Abstract
Increasing evidence demonstrates the transmissibility of fibrillar species of tau protein, but this has never been directly tested in neurons, the cell type most affected by formation of tau inclusions in neurodegenerative tauopathies. Here we show that synthetic tau fibrils made from recombinant protein not only time-dependently recruit normal tau into neurofibrillary tangle-like insoluble aggregates in primary hippocampal neurons over-expressing human tau, but also induce neuritic tau pathology in non-transgenic neurons. This study provides highly compelling support for the protein-only hypothesis of pathological tau transmission in primary neurons and describes a useful neuronal model for studying the pathogenesis of tauopathies.
Keywords: Tau, Protein Aggregation, Tauopathy, Transmission
Introduction
Tau, a microtubule-binding protein enriched in axons, accumulates into intracellular hyperphosphorylated aggregates known as neurofibrillary tangles (NFT) in a wide variety of neurodegenerative diseases, including Alzheimer’s disease, frontotemporal dementia with Parkinsonism linked to chromosome 17, progressive supranuclear palsy, corticobasal degeneration and Pick’s disease, which are collectively termed neurodegenerative tauopathies [1]. It has always been enigmatic what initiates the conversion of tau from a highly soluble protein without defined secondary structure into insoluble beta-sheet rich fibrils. Recently, numerous studies have shown that exogenously supplied pathological tau, including synthetic tau fibrils, can drive soluble tau into tangle-like inclusions in both cell culture systems and mouse models [2–5], implicating a seeding-recruitment process as well as cell-to-cell transmission of pathology as possible underlying mechanisms for the initiation and progression of tauopathies. Similar results have been demonstrated for other amyloidogenic proteins, such as β-amyloid and α-synuclein (α-syn) [6–9], therefore suggesting a unifying pathogenic mechanism for neurodegenerative diseases involving protein aggregation.
We previously found that synthetic tau fibrils can spontaneously enter non-neuronal cells and recruit normal tau into pathological accumulations [4], but it was unclear whether this phenomenon could also happen in post-mitotic neurons, which are the more relevant cell type for studying tauopathies. While a recent study from our lab showed that intracerebral inoculation of preformed tau fibrils (tau pffs) into transgenic (Tg) mice overexpressing human mutant P301S tau (PS19) dramatically accelerated development of tau pathology [10] thereby providing in vivo evidence for transmissibility of synthetic tau fibrils, a study in cultured neurons would provide an in vitro system more amenable for mechanistic interrogations and investigation of novel therapies. Here, we demonstrate that synthetic tau fibrils made from recombinant protein triggered robust aggregation of otherwise soluble endogenous tau into NFT-like inclusions in primary neurons dissociated from embryonic mouse hippocampus.
Materials and Methods
Recombinant Tau Purification and in Vitro Fibrillization Assays
The cDNAs coding for (1) the longest isoform of wildtype (wt) human tau with a myc tag at the 3′ end (myc-T40), (2) truncated human tau containing four MT-binding repeats with a myc tag at the 5′ end (myc-K18), (3) 3′myc-T40 with P301S mutation (myc-T40/P301S), (4) 5′myc-K18 containing P301L mutation (myc-K18/P301L) were cloned into pRK172 bacterial expression vector. Each protein was expressed in BL21 (DE3) RIL cells and purified by cationic exchange using a Fast Protein Liquid Chromatography (FPLC) as previously described [11].
For in vitro fibrillization, 40 μM recombinant tau was incubated with 40 μM low-molecular-weight heparin and 2 mM DTT in 100 mM sodium acetate buffer (pH 7.0) at 37°C. Myc-T40 and myc-T40/P301S were agitated at 1000 rpm for 5 to 7 d and 1 d respectively, but myc-K18 and myc-K18/P301L were incubated without agitation for 2–3 d. Before they were used for transduction in neurons, fibrillization mixtures were centrifuged at 100,000 g for 30 min at 22°C, and the resulted pellet was re-suspended in equal volume of 100 mM sodium acetate buffer (pH 7.0) without heparin and DTT. Successful fibrillization was verified by sedimentation test, ThT test and negative stain EM.
Primary Neuron Cultures and Fibril Transduction
Primary neuron cultures were prepared from E15–E17 embryos from het/het or homo/het crosses of PS19 mice [12], non-Tg CD1 mice (Charles River, Wilmington, MA) and tau knock-out mice [13]. Dissociated hippocampal neurons were plated onto poly-D-lysine coated 13 mm coverslips or 6-well plates. Pff transduction was performed at 6 d in vitro, whereby tau pffs were diluted in PBS and sonicated with 60 pulses before being added to neuron medium. Neurons on each coverslip received 1.5 μg of tau pffs and those on the 6-well plate received 6 μg of pffs per well. Transduced neurons were harvested for immunocytochemistry or sequential extraction at 18 d post-pff transduction unless otherwise indicated.
Immunocytochemistry
Neurons were fixed in 4% paraformaldehyde (PFA) containing 2% sucrose for 15 min and permeabilized with 0.1% Triton-X100 for 15 min, or fixed with 4% PFA containing 2% sucrose and 1% Triton-X100 for 15 min to remove soluble proteins. After blocking with 3% BSA and 3% FBS for at least one hour at RT, neurons were incubated with specific primary antibodies (Supplementary Table 1) overnight at 4°C followed by staining with appropriate Alexa fluor 594 or 488-conjugated secondary antibodies (Invitrogen; Carlsbad, CA) for 2 hr at RT. DAPI was added to PBS wash after secondary antibody incubation to label cell nuclei. Quantification for area occupied by pff-induced pathology was performed on 20× images using Image J (National Institute of Health).
Sequential Extraction and Western Blot Analysis
Neurons were scraped into Triton lysis buffer (1% TritonX-100 in 50 mM Tris, 150 mM NaCl, pH 7.6) containing phosphatase and protease inhibitor cocktail, sonicated, and centrifuged at 100,000 g for 30 min at 4°C. Pellets were washed once in Triton lysis buffer, re-suspended into SDS lysis buffer (1% SDS in 50 mM Tris, 150 mM NaCl, pH7.6) at a volume that is ¼ of the Triton lysis buffer and centrifuged at 100,000 g for 30 min at 22°C. Supernatants from Triton and SDS extractions were resolved on SDS-PAGE, transferred to nitrocellulose membranes, and blocked in 5% milk or 7.5% BSA in TBS before immunoblotted with specific antibodies (Supplementary Table 1). 10 μg of proteins from Triton fractions and equal volume of corresponding SDS fractions were loaded per lane.
Electron Microscopy (EM)
Negative stain transmission EM (TEM) of tau pffs, TEM and immuno-EM of pff transduced primary hippocampal neurons were performed as previously described [4, 8]. Monoclonal antibody (mAb) MC-1 was used in the immuno-EM of neurons.
Results
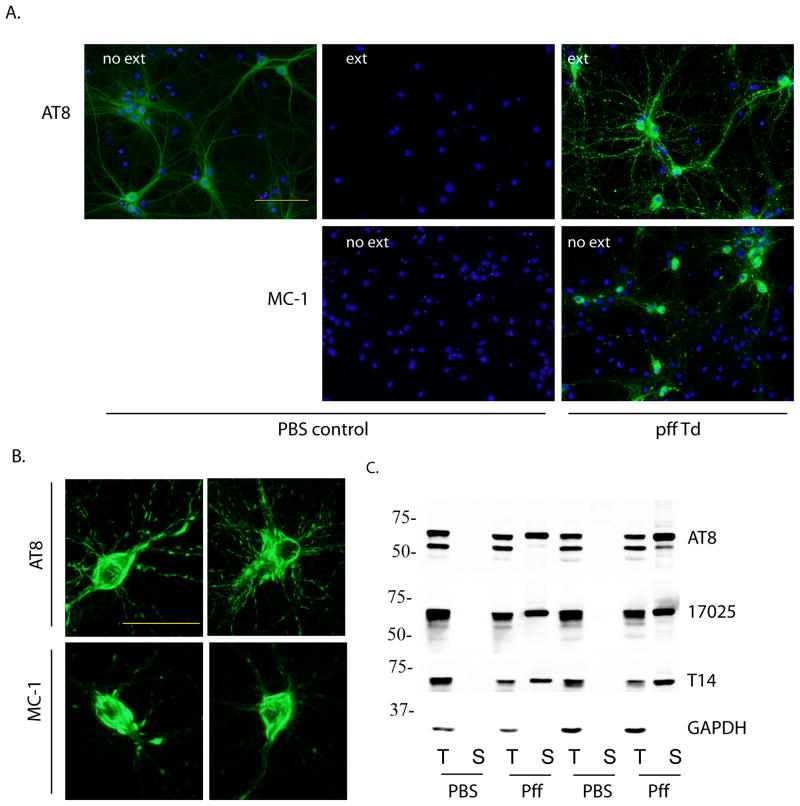
Despite the over-expression of human tau harboring P301S mutation, primary hippocampal neurons dissociated from PS19 mouse embryos showed phosphorylated tau recognized by AT8 that remained Triton X-100 soluble up to 24 d in vitro (Figure 1A). Remarkably, addition of tau pffs consisting of only the MT-binding domain with P301L mutation (myc-K18/P301L) to neuron medium at 6 d in vitro, without any transduction reagent, led to robust accumulation of phosphorylated tau throughout soma and neurites that resisted detergent extraction at 18 d post-transduction (24 d in vitro) (Figure 1A). In addition, perikaryal aggregates in pff-treated neurons were intensely labeled by disease-specific conformational antibody MC-1 [14], indicating pathological conformation acquired by pff-induced aggregates. Higher magnification images revealed AT8 and MC-1 labeled inclusions as bundles of aggregates highly reminiscent of NFTs filling up the soma of a subset of neurons (Figure 1B). Since both AT8 and MC-1 recognize epitopes absent from myc-K18/P301L pffs and pff-transduced tau knock-out neurons displayed no immunoreactivity for any tau antibodies tested (Supplementary Figure 1 and Figure 3C), the observed tau aggregates in PS19 neurons must represent fibrillization of endogenous full-length tau seeded by exogenous pffs that spontaneously entered neurons. Furthermore, K18/P301L pffs without a myc tag also induced widespread accumulation of pathological tau within the same time frame, ruling out non-specific effects associated with the tag (Supplementary Figure 2).
Figure 1. Insoluble tau accumulated in PS19 primary hippocampal neurons after incubation with myc-K18/P301L pffs.
(A) PS19 neurons treated with PBS (PBS control) or myc-K18/P301L fibrils (pff Td) were immunostained with phospho-tau mAb AT8 and conformational mAb MC-1 with or without 1% Triton-X100 extraction during fixing (ext, no ext, respectively). DAPI staining was used to visualize nuclei (blue). Scale bar: 100 μm. (B) Perikaryal tau aggregates recognized by AT8 and MC-1. Scale bar: 50 μm. (C) Neurons treated with PBS or myc-K18/P301L fibrils (pff) were sequentially extracted with 1% Triton-X100 lysis buffer (T) followed by 1% SDS lysis buffer (S) and immunoblotted with AT8, polyclonal antibody against total tau (17025), and mAb against human tau (T14). GAPDH served as loading control. Results from two independent sets of neurons were shown.
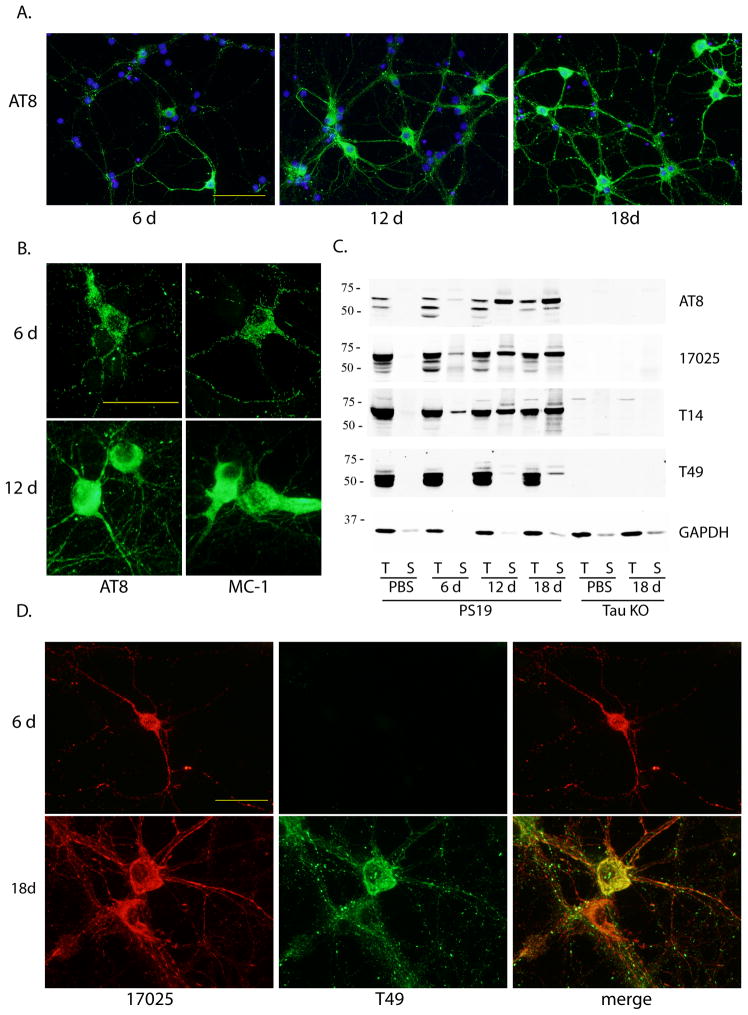
Figure 3. Time-dependent increase in pff-induced tau pathology and recruitment of mouse tau into aggregates.
(A) Increasing population of cells developed tau pathology from 6 d to 18 d incubation after pff addition. Green: AT8. Blue: DAPI. Scale bar: 100 μm. (B) Morphological difference between 6 d and 12 d post-transduction aggregates immunostained with AT8 and MC-1. Scale bar: 50 μm. (C) Sequentially extracted PS19 neuron lysates at different time points after pff addition (6 d, 12 d, 18 d) or at 18 d after PBS treatment (PBS) and tau knock-out neuron lysates (tau KO) at 18 d post-addition of PBS or pffs were immunoblotted with AT8, 17025, T14 as well as mAb specific for mouse tau (T49). (D) Double-labeling of aggregates by T49 and 17025 at different time points after pff addition. Scale bar: 50 μm. For (A), (B) and (D), soluble proteins were removed by 1% Triton-X100 during fixing.
Pff-induced solubility change of endogenous tau was further assessed by sequentially extracting pff-transduced neurons as well as PBS-treated control neurons into Triton X-100 lysis buffer followed by SDS lysis buffer and immunoblotting with different tau antibodies. Prominent Triton-soluble tau was present in PBS- and pff treated cultures but SDS-soluble tau was recovered only in pff-transduced neurons (Figure 1C).
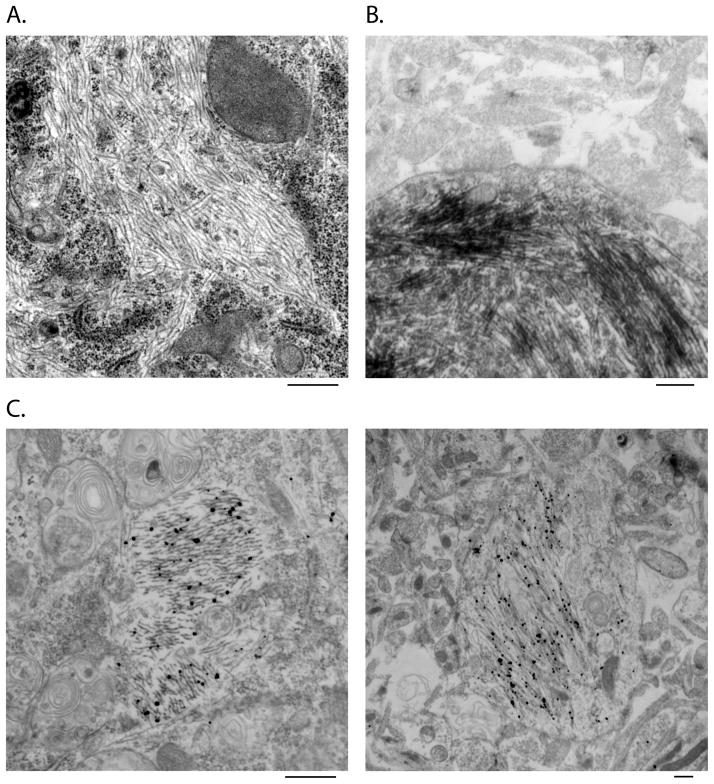
Ultrastructural analysis using routine transmission EM revealed cytoplasmic filaments in neurons treated with pffs (Figure 2A) but not in PBS-treated control neurons (data not shown). Immuno-EM with both HRP-labeling and nanogold-amplification demonstrated MC-1 immunoreactivity of pff-induced fibrillar accumulations, further supporting the recruitment of endogenous full-length tau into NFT-like filamentous aggregates (Figure 2B and C).
Figure 2. Ultrastructural analysis of tau pff-induced aggregates by EM and immune-EM.
Routine EM (A) revealed filamentous structures in the cytoplasm of tau pff transduced neurons, which were recognized by MC-1 in immuno-EM using both HRP-labeling (B) and nanogold-amplification (C) detecting methods. Scale bar: 500 nm.
A time-course analysis demonstrated that the prevalence of aggregate-bearing cells increased over time, from sparse distribution at 6 d post-pff addition to about 20% of neurons carrying perikaryal aggregates at 18 d post-transduction (Figure 3A). Moreover, diffuse tau accumulations seen as punctate AT8 and MC-1 immunoreactivity at 6 d turned into densely packed aggregates at 12 d (Figure 3B), while NFT-like aggregate bundles described earlier were only observed at 18 d post-transduction (Figure 1C). The progressive augmentation in insoluble tau aggregates was also evident on immunoblots of sequentially extracted neuron lysates at different time points with about 33% of total human tau sequestered into Triton-insoluble fraction at 18 d post-transduction (Figure 3C).
Interestingly, early inclusions did not contain endogenous mouse tau recognized by mAb T49, which appeared at 12 d post-pff transduction and became more abundant after 18 d incubation in aggregates co-labeled with polyclonal tau antibody 17025 that preferentially recognizes human tau on immunostaining (Figure 3D). Therefore, mouse tau can be recruited into pff-seeded aggregates although the process occurred more slowly compared to over-expressed human tau. Unlike immunostaining by human tau specific mAb T14 which completely overlapped with 17025 staining (Supplementary Figure 3), mouse tau immunoreactivity only partially colocalized with that of 17025 even at 18 d post-transduction (Figure 3D), suggesting not all inclusions contained misfolded mouse tau. The time-dependent emergence of insoluble mouse tau was also detected on immunoblot, although the insoluble pool only constituted a very small fraction of total mouse tau (Figure 3C).
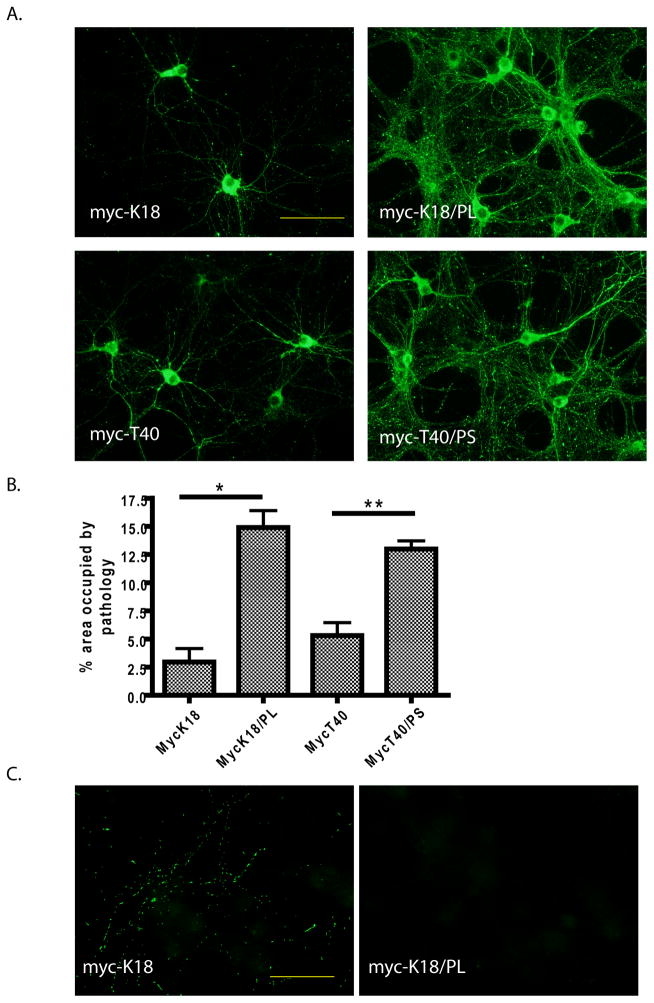
The differential seeding capacity of wt versus mutant and truncated versus full-length tau pffs was compared in PS19 neurons. Full-length tau pffs carrying P301S mutation (myc-T40/P301S pffs) were similarly efficient as myc-K18/P301L pffs (Figure 4A and B). In contrast, wt tau pffs, both full-length and truncated (myc-T40 and myc-K18), demonstrated significantly lower seeding efficiency with especially pronounced reduction in neuritic pathology than mutant pffs, although myc-T40 pffs appeared to be slightly more potent than myc-K18 pffs (Figure 4A and B).
Figure 4. Differential seeding capacity of distinct forms of tau pffs on PS19 and non-Tg primary neurons.
(A) Tau pathology (green: AT8) induced in PS19 neurons by myc-K18, myc-K18/P301L (myc-K18/PL), myc-T40 and myc-T40/P301S (myc-T40/PS) fibrils. Scale bar: 100 μm. (B) Quantification of percentage area occupied by AT8-positive pathology induced by the different pffs (mean + SEM). Number of independent sets of PS19 neurons tested: n = 2 for myc-K18, n = 3 for the other three. *: < 0.05. **: < 0.005. (C) Transduction of myc-K18 but not myc-K18/P301L pffs on non-Tg neurons resulted in neuritic tau pathology (green: AT8). Scale bar: 50 μm. For both (A) and (C), soluble proteins were removed by 1% Triton-X100 during fixing.
Myc-K18 and myc-K18/P301L pffs were also tested in non-Tg primary neurons for their capability to seed endogenous mouse tau fibrillization without over-expressed human mutant tau. Interestingly, wt myc-K18 pffs, but not mutant myc-K18/P301L pffs, were able to induce punctate accumulations of insoluble mouse tau in the neurites of non-Tg neurons after 18 d incubation (Figure 4C). Notably, the amount of tau inclusions induced in non-Tg neurons was highly limited as compared to that observed in PS19 neurons, most likely owing to lack of tau over-expression and mutation. In addition, unlike pff-induced tau pathology that were throughout the soma and processes of PS19 neurons, mouse tau aggregation was never found in the soma of pff-transduced non-Tg neurons, likely due to predominant expression of endogenous tau in the axons.
Discussion
The current study demonstrated induction of robust tau aggregation inside primary neurons by the simple addition of synthetic tau fibrils to neuronal culture medium without any transduction reagent, suggesting that the previously described phenomenon of spontaneous uptake of fibrillar tau and templated conversion of normal tau into pathological aggregates [2–4] is also applicable to neurons, the cell type most relevant for neurodegenerative tauopathies. Importantly, pff-induced tau aggregation also occurred in non-Tg neurons in the absence of over-expression, albeit to a much less extent than in PS19 neurons which over-express mutant tau, suggesting that the seeding-recruitment process can indeed take place at physiological conditions and underlie the etiology of NFT formation in tauopathies.
As a highly soluble protein, tau resists aggregation under normal conditions. It was therefore a breakthrough in the field when polyanionic cofactors, such as heparin, RNA, and fatty acids, were found to greatly promote in vitro fibrillization of tau [15–17]. Even though preformed tau fibrils can dramatically accelerate the kinetics of in vitro fibril assembly by acting as seeds, efficient seeding reaction still requires the presence of cofactors [10]. Curiously, tau pffs alone are sufficient to drive aggregation of soluble tau inside the cells without the addition of any cofactor, especially considering the fact that pffs we added to neurons were re-suspended in heparin-free buffer. Therefore, intracellular environment appears to be more permissive for seeded fibrillization than a cell-free system. Consequently, while polyanionic cofactors may catalyze the initial formation of misfoded tau seeds in human brains, their involvement in NFT genesis may not be obligatory, since seeded fibrillization of tau is a self-perpetuating process in cells once pathological conformers are generated.
Although mutant tau pffs resulted in dramatically more abundant pathology than wt tau pffs in PS19 neurons overexpressing mutant tau, they showed even lower seeding capacity than wt pffs in non-Tg neurons expressing wt mouse tau. The asymmetrical seeding between wt and P301L mutant tau was also observed in our previous non-neuronal model albeit less dramatically [4]. Together with the finding that NFTs in human patients harboring P301L mutation are nearly exclusively composed of mutant tau [1], these studies point to possible conformational disparities between wt and mutant tau fibrils. Since we have never detected obvious differences in the morphologies of the two types of fibrils at EM level, we speculate any structural differences are likely more subtle as suggested by a previous study [18].
Interestingly, full-length wt tau (myc-T40) pffs showed a tendency to seed better than truncated wt (myc-K18) pffs despite equal mass thus a lower molarity of the former being added to PS19 neurons. In our previous study when transduction reagent was used to facilitate pff uptake in non-neuronal cells [4], wt T40 pffs actually demonstrated less efficient seeding than wt K18 pffs (unpublished data), therefore the observed difference between full-length and truncated tau pffs in primary neurons is probably not due to differential recruiting capacity per se, but possibly due to more efficient uptake of full-length pffs. With the N-terminus of tau was shown to be critical for extracellular secretion [19], it would be interesting to explore whether the same domain is playing a role in the uptake of fibrillar tau into neurons.
Unlike the recently developed neuronal model of synucleinopathy in which robust Lewy body- and Lewy neurite-like accumulations composed of mouse α-syn can be induced in non-Tg neurons by α-syn pffs [8], low abundance of tau aggregates form upon wt tau pff transduction on non-Tg neurons. The difference could be attributed to several reasons. First, non-Tg mice naturally express α-syn of the A53T variant which is a pathogenic mutation in human. Not only does A53T mutation greatly enhance fibrillization of α-syn [20], but mouse α-syn even fibrillizes much more readily than human α-syn carrying the A53T mutation (unpublished data). Lack of such a “natural” mutation in mouse tau limits the extent of tau pathology that can develop within our experimental time window. Second, α-syn is highly concentrated at presynaptic terminals in mature neurons [21] and the local high concentration probably facilitates efficient recruitment by internalized pff seeds. In contrast, tau is more diffusely located throughout axons in non-Tg neurons. Third, the adult isoform of tau with four repeats (4R) only constitutes a small fraction of total tau in developing neurons [22, 23], while the predominantly expressed 3R-tau has lower propensity to aggregate than 4R-tau [24] in addition to a seeding barrier between 3R- and 4R-tau as previously reported [25]. Nonetheless, seeding endogenous mouse tau in non-Tg neurons provides a more physiological system for studying tau aggregation and pathology spreading.
In summary, our study directly demonstrates the transmissibility of fibrillar tau in neurons, thereby presenting a neuronal model suitable for both mechanistic studies on the pathogenesis of NFTs and therapeutic investigation to identify drugs that can reverse or prevent tau pathology.
Supplementary Material
Highlights.
Synthetic tau fibrils induce tau pathology in primary neurons.
Fibrils were directly added to neuron medium without any transduction reagent.
Endogenous mouse tau is recruited into insoluble aggregates.
Wt and mutant tau demonstrated preferential self-seeding over cross-seeding.
Acknowledgments
The authors would like to thank Anna Stieber for performing EM for this study, Ashley Chen and Joshua Daniels for mouse husbandry and technical support, Chi Li for assistance in molecular cloning, Andrea Asimoglou for helping with protein purification, Dr. John Trojanowski for critical reading of the manuscript, and all the other members of the Center for Neurodegenerative Disease Research for their help and support. MAb MC-1 is a generous gift from Dr. Peter Davies. We also thank Dr. Gerard Schellenberg for generously providing tau knock-out mice. This study was supported by NIH AG17586.
Abbreviations
- α-syn
alpha synuclein
- ICC
immunocytochemistry
- mAb
monoclonal antibody
- NFT
neurofibrillary tangle
- Tg
transgenic
- Pff
preformed fibril
- ThT
thioflavin T
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature reviews Neuroscience. 2007;8:663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 2.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. The Journal of biological chemistry. 2009;284:12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of {alpha}-synuclein and tau: cellular models of neurodegenerative diseases. The Journal of biological chemistry. 2010;285:34885–98. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo JL, Lee VM. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. The Journal of biological chemistry. 2011;286:15317–31. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nature cell biology. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–2. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stohr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, Dearmond SJ, Prusiner SB, Giles K. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11025–30. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. The Journal of experimental medicine. 2012;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM-Y. Synthetic Tau Fibrils Mediate Transmission of Neurofibrillary Tangles in a Transgenic Mouse Model of Alzheimer’s-like Tauopathy. Journal of Neuroscience. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Lee VM. Characterization of two VQIXXK motifs for tau fibrillization in vitro. Biochemistry. 2006;45:15692–701. doi: 10.1021/bi061422+. [DOI] [PubMed] [Google Scholar]
- 12.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. Journal of cell science. 2001;114:1179–87. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- 14.Jicha GA, Bowser R, Kazam IG, Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. Journal of neuroscience research. 1997;48:128–32. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–3. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 16.Kampers T, Friedhoff P, Biernat J, Mandelkow EM, Mandelkow E. RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS letters. 1996;399:344–9. doi: 10.1016/s0014-5793(96)01386-5. [DOI] [PubMed] [Google Scholar]
- 17.Chirita CN, Necula M, Kuret J. Anionic micelles and vesicles induce tau fibrillization in vitro. The Journal of biological chemistry. 2003;278:25644–50. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- 18.Aoyagi H, Hasegawa M, Tamaoka A. Fibrillogenic nuclei composed of P301L mutant tau induce elongation of P301L tau but not wild-type tau. The Journal of biological chemistry. 2007;282:20309–18. doi: 10.1074/jbc.M611876200. [DOI] [PubMed] [Google Scholar]
- 19.Kim W, Lee S, Jung C, Ahmed A, Lee G, Hall GF. Interneuronal transfer of human tau between Lamprey central neurons in situ. Journal of Alzheimer’s disease : JAD. 2010;19:647–64. doi: 10.3233/JAD-2010-1273. [DOI] [PubMed] [Google Scholar]
- 20.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–63. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 21.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3214–20. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dotti CG, Banker GA, Binder LI. The expression and distribution of the microtubule-associated proteins tau and microtubule-associated protein 2 in hippocampal neurons in the rat in situ and in cell culture. Neuroscience. 1987;23:121–30. doi: 10.1016/0306-4522(87)90276-4. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira A, Lu Q, Orecchio L, Kosik KS. Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar A beta. Molecular and cellular neurosciences. 1997;9:220–34. doi: 10.1006/mcne.1997.0615. [DOI] [PubMed] [Google Scholar]
- 24.Zhong Q, Congdon EE, Nagaraja HN, Kuret J. Tau isoform composition influences rate and extent of filament formation. The Journal of biological chemistry. 2012;287:20711–9. doi: 10.1074/jbc.M112.364067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norris EH, Giasson BI, Lee VM. Alpha-synuclein: normal function and role in neurodegenerative diseases. Current topics in developmental biology. 2004;60:17–54. doi: 10.1016/S0070-2153(04)60002-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.