Abstract
Although signal transducer and activator of transcription 3 (Stat3) is a key second messenger by which leptin regulates appetite and body weight, its role in specific neuronal populations in metabolic regulation and in mediating the chronic effects of leptin on blood pressure are unknown. The current study tested the hypothesis that Stat3 signaling in proopiomelanocortin (POMC) neurons mediates the chronic effects of leptin on mean arterial pressure (MAP) as well as on glucose regulation, energy expenditure, and food intake. Stat3flox/flox mice were crossed with POMC-Cre mice to generate mice with Stat3 deletion specifically in POMC neurons (Stat3flox/flox/POMC-Cre). Oxygen consumption (VO2), carbon dioxide respiration (VCO2), motor activity, heat production, food intake and MAP were measured 24hrs/day. After baseline measurements, leptin was infused (4μg/kg/min, IP) for 7 days. Stat3flox/flox/POMC-Cre mice were hyperphagic, heavier, and had increased respiratory quotients compared to control Stat3flox/flox mice. Baseline MAP was not different between the groups and chronic leptin infusion reduced food intake similarly in both groups (27 vs. 29%). VO2, VCO2, and heat production responses to leptin were not significantly different in control and Stat3flox/flox/POMC-Cre mice. However, leptin-mediated increases in MAP were completely abolished and blood pressure responses to acute air-jet stress were attenuated in male Stat3flox/flox/POMC-Cre mice. These results indicate that Stat3 signaling in POMC neurons is essential for leptin-mediated increases in MAP but not for leptin’s anorexic or thermogenic effects.
Keywords: Obesity, hypertension, appetite regulation, blood pressure, glucose, insulin, motor activity, sex differences
INTRODUCTION
Most of the known physiological actions of leptin, including regulation of appetite, thermogenesis, and sympathetic nervous system (SNS) activity, are mediated by activation of the long form of the leptin receptor (LRb).1 LRb, a cytokine receptor, is expressed in many areas of the brain and peripheral tissues and activates janus tyrosine kinase (Jak). Jak phosphorylates three major tyrosine residues to elicit three distinct signaling pathways.1,2 Signal transducer and activator of transcription 3 (Stat3) is one of the key signaling pathways activated in the hypothalamus by leptin and appears to be important in regulating appetite and body weight. Mice with conditional deletion of Stat3 in the entire CNS are hyperphagic, obese, and display many of the metabolic abnormalities found in leptin receptor deficient mice.3 Moreover, leptin-mediated appetite suppression is markedly attenuated in mice with Stat3 deficiency in the entire brain.3 Although these observations indicate that LRb signaling through Stat3 is important for regulating appetite and body weight, the role of Stat3 in mediating the chronic blood pressure effects of leptin are unknown. Furthermore, there have been no previous studies, to our knowledge, that have determined the role of Stat3 signaling in different regions of the CNS in mediating the chronic actions of leptin on appetite, thermogenesis, glucose homeostasis, and cardiovascular regulation.
In the current study, we used a genetic approach to inactivate Stat3 specifically in proopiomelanocortin (POMC) neurons. The generation of mice with targeted deletion of Stat3 allowed us to test the hypothesis that Stat3 in POMC neurons is essential for leptin’s chronic effects on cardiovascular and metabolic functions. Our results indicate that Stat3 signaling in POMC neurons plays a key role in the chronic effects of leptin to raise arterial pressure as well as in modulating the acute blood pressure responses to stress in male mice, but may not be essential for mediating leptin’s chronic effects on appetite or glucose regulation.
METHODS
The experimental protocols of this study followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Animals
Stat3flox/flox mice (generously provided by Dr. Xin-Yuan Fu, Indiana University School of Medicine) have loxP sites flanking exons 18-20 that contain the SH2 domain essential for phosphorylation of Stat3. These mice were crossed with heterozygotic POMC-Cre mice (generously provided by Dr. Joel Elmquist, University of Texas Southwestern) that express Cre-recombinase specifically in POMC neurons. Mice that were homozygous for Stat3flox/flox and expressed Cre-recombinase were labeled Stat3flox/flox/POMC-Cre and littermate homozygous Stat3flox/flox mice not expressing Cre-recombinase were used as controls. We previously reported that the cardiovascular and metabolic responses to leptin were not different in POMC-Cre mice and wild type (C57BL/6J) controls.4
The different groups of mice (n=15 for each group) were followed from weaning to obtain a growth curve and a subset of mice (N=6) randomly selected from each parent group was studied at 8-10 weeks of age to assess early metabolic parameters. Then at 20 weeks of age subsets of Stat3flox/flox and Stat3flox/flox/POMC-Cre mice (n=6 for each group) were used to investigate the chronic cardiovascular and metabolic effects of leptin.
The use of Cre-recombinase technology for selective inactivation of Stat3 in POMC neurons has been previously reported.5 To further validate that Stat3 was inactivated specifically in POMC neurons of Stat3flox/flox-POMC-Cre mice, immunohistochemistry was performed in a subset of POMC-Cre mice that were bred with mice that carried the R26R Lac Z reporter allele (Gt(ROSA)26Sortm1Sor; Jackson Lab.). These mice, Rosa/POMC-Cre and Stat3flox/floxRosa/POMC-Cre mice, allowed us to assess localization of POMC and phosphorylated-Stat3 (p-Stat3) immunoreactivity following intraperitoneal leptin injection.
Surgical implantation of telemetry probes
Mice from each group were anesthetized with 1% isoflurane and, using sterile techniques, a radiotelemeter (TA11PA-C10 DSI systems, St. Paul, MN) was inserted into the carotid artery and advanced to the aorta for measurement of mean arterial pressure (MAP) and heart rate (HR), 24 hrs/day, using computerized methods (Dataquest 4.0: DSI systems, St. Paul, MN) for data collection as described previously.4 Mice were allowed to recover for at least 7 days before starting any experimental protocols.
Experimental protocol
General
Mice were fed ad libitum throughout the study, except during the fasting periods (24 hours) for the refeeding experiment and for 4 hours preceding blood collection for measurements of leptin, insulin and glucose as described later. Body weight was measured weekly in each mouse beginning at 5 weeks of age and for the duration of the study. Body fat mass, lean mass, and water content of the mice were measured using EchoMRI-4in1 system (EchoMRI, TX) (n=6 from each group) at 8 and 20 weeks of age. Body composition data represent the average of 3 measurements in each mouse.
Studies at 8-10 weeks of age
Between 8-10 weeks of age, subsets of mice (n=6) randomly selected from each group were placed in individual metabolic cages to determine baseline food intake, fasting/refeeding responses, and metabolic profiles. Food intake was measured for 3 consecutive days followed by a 24-hour fast. Then the mice were permitted to eat ad libitum and food intake was measured for an additional 48-72 hours post fasting and a blood sample (approximately 150 μl) was collected at the end of this period. Mice were then transferred to metabolic cages (Accuscan Instruments Inc, Columbus, OH) to measure VO2, VCO2, respiratory quotient (RQ), motor activity, and heat production for 3 consecutive days.
Studies at 20 weeks of age
Radiotelemeters were implanted at 18 weeks of age, and after recovery for 7-10 days the mice were placed back into metabolic cages (Accuscan) and allowed to acclimate for 2 days. MAP and HR were recorded 24 hrs/day using computerized methods as previously described.4,6 Briefly, 500 samples/second were taken in bursts of 10 seconds every 10 minutes and average values were recorded for each day. Food and water consumption were recorded daily. After 5 days of stable control measurements, osmotic minipumps (Alzet, Cupertino, CA, model 1007D) were implanted intraperitoneally to infuse leptin (R&D Systems, Minneapolis, MN) at 4 μg/kg/min for 7 days. This dose was chosen because it increases plasma leptin to concentrations comparable to those found in severe obesity and we have previously shown that these levels significantly decrease food intake and elevate blood pressure in control mice.4,6 After leptin infusion was stopped, measurements were continued for 5 additional days of post-treatment recovery. A blood sample (150 μl) was taken via a small tail snip after a 4 hour fast on the last day of control, the last day of leptin infusion, and the last day of the recovery periods. Benzocaine was used as a topical analgesic to alleviate pain following tail snips. Mice were allowed to recover for 2 weeks after the leptin infusions were stopped before acute blood pressure responses to stress were evaluated, as described below.
Acute Air-Jet Stress Studies
The acute blood pressure and heart rate responses to stress were measured in Stat3flox/flox and Stat3flox/flox/POMC-Cre mice following recovery for 2 weeks after the chronic leptin infusion was stopped. Mice were placed in special cages and allowed two hours to acclimate. MAP and HR were then monitored continuously for the duration of the test. Following 30 minutes of baseline measurements, mice were abruptly awakened with an air-jet delivered near the mouse’s head using a 14-gauge needle attached to a container of compressed air. The air-jet was delivered intermittently for 5 seconds on and 10 seconds off for an additional 5 minutes. MAP and HR were recorded for an additional 30 minutes after stopping the air-jet stress.
Glucose Tolerance Test
Following completion of the experimental protocols, when the mice were approximately 20 weeks old, glucose tolerance tests (GTT) were conducted in Stat3flox/flox and Stat3flox/flox/POMC-Cre (n=6 per group). Briefly, mice were fasted for 6 hours and then given a single intraperitoneal injection of 15% glucose (1.5 g glucose/kg body weight). Blood samples (5 μl) for glucose measurements were taken at 0, 15, 30, 60, 90, and 120 minutes from a small tail snip, and analyzed using a glucose meter and strips (Reli OnAbbott, CA). The reported values represent averages of 3 measurements in each mouse.
Analytical Methods
Polymerase Chain Reaction (RT-PCR)
After weaning, mice were genotyped by using RT-PCR of DNA obtained from a tail snip. DNA was purified using DirectPCR Lysis Reagent (Viagen, CA) with proteinaseK solution (Sigma, MO) then mixed with iQSupermix (BioRad, CA) and Primers. Stat3 primers were ATTGGAACCTGGGACCAAGTGG and ACATGTACTTACAGGGTGTGTGC. Cre primers were CTGCCACGACCAAGGTGACAGC and CTTCTCTACACCTGCGGTGCT. Rosa primers were GCGAAGAGTTTGTCCTCAACC, GGAGCGGGAGAAATGGATATG, and AAAGTCGCTCTGAGTTGTTAT. Each sample was then PCR amplified using a Thermal Cycler (BioRad, CA), and separated on a 1.5% agarose gel.
Tissue collection and Immunohistochemistry
To confirm loss of Stat3 activity specifically in POMC neurons of Stat3flox/flox/POMC-Cre mice, we used immunohistochemistry to double-label POMC neurons and p-Stat3 in Rosa/POMC-Cre and Stat3flox/floxRosa/POMC-Cre mice. Weight-matched mice between 10-12 weeks of age (n=3 per group) were injected intraperitoneally with recombinant mouse leptin (R&D Systems; 5 mg/kg), and 45 minutes later the mice were anesthetized with isoflurane and perfused via a left ventricle puncture with 4% paraformaldehyde containing phosphatase inhibitor. Tissues were collected and brains were placed in 4% paraformaldehyde overnight and then infiltrated with 30% sucrose in PBS at 4°C. Frozen coronal sections (25-μm thick) were prepared, free-floating sections were washed with PBS, and then incubated for X-gal staining (β-Gal Staining Set, Roche, IN) for 3 hours. After X-gal staining, sections were washed and immunostained with pStat3 antibody (Cell Signaling Technology, MA). Upon completion of staining, sections were mounted on Superfrost/Plus slides (Fisher). Co-localization of pStat3 with POMC neurons were quantified in 100 randomly chosen lacZ expressing POMC neurons (perinuclear blue dot) at 10X magnification, and a blinded investigator then counted the number of positive POMC neurons expressing pStat3 (brown nuclear staining) cells.
Plasma Hormones and Glucose Measurements
Fasting plasma leptin and insulin concentrations were measured with ELISA (R&D Systems and Crystal Chem Inc, respectively), and plasma glucose concentrations were determined using the glucose oxidation method (Beckman Coulter, CA), except during the GTT where glucose was measured using a glucose meter and strips as previously described.
Statistical analyses
Data are expressed as means ± SEMs and analyzed by using 2-factor ANOVA with repeated measures. The Bonferroni post-hoc test was used for comparisons between groups. Mann-Whitney’s t-test was used to compare baseline data of the different groups of mice. Statistical significance was accepted at a level of P<0.05.
RESULTS
Confirmation of Stat3 inactivation in POMC neurons
At 3 weeks of age, mice were genotyped for Stat3flox and Cre-recombinase using DNA obtained from a tail snip. Figure 1A shows gels following RT-PCR amplification for Stat3flox and Cre-recombinase from 9 tail snip samples with analysis indicating presence or absence of Cre-recombinase and Stat3flox expression. To confirm inhibition of Stat3 phosphorylation in POMC we also performed double-labeling of LacZ reporter gene and p-Stat3 in Stat3flox/floxRosa/POMC-Cre and Rosa/POMC-Cre control mice. We observed a 74% reduction in p-Stat3 immunoreactivity in POMC neurons of Stat3flox/floxRosa/POMC-Cre compared to Rosa/POMC-Cre controls following an IP injection of leptin (Figure 1B, D-F). We found no significant differences in the number of POMC neurons in Stat3flox/floxRosa/POMC-Cre compared to Rosa/POMC-Cre controls (Figure 1C).
Figure 1.
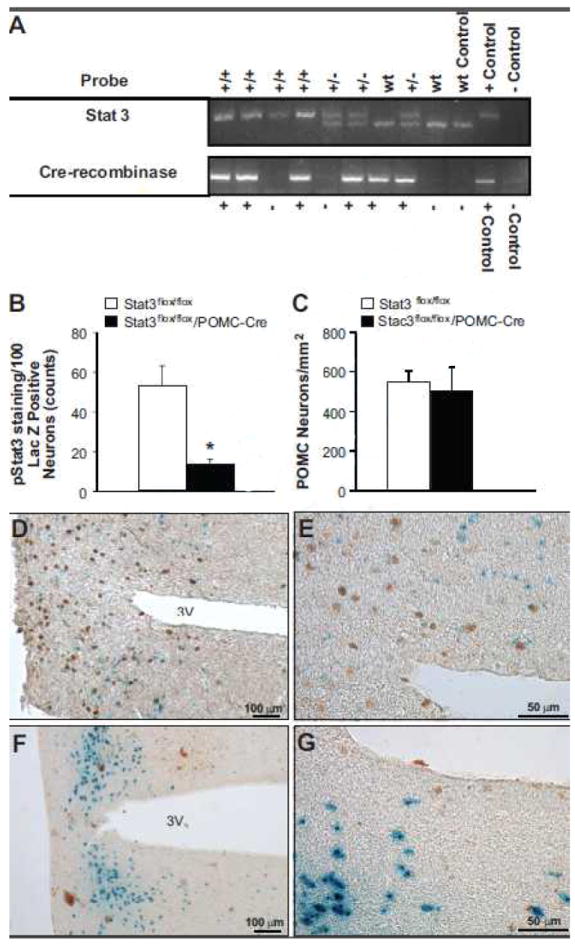
(A) PCR gels for Stat3flox and Cre-recombinase from 9 tail snip samples with analysis indicating presence or absence of Cre-recombinase and Stat3flox. Top line: +/+ denotes a homozygous result, +/- denotes a heterozygous result, and wt denotes wild-type result based on the three control bands for wt, +, and − negative primers (far left). Bottom line: + expresses Cre-recombinase and – does not express Cre-recombinase based on + and − control bands (far left). (B) Quantitative analysis of POMC neurons which are positive for pStat3 45 min after leptin administration (5 mg/kg, IP). *p<0.05, Stat3flox/flox/POMC-Cre mice vs. Stat3flox/flox control group. (C) Quantitative analysis of POMC neurons that stained for LacZ. (D and F) 10X and (E and G) 40X view of representative immunohistochemical staining of pStat3 (brown nuclear staining) and POMC neurons (perinuclear blue dot staining) in the arcuate nucleus of Rosa/POMC-Cre (D/E) and Stat3flox/floxRosa/POMC-Cre (F/G).
Effect of POMC neuron Stat3 inactivation on body weight, appetite and metabolic profile at 8-10 weeks of age
Body weight was significantly increased in male and female Stat3flox/flox/POMC-Cre mice, compared to controls, starting at 5 weeks of age and remained elevated throughout adulthood (Figures 2A and 2B, respectively).
Figure 2.

Stat3 inactivation in POMC neurons mildly increased body weight in (A) male and (B) female mice from 5 weeks of age till 18 weeks of age. *p<0.05, Stat3flox/flox/POMC-Cre mice vs. Stat3flox/flox sex-matched control mice.
Average daily food intake was also significantly increased in male and female Stat3flox/floxRosa/POMC-Cre mice compared to controls at 8-10 weeks of age (Figures 3A and 3B). However, the usual rise in food intake when mice were permitted to eat ad libitum following a 24-hour fast was attenuated in mice with Stat3 inactivation in POMC neurons (Figures 3A and 3B). In control mice, food intake increased significantly when they were permitted to eat ad libitum following a 24 hr fast, but in Stat3flox/flox/POMC-Cre mice, food intake remained similar to baseline values at 24 and 48 hours post-fasting.
Figure 3.
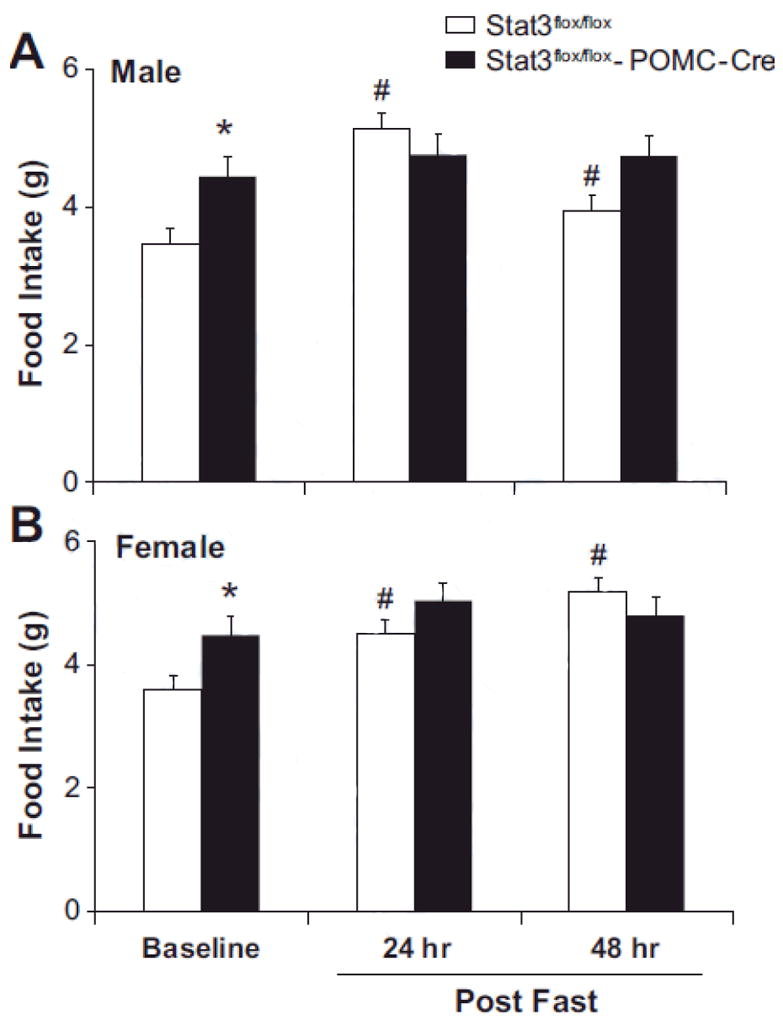
Inactivation of Stat3 in POMC neurons induces hyperphagia and inhibits refeeding after a 24-hour fast. Baseline control food intake and food intake at 24 and 48 hours following a 24 hour fast in 8-10 week old male (A) and female (B) mice n=6/group. * p<0.05, Stat3flox/flox/POMC-Cre vs. Stat3flox/flox sex-matched control mice. # p<0.05 refeeding during 24 or 48 hour post-fast vs. baseline control.
We also examined metabolic parameters in Stat3flox/flox/POMC-Cre mice and Stat3flox/flox control mice at 8-10 weeks of age. Body weight and fat mass were higher in male and female mice with Stat3 deletion in POMC neurons (Table 1). Leptin levels were significantly increased only in female Stat3flox/flox/POMC-Cre mice, and although leptin tended to be higher in male Stat3flox/flox/POMC-Cre compared to male control mice this difference was not statistically significant. We observed no differences in total body lean mass or water content, fasting insulin, or glucose levels in control mice and Stat3flox/flox/POMC-Cre mice at 8-10 weeks of age. RQ and motor activity were higher in Stat3flox/flox/POMC-Cre than in control mice (Table 1). Female Stat3flox/flox/POMC-Cre mice also exhibited higher heat production than female control Stat3flox/flox mice (Table 1).
Table 1.
Metabolic parameters in male and female Stat3flox/flox control and Stat3flox/flox/POMC-Cre mice at 8-10 weeks of age
| Parameter | Stat3flox/flox Control Male | Stat3flox/flox POMC-Cre Male | Stat3flox/flox Control Female | Stat3flox/flox POMC-Cre Female |
|---|---|---|---|---|
| Body Weight (g) | 23.4 ± 1.0 | 25.7 ± 0.6* | 17.4 ± 0.6 | 21.8 ± 0.8* |
| Fat Mass (g) | 1.4 ± 0.1 | 2.1 ± 0.2* | 1.5 ± 0.2 | 5.0 ± 0.6* |
| Lean Mass (g) | 20.7 ± 0.9 | 21.9 ± 0.6 | 14.7 ± 0.4 | 15.4 ± 0.7 |
| Water Content (g) | 16.6 ± 0.8 | 17.5 ± 0.5 | 12.0 ± 0.4 | 12.0 ± 0.5 |
| Leptin (ng/mL) | 13 ± 4 | 19 ± 4 | 10 ± 5 | 25 ± 2* |
| Insulin (μU/mL) | 20 ± 2 | 24 ± 7 | 19 ± 2 | 19 ± 3 |
| Glucose (mg/dL) | 171 ± 10 | 160 ± 13 | 161 ± 26 | 167 ± 19 |
| RQ (VCO2/VO2) | 0.85 ± 0.02 | 0.90 ± 0.01* | 0.79 ± 0.03 | 0.83 ± 0.02* |
| VO2 (ml/kg/min) | 64.8 ± 9.6 | 56.8 ± 6.4 | 79.6 ± 15.1 | 82.8 ± 6.7 |
| Motor Activity (m/d) | 86 ± 13 | 181 ± 33* | 132 ± 19 | 230 ± 48* |
| Heat Production (cal/hr) | 491 ± 58 | 525 ± 64 | 537 ± 44 | 625 ± 37* |
Body weight, RQ, VO2, motor activity, and heat production represent the average values for three consecutive days. Data are expressed as mean ± SEM. n=6 mice in each group.
p<0.05, Stat3flox/flox/POMC-Cre mice vs. sex-matched Stat3flox/flox control group.
Metabolic and cardiovascular profiles and responses to air jet stress of Stat3flox/flox/POMC-Cre and Stat3flox/flox mice at 20 weeks of age
At 20 weeks of age, body weight, food intake, and fat mass were still higher in male and female Stat3flox/flox/POMC-Cre mice compared to control mice (Tables 2 and 3, and Figures 4B and 4F). There were no significant differences in lean mass between groups at 20 weeks of age (Figures 4A and 4E). Fasting leptin levels tended to be higher in Stat3flox/flox/POMC-Cre compared to control mice although differences were statistically significant only in female mice (Tables 2 and 3). Fasting plasma insulin and glucose were not significantly different in control and Stat3flox/flox/POMC-Cre mice, although female mice had lower fasting insulin and glucose levels compared to male mice (Tables 2 and 3). We observed no differences in the area under the blood glucose curve during glucose tolerance testing in male or female Stat3flox/flox/POMC-Cre compared to control Stat3flox/flox mice (Figures 4D and 4H).
Table 2.
Effect of leptin infusion (4 μg/kg/min, IP) for 7 days in male Stat3flox/flox control and Stat3flox/flox/POMC-Cre mice at 20 weeks of age
| Parameter | Stat3flox/flox Male | Stat3flox/flox POMC-Cre Male | Leptin in Stat3flox/flox Male | Leptin in Stat3flox/flox POMC-Cre Male |
|---|---|---|---|---|
| Body Weight (g) | 31.7 ± 0.9 | 36.5 ± 2.0* | 27.6 ± 0.9# | 33.5 ± 2.0# |
| Food Intake (g) | 3.4 ± 0.2 | 4.4 ± 0.5* | 2.4 ± 0.3# | 3.2 ± 0.3# |
| Leptin (ng/mL) | 16 ± 5 | 23 ± 4 | 51 ± 11# | 64 ± 7 |
| Insulin (μU/mL) | 29 ± 5 | 21 ± 5 | 16 ± 4# | 18 ± 3 |
| Glucose (mg/dL) | 215 ± 28 | 242 ± 12 | 150 ± 12# | 185 ± 15# |
| RQ (VCO2/VO2) | 0.78 ± 0.03 | 0.87 ± 0.03* | 0.77 ± 0.01 | 0.89 ± 0.03 |
| VO2 (ml/kg/min) | 65.9 ± 6.8 | 56.8 ± 3.8 | 63.7 ± 5.8 | 54.9 ± 2.8 |
| Motor Activity (m/d) | 87 ± 19 | 44 ± 9* | 74 ± 9 | 37 ± 9 |
| Heat Production (cal/hr) | 607 ± 44 | 586 ± 33 | 532 ± 40 | 532 ± 14 |
| MAP (mmHg) | 105 ± 11 | 108 ± 5 | 115 ± 5# | 109 ± 5 |
| HR (bpm) | 555 ± 19 | 533 ± 22 | 570 ± 17 | 551 ± 25 |
RQ, VO2, Motor Activity, Heat Production, MAP, and HR represent the average values of 3 consecutive days at the end of the control (open) and leptin infusion periods (shaded). Data are expressed as mean ± SEM.
p<0.05, Stat3flox/flox/POMC-Cre mice vs. sex-matched Stat3flox/flox control mice.
p<0.05, leptin infusion vs. control period in sex-matched mice. RQ indicates respiratory quotient; VO2 indicates oxygen consumption.
Table 3.
Effect of leptin infusion (4 μg/kg/min, IP) for 7 days in female Stat3flox/flox control and Stat3flox/flox/POMC-Cre mice at 20 weeks of age
| Parameter | Stat3flox/flox Female | Stat3flox/flox POMC-Cre Female | Leptin in Stat3flox/flox Female | Leptin in Stat3flox/flox POMC-Cre Female |
|---|---|---|---|---|
| Body Weight (g) | 27.4 ± 1.1 | 32.0 ± 0.8* | 23.0 ± 0.7# | 29.3 ± 0.8# |
| Food Intake (g) | 3.4 ± 0.3 | 4.4 ± 0.3* | 2.7 ± 0.3# | 3.0 ± 0.3# |
| Leptin (ng/mL) | 6 ± 3 | 15 ± 4 | 53 ± 16# | 64 ± 9# |
| Insulin (μU/mL) | 12 ± 2 | 15 ± 2 | 14 ± 9 | 20 ± 5 |
| Glucose (mg/dL) | 199 ± 23 | 175 ± 19 | 141 ± 12# | 169 ± 10 |
| RQ (VCO2/VO2) | 0.83 ± 0.05 | 0.82 ± 0.02 | 0.79 ± 0.03# | 0.76 ± 0.03# |
| VO2 (ml/kg/min) | 73.9 ± 4.7 | 69.7 ± 3.9 | 76.0 ± 6.8 | 65.0 ± 3.6 |
| Motor Activity (m/d) | 82 ± 16 | 130 ± 25* | 44 ± 11# | 136 ± 30 |
| Heat Production (cal/hr) | 558 ± 43 | 623 ± 36 | 500 ± 46 | 581 ± 32 |
| MAP (mmHg) | 111 ± 5 | 114 ± 3 | 121 ± 9# | 115 ± 4 |
| HR (bpm) | 560 ± 15 | 603 ± 11* | 578 ± 20 | 608 ± 6 |
RQ, VO2, Motor Activity, Heat Production, MAP, and HR represent the average values of 3 consecutive days at the end of the control (open) and leptin infusion periods (shaded). Data are expressed as mean ± SEM.
p<0.05, Stat3flox/flox/POMC-Cre mice vs. sex-matched Stat3flox/flox control mice.
p<0.05, leptin infusion vs. control period in sex-matched mice. RQ indicates respiratory quotient; VO2 indicates oxygen consumption.
Figure 4.

Stat3 inactivation in POMC neurons increases fat mass without altering the tolerance to an acute glucose load. Lean mass and fat mass in male (A and B) and female (E and F) Stat3flox/flox (open bars) and Stat3flox/flox-POMC-Cre (black bars) mice at 20 weeks of age (n=6/group). Glucose tolerance test (C and G) in Stat3flox/flox (open squares) and Stat3flox/flox-POMC/Cre (black squares) mice, and the area under the curve of the glucose tolerance test (AUC) (D and H). * p<0.05, Stat3flox/flox/POMC-Cre vs. Stat3flox/flox sex-matched control mice.
RQ was significantly elevated in male but not female Stat3flox/flox/POMC-Cre mice compared to control mice (Table 2 and 3). We also observed sex differences at 20 weeks of age in motor activity which was reduced in male Stat3flox/flox/POMC-Cre mice compared to controls whereas female Stat3flox/flox/POMC-Cre mice showed increased motor activity compared to controls (Tables 2 and 3). No differences were observed in oxygen consumption or heat production in control and Stat3flox/flox/POMC-Cre mice (Tables 2 and 3).
Baseline MAP and HR were not significantly different among groups except in female Stat3flox/flox/POMC-Cre mice which exhibited slightly increased HR compared to control female mice (Tables 2 and 3).
In male mice with Stat3 deletion in POMC neurons, increases in MAP during acute air-jet stress were attenuated by approximately 50% compared to control male Stat3flox/flox mice (Figure 5A). No differences were observed in the MAP responses to air-jet stress in Stat3flox/flox and Stat3flox/flox/POMC-Cre female mice. However, the MAP responses to air-jet stress test were reduced in female compared to male control mice. No differences in the HR responses to acute air-jet stress were observed in either male or female Stat3flox/flox and Stat3flox/flox/POMC-Cre mice (data not shown).
Figure 5.
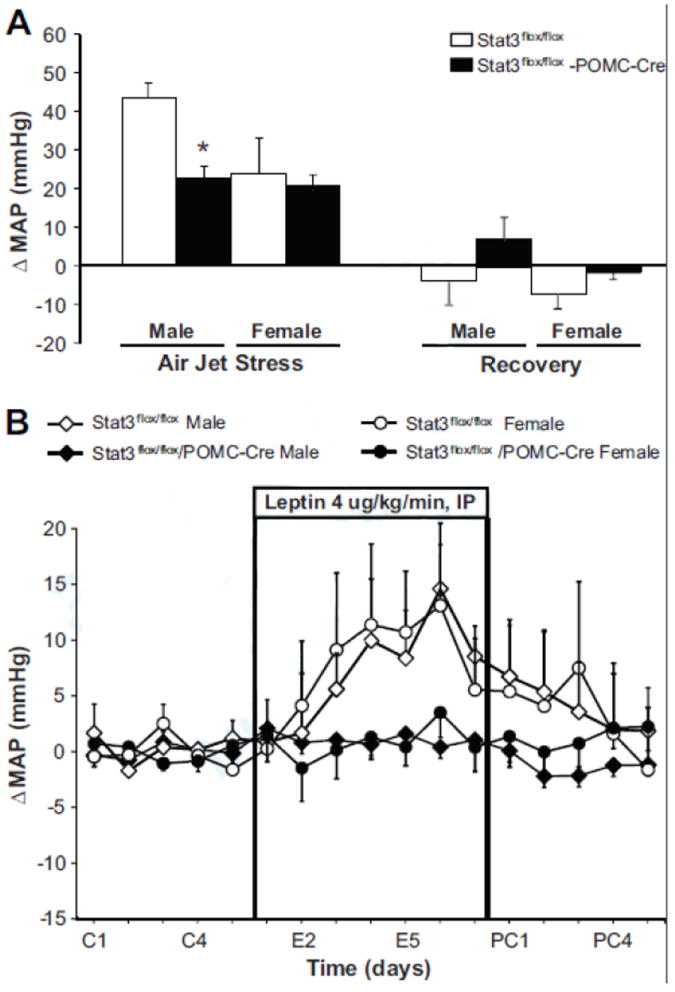
Inactivation of Stat3 in POMC neurons alters blood pressure responses to stress and leptin. (A) Acute mean arterial pressure (MAP) responses to air-jet stress. Each value represents the average blood pressure for 5 minutes (n=6 animals/group). (B) Change in mean blood pressure (MAP) during chronic leptin infusion (4 μg/kg/min, IP) for 7 days (n=6/group). * p<0.05, Stat3flox/flox/POMC-Cre mice vs. Stat3flox/flox sex-matched control mice.
Metabolic and cardiovascular responses to chronic leptin infusion in Stat3flox/flox/POMC-Cre and Stat3flox/flox mice
Leptin infusion for 7 days increased plasma leptin by a similar amount (35-41 ng/ml) in all groups; after 7 days of leptin infusion plasma leptin concentration averaged 51-64 ng/ml (Tables 2 and 3). Leptin infusion reduced food intake by ~30% and decreased body weight in control mice as well as in Stat3flox/flox/POMC-Cre mice (Tables 2 and 3). Food intake during the recovery period was not significantly different than food intake measured during baseline prior to leptin administration (data not shown).
Leptin treatment did not alter RQ, oxygen consumption, or heat production in male or female mice from both groups (Tables 2 and 3). Although leptin treatment reduced motor activity in female control mice, motor activity did not change significantly in any of the other groups during leptin infusion.
Leptin treatment lowered fasting plasma insulin and glucose concentrations in control male Stat3flox/flox mice as well as in male Stat3flox/flox/POMC-Cre mice (Table 2). Leptin administration reduced blood glucose in control female mice but not in Stat3flox/flox/POMC-Cre female mice (Table 3). In addition, leptin infusion did not significantly alter plasma insulin levels in either group of female mice (Table 3).
Leptin infusion for 7 days caused a gradual rise in blood pressure in male and female Stat3flox/flox control mice (Figure 5B); during the last 3 days of leptin infusion the average increase in MAP was 10 mmHg in both male and female control mice (Tables 2 and 3). In contrast, there were no significant increases in MAP in male or female Stat3flox/flox/POMC-Cre mice during leptin administration (Figure 5B). Although HR tended to increase during leptin infusion the changes were not statistically significant (Tables 2 and 3).
DISCUSSION
The most important findings of this study are that inactivation of Stat3 in POMC neurons abolished the rise in blood pressure during chronic leptin treatment and attenuated the pressor response to acute air-jet stress in male mice. We also found that deletion of Stat3 signaling in POMC neurons caused only a modest increase in body weight and did not significantly alter leptin’s chronic anorexic actions.
Role of POMC neuron Stat3 in mediating chronic blood pressure effects of leptin
We previously found that intact leptin receptors on POMC neurons as well as intact MC4R are necessary for leptin to raise blood pressure.4,6 These and other studies indicate that the CNS POMC-MC4R system mediates the increases in renal sympathetic nerve activity and the chronic hypertensive actions of leptin.7,8 Yet, leptin is known to elicit multiple post-receptor signaling events in POMC neurons that could contribute to increases in sympathetic activity and blood pressure.1 Although Stat3 activation clearly contributes to the anorexic effects of leptin9,10,11, the role of Stat3 signaling and the neuronal sites involved in mediating the chronic blood pressure effects of leptin have not, to our knowledge, been previously determined. Our current results indicate a key role for POMC neuron Stat3 signaling in contributing to the chronic hypertensive effects of leptin.
These findings are consistent with the observation that leptin’s blood pressure effects are slow to develop and appear to increase over several days.12 It is possible that the transcriptional activity of Stat3 to increase production of α-melanocyte stimulating hormone, a key POMC neurotransmitter that activates MC4R in downstream neurons, is vital for leptin to promote a long-term rise in sympathetic activity and blood pressure. However, further studies are needed to test this hypothesis.
Stat3 deletion in POMC neurons of male mice also attenuated the blood pressure responses to an acute pressor stimulus (i.e. air-jet stress) in male mice. This finding is consistent with our previous observation that male mice with leptin receptors deleted in POMC neurons also exhibit an attenuated pressor response to acute stress.4 Female mice, however, had an attenuated blood pressure response to acute air-jet stress compared to male mice and this was not affected by Stat3 deletion in POMC neurons. Further experiments are needed to unravel the mechanisms responsible for these sex differences in the blood pressure responses to acute stress and their pathophysiological significance.
Previous studies have suggested that other factors besides leptin, such as angiotensin II (Ang II) and interleukin-6 (IL-6), may also cause hypertension via phosphorylation of Jak2 and Stat3.13,14 The results of our current study indicate that Stat3 deletion in POMC neurons did not alter baseline blood pressure. However, the importance of this pathway in mediating the hypertensive effects of factors other than leptin, such as high levels of Ang II or IL-6 has not, to our knowledge, been previously reported.
Although our results suggest that Stat3 activation in POMC neurons is important in mediating the chronic hypertensive effects of leptin, they do not rule out the possibility that leptin may influence blood pressure via other signaling pathways in other neuronal populations. For instance, leptin may have effects on other neuronal populations that tend to lower blood pressure. Further studies are needed to unravel the complex actions of leptin on sympathetic activity and blood pressure regulation.
Role of POMC neuron Stat3 in regulating body weight and glucose homeostasis
Deletion of Stat3 in the entire brain of mice has been shown to cause marked hyperphagia and severe obesity with body weight averaging twice as much as in control littermates and body fat content increasing by 5-fold.3 In the present study, however, Stat3flox/flox/POMC-Cre male and female mice were only moderately overweight at 8 and 20 weeks of age, compared to sex-matched Stat3flox/flox control mice. This increase in body weight observed with Stat3 deletion specifically in POMC neurons was associated with mild hyperphagia without major alterations in oxygen consumption or heat production. Thus, our findings suggest that Stat3 signaling in POMC neurons contributes to regulation of body weight but may be considerably less important than Stat3 signaling in other neuronal populations for control of energy balance.
Our findings are consistent with previous studies suggesting that Stat3 signaling in POMC neurons plays a role in body weight regulation, albeit modest. Ernst et al 15, surprisingly, and in apparent contrast to other studies showing that Stat3 deficiency causes obesity, reported that constitutive nuclear overexpression of Stat3 signaling in POMC neurons caused mild (10% or less) increases in body weight and decreased POMC expression, even though Stat3 serves as a transcriptional activator of POMC expression. Although the mechanisms for these unexpected findings are unclear, they may be related to impaired POMC neuron function or to the effect of excess Stat3 signaling to increase expression of SOCS3 (suppressor of cytokine signaling 3) which is a negative regulator of leptin signaling.15 Gamber et al 16 reported that overexpression of leptin receptors in POMC neurons caused leptin resistance and exacerbated the obesity observed in mice fed a high fat diet, but not in mice fed a normal diet. These studies suggest that overstimulation of the leptin receptor-Stat3 signaling pathway in POMC neurons can, paradoxically, cause mild obesity similar to the effects of deletion of Stat3 signaling in POMC neurons. In both cases, however, the impact of Stat3 signaling on body weight regulation appears to be modest compared to the effects of Stat3 in other neuronal populations.
Despite having a modest effect on body weight, Stat3 deletion in POMC neurons substantially increased fat mass in male and female mice. Although the mechanisms responsible for the accumulation of adipose tissue are unclear and were not the focus of the present study, we observed hyperphagia and higher RQ in Stat3flox/flox/POMC-Cre compared to control mice, suggesting increased carbohydrate utilization for energy substrate while favoring fat storage.
Deletion of Stat3 in POMC neurons caused no major changes in fasting plasma glucose or insulin compared to control mice. This contrasts with the effects of disrupting Stat3 signaling in the entire brain which elicits marked elevations of fasting plasma glucose and insulin associated with severe obesity.3 We also found that Stat3flox/flox/POMC-Cre mice had nearly normal responses to glucose tolerance tests. Thus, our findings suggest that Stat3 signaling in other neuronal populations besides POMC neurons may be more critical for CNS regulation of glucose homeostasis although the specific neurons involved are still unclear and remain an important area for further investigation.
Role of POMC neuron Stat3 in mediating chronic metabolic effects of leptin
The specific neuronal populations involved in mediating the anorexic effects of leptin on appetite and body weight regulation via Stat3 signaling have not been fully elucidated. In the present study, we found that Stat3 deletion in POMC neurons had no major effect on leptin’s chronic anorexic effects. This finding is consistent with our previous report that deletion of leptin receptors in POMC neurons did not significantly attenuate the acute or chronic effects of leptin to reduce food intake.4 These observations suggest that other neurons besides those expressing POMC mediate a major share of leptin’s effects to suppress appetite.
In addition to regulating appetite, leptin’s CNS actions also play a major role in glucose homeostasis. For example, we and others have shown that the leptin’s CNS actions can completely normalize plasma glucose levels in streptozotocin-induced type 1 diabetes mellitus.17,18 Moreover, this powerful antidiabetic effect of leptin is abolished by blockade of CNS melanocortin 4 receptors (MC4R).18 The effects of leptin to reduce plasma glucose and insulin levels are also abolished in mice with leptin receptors deleted specifically in POMC neurons.4 These observations indicate that leptin’s CNS-mediated antidiabetic effects are due primarily to activation of leptin receptors in POMC neurons and subsequent stimulation of MC4R. Whether leptin mediates this antidiabetic effect by activating Stat3 or one of its other main signaling pathways, insulin receptor substrate 2 (Irs2) or the tyrosine phosphatase Shp2, in POMC neurons has not been previously determined.
Results from the present study suggest that LepR-mediated activation of Stat3 in POMC neurons is unlikely to explain a major share of the leptin’s CNS-mediated effects on glucose homeostasis, and are consistent with the possibility that other signaling pathways (i.e. Shp2 or Irs2) in POMC neurons may contribute importantly to leptin’s CNS-mediated antidiabetic effects. However, the contribution of these signaling pathways in POMC neurons to leptin’s effects on glucose homeostasis is still unclear and is an important area for further investigation.
Sex differences in Metabolic Effects of Stat3 Deletion in POMC Neurons
Another finding of our study is that there were sex differences in some of the metabolic effects of POMC neuron Stat3 deletion. For example, female mice with POMC neuron Stat3 deletion had earlier increases in fat mass compared with male mice with POMC Stat3 deletion at 10 weeks of age. Also, there were sex differences in some of the metabolic responses to chronic leptin infusion. In male mice with POMC Stat3 deletion, leptin infusion caused significant reductions in plasma insulin and glucose levels; however, in female mice at 20 weeks of age baseline insulin and glucose levels were lower than in males and chronic leptin infusion failed to significantly lower plasma insulin concentration in either Stat3flox/flox or Stat3flox/flox/POMC-Cre mice. Another sex difference is that 20-week old female mice with POMC Stat3 deletion had substantially higher motor activity than male mice with POMC Stat3 deletion. Quantitative differences in Stat3 expression and/or deletion in males and females might explain some of the sex differences observed, but are unlikely to account for qualitative differences, for example, in motor activity, during leptin infusion. Also, control female mice had a markedly attenuated pressor response to air jet stress, compared to male mice and this was not altered by Stat3 deletion in POMC neurons.
Although our studies were not designed to investigate the mechanisms responsible for sex differences in Stat3 signaling and POMC neuronal control of metabolism, they emphasize the need for further investigation. Accounting for sex differences in the design of experimental studies and interpretation of results is increasingly recognized as an important step in developing translational approaches to prevention and treatment of human diseases. 19
Perspectives
Previous studies indicate that increased leptin levels may contribute to sympathetic activation and hypertension in obesity even though obese subjects appear to be resistant to some of the metabolic effects of leptin, including appetite suppression.7 Our current results indicate that intact Stat3 signaling in POMC neurons is essential for leptin’s chronic hypertensive effects but not for its effects on appetite and body weight regulation. These findings, however, do not imply that Stat3 signaling is unimportant in regulating appetite and body weight. In fact, total brain deficiency of Stat3 causes extreme obesity, comparable to that found with leptin deficiency.3 However, POMC neuronal Stat3 signaling apparently plays only a modest role in body weight regulation and in mediating leptin’s effects on appetite, energy expenditure, body weight, and glucose regulation. These metabolic effects of leptin appear to be mediated, at least in part, either by Stat3 in other neuronal populations besides those expressing POMC or by another signaling pathway. This differential regulation of blood pressure and various metabolic functions by POMC Stat3 signaling may help explain how leptin is capable of regulating sympathetic activity and blood pressure independently from appetite and other metabolic functions in obesity.
NOVELTY AND SIGNIFICANCE.
-
What Is New?
Stat3 activation in POMC neurons is essential for leptin’s ability to chronically raise blood pressure.
Stat3 activation in POMC neurons modulates the pressor response to acute stress.
Stat3 activation in POMC neurons does not play a key role in mediating leptin’s effects on appetite and body weight.
We observed sex differences in the effects of deletion of Stat3 signaling in POMC neurons on glucose regulation and motor activity
-
What Is Relevant?
Leptin appears to be a critical link between obesity, sympathetic activation and hypertension, and this study suggest that activation of Stat3 signaling pathway in POMC neurons is required for the effects of leptin on blood pressure regulation.
Stat3 activation in POMC neurons may contribute to the differential regulation of appetite and blood pressure by leptin which may help explain the development of selective leptin resistance in obesity-induced hypertension.
Summary
Stat3 signaling in POMC neurons plays a key role in the chronic effects of leptin to raise arterial pressure as well as in modulating the blood pressure response to acute stress, but may not be essential for mediating leptin’s chronic effects on appetite, body weight regulation or glucose homeostasis.
Acknowledgments
We thank Haiyan Zhang, Stephanie Peters, Calvin Torrey, Benjamin Pace, John Rushing, Sabira Ebaady, and Price Sessums for technical assistance.
SOURCES OF FUNDING
This research was supported by the National Heart, Lung, and Blood Institute grant PO1HL-51971.
Footnotes
Conflict of interest: none
DISCLOSURES
None.
References
- 1.Münzberg H, Myers MG. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 2.Yang R, Barouch LA. Leptin Signaling and Obesity: Cardiovascular Consequences. Circulation Research. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 3.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility and thermal dysregulation. PNAS. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 201;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 6.Tallam LS, da Silva AA, Hall JE. The melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 7.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec D. Obesity-induced hypertension: role of sympathetic nervous system, leptin and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 10.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Piper ML, Unger EK, Myers MG, Jr, Xu AW. Specific physiological roles of signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol. 2008;22:751–759. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 13.Banes-Berceli AK, Al-Azawi H, Proctor D, Qu H, Femminineo D, Hill-Pyror C, Webb RC, Brands MW. Angiotensin II utilizes Janus kinase 2 in hypertension, but not in the physiological control of blood pressure, during low-salt intake. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1169–R1176. doi: 10.1152/ajpregu.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–884. doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Münzberg H, Hampel B, Alber J, Kloppenburg P, Brüning JC, Wunderlich FT. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamber KM, Huo L, Ha S, Hairston JE, Greeley S, Bjørbæk C. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS One. 2012 doi: 10.1371/journal.pone.0030485. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. Am J Physiology Regul Integr Comp Physiol. 2006;291:1275–1282. doi: 10.1152/ajpregu.00187.2006. [DOI] [PubMed] [Google Scholar]
- 18.da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE. A functional melanocortin system is required for CNS mediated chronic antidiabetic and cardiovascular actions of leptin. Diabetes. 2009;58:1749–1756. doi: 10.2337/db08-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller VM. In pursuit of scientific excellence: sex matters. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1023–R1024. doi: 10.1152/ajpregu.00105.2012. [DOI] [PubMed] [Google Scholar]


