First demonstration of associations among white matter, executive, and Phe control
Acknowledgements
This research was supported by an Investigator Sponsored Trial grant from BioMarin Pharmaceutical Inc. This research was also supported by a grant from the National Institute on Drug Abuse (T32DA007261) and the Human Clinical Core of the Washington University Intellectual and Developmental Disabilities Research Center which is funded by the National Institute of Child Health and Human Development (P30HD062171) and the James S. McDonnell Foundation. The authors wish to thank those who participated in our study for their contributions to our research. We also thank Suzin Blankenship and Laurie Sprietsma for their contributions to study management, as well as the physicians, faculty, and staff of Washington University, University of Missouri, University of Florida, St. Louis University, New York Medical College, and University of Nebraska who generously contributed through recruitment and phenylalanine monitoring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol. Genet. Metab. 2010;99(Suppl 1):S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Paine RS, Hsia DY. The dietary phenylalanine requirements and tolerances of phenylketonuric patients. AMA. J. Dis. Child. 1957;94:224–230. doi: 10.1001/archpedi.1957.04030040006002. [DOI] [PubMed] [Google Scholar]
- 3.Moyle JJ, Fox AM, Bynevelt M, Arthur M, Burnett JR. A neuropsychological profile of off-diet adults with phenylketonuria. J. Clin. Exp. Neuropsychol. 2007;29:436–441. doi: 10.1080/13803390600745829. [DOI] [PubMed] [Google Scholar]
- 4.Brumm VL, Grant ML. The role of intelligence in phenylketonuria: a review of research and management. Mol. Genet. Metab. 2010;99(Suppl 1):S18–S21. doi: 10.1016/j.ymgme.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Christ SE, Huijbregts SC, De Sonneville LM, White DA. Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol. Genet. Metab. 2010;99(Suppl 1):S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.DeRoche K, Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: intelligence and executive function. Dev. Neuropsychol. 2008;33:474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- 7.Welsh MC, Pennington BF, Ozonoff S, Rouse B, McCabe ER. Neuropsychology of early-treated phenylketonuria: specific executive function deficits. Child. Dev. 1990;61:1697–1713. [PubMed] [Google Scholar]
- 8.Anderson PJ, Wood SJ, Francis DE, Coleman L, Anderson V, Boneh A. Are neuropsychological impairments in children with early-treated phenylketonuria (PKU) related to white matter abnormalities or elevated phenylalanine levels? Dev. Neuropsychol. 2007;32:645–668. doi: 10.1080/87565640701375963. [DOI] [PubMed] [Google Scholar]
- 9.Cleary MA, Walter JH, Wraith JE, Jenkins JP, Alani SM, Tyler K, Whittle D. Magnetic resonance imaging of the brain in phenylketonuria. Lancet. 1994;344:87–90. doi: 10.1016/s0140-6736(94)91281-5. [DOI] [PubMed] [Google Scholar]
- 10.Dezortova M, Hajek M, Tintera J, Hejcmanova L, Sykova E. MR in phenylketonuria-related brain lesions. Acta Radiol. 2001;42:459–466. doi: 10.1080/028418501127347179. [DOI] [PubMed] [Google Scholar]
- 11.Ding XQ, Fiehler J, Kohlschutter B, Wittkugel O, Grzyska U, Zeumer H, Ullrich K. MRI abnormalities in normal-appearing brain tissue of treated adult PKU patients. J. Magn. Reson. Imaging. 2008;27:998–1004. doi: 10.1002/jmri.21289. [DOI] [PubMed] [Google Scholar]
- 12.Kono K, Okano Y, Nakayama K, Hase Y, Minamikawa S, Ozawa N, Yokote H, Inoue Y. Diffusion-weighted MR imaging in patients with phenylketonuria: relationship between serum phenylalanine levels and ADC values in cerebral white matter. Radiology. 2005;236:630–636. doi: 10.1148/radiol.2362040611. [DOI] [PubMed] [Google Scholar]
- 13.Leuzzi V, Tosetti M, Montanaro D, Carducci C, Artiola C, Carducci C, Antonozzi I, Burroni M, Carnevale F, Chiarotti F, Popolizio T, Giannatempo GM, D'Alesio V, Scarabino T. The pathogenesis of the white matter abnormalities in phenylketonuria. A multimodal 3.0 tesla MRI and magnetic resonance spectroscopy (1H MRS) study. J. Inherit. Metab. Dis. 2007;30:209–216. doi: 10.1007/s10545-006-0399-4. [DOI] [PubMed] [Google Scholar]
- 14.Manara R, Burlina AP, Citton V, Ermani M, Vespignani F, Carollo C, Burlina AB. Brain MRI diffusion-weighted imaging in patients with classical phenylketonuria. Neuroradiology. 2009;51:803–812. doi: 10.1007/s00234-009-0574-z. [DOI] [PubMed] [Google Scholar]
- 15.Scarabino T, Popolizio T, Tosetti M, Montanaro D, Giannatempo GM, Terlizzi R, Pollice S, Maiorana A, Maggialetti N, Carriero A, Leuzzi V, Salvolini U. Phenylketonuria: white-matter changes assessed by 3.0-T magnetic resonance (MR) imaging, MR spectroscopy and MR diffusion. Radiol. Med. 2009;114:461–474. doi: 10.1007/s11547-009-0365-y. [DOI] [PubMed] [Google Scholar]
- 16.Thompson AJ, Tillotson S, Smith I, Kendall B, Moore SG, Brenton DP. Brain MRI changes in phenylketonuria. Associations with dietary status. Brain. 1993;116:811–821. doi: 10.1093/brain/116.4.811. [DOI] [PubMed] [Google Scholar]
- 17.Vermathen P, Robert-Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magn. Reson. Med. 2007;58:1145–1156. doi: 10.1002/mrm.21422. [DOI] [PubMed] [Google Scholar]
- 18.Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimony JS, Sheline YI, D'Angelo G, Epstein AA, Benzinger TL, Mintun MA, McKinstry RC, Snyder AZ. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol. Psychiatry. 2009;66:245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White DA, Connor LT, Nardos B, Shimony JS, Archer R, Snyder AZ, Moinuddin A, Grange DK, Steiner RD, McKinstry RC. Age-related decline in the microstructural integrity of white matter in children with early- and continuously-treated PKU: a DTI study of the corpus callosum. Mol. Genet. Metab. 2010;99(Suppl 1):S41–S46. doi: 10.1016/j.ymgme.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng H, Peck D, White DA, Christ SE. Tract-based evaluation of white matter damage in individuals with early-treated phenylketonuria. J. Inherit. Metab. Dis. doi: 10.1007/s10545-013-9650-y. in press. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PC, Mazziotta J, Mori S. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. Int. J. Adolesc. Med. Health. 2004;16:41–45. doi: 10.1515/ijamh.2004.16.1.41. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PJ, Wood SJ, Francis DE, Coleman L, Warwick L, Casanelia S, Anderson VA, Boneh A. Neuropsychological functioning in children with early-treated phenylketonuria: impact of white matter abnormalities. Dev. Med. Child Neurol. 2004;46:230–238. doi: 10.1017/s0012162204000386. [DOI] [PubMed] [Google Scholar]
- 29.Pitkonen M, Abo-Ramadan U, Marinkovic I, Pedrono E, Hasan KM, Strbian D, Durukan A, Tatlisumak T. Long-term evolution of diffusion tensor indices after temporary experimental ischemic stroke in rats. Brain Res. 2012;1445:103–110. doi: 10.1016/j.brainres.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Kashem MA, Sarker R, Des EH, Machaalani R, King N, McGregor IS, Matsumoto I. Comparative proteomics in the corpus callosal sub-regions of postmortem human brain. Neurochem. Int. 2009;55:483–490. doi: 10.1016/j.neuint.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Individual differences in brain asymmetries and fiber composition in the human corpus callosum. Brain Res. 1992;598:154–161. doi: 10.1016/0006-8993(92)90179-d. [DOI] [PubMed] [Google Scholar]
- 32.Lomber SG, Payne BR, Rosenquist AC. The spatial relationship between the cerebral cortex and fiber trajectory through the corpus callosum of the cat. Behav. Brain Res. 1994;64:25–35. doi: 10.1016/0166-4328(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 33.Dudink J, Kerr JL, Paterson K, Counsell SJ. Connecting the developing preterm brain. Early Hum. Dev. 2008;84:777–782. doi: 10.1016/j.earlhumdev.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee P, Grange DK, Steiner RD, White DA. Executive strategic processing during verbal fluency performance in children with phenylketonuria. Child Neuropsychol. 2011;17:105–117. doi: 10.1080/09297049.2010.525502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janos AL, Grange DK, Steiner RD, White DA. Processing speed and executive abilities in children with phenylketonuria. Neuropsychology. 2012;26:735–743. doi: 10.1037/a0029419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White DA, Nortz MJ, Mandernach T, Huntington K, Steiner RD. Age-related working memory impairments in children with prefrontal dysfunction associated with phenylketonuria. J. Int. Neuropsychol. Soc. 2002;8:1–11. [PubMed] [Google Scholar]
- 37.White DA, Nortz MJ, Mandernach T, Huntington K, Steiner RD. Deficits in memory strategy use related to prefrontal dysfunction during early development: evidence from children with phenylketonuria. Neuropsychology. 2001;15:221–229. doi: 10.1037//0894-4105.15.2.221. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J. Cogn. Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banich MT, Passarotti AM, White DA, Nortz MJ, Steiner RD. Interhemispheric interaction during childhood: II. children with early-treated phenylketonuria. Dev. Neuropsychol. 2000;18:53–71. doi: 10.1207/S15326942DN1801_4. [DOI] [PubMed] [Google Scholar]
- 41.Gourovitch ML, Craft S, Dowton SB, Ambrose P, Sparta S. Interhemispheric transfer in children with early-treated phenylketonuria. J. Clin. Exp. Neuropsychol. 1994;16:393–404. doi: 10.1080/01688639408402650. [DOI] [PubMed] [Google Scholar]
- 42.Cleary MA, Walter JH, Wraith JE, White F, Tyler K, Jenkins JP. Magnetic resonance imaging in phenylketonuria: reversal of cerebral white matter change. J. Pediatr. 1995;127:251–255. doi: 10.1016/s0022-3476(95)70303-9. [DOI] [PubMed] [Google Scholar]