Abstract
Microneedles provide a minimally invasive means to enhance skin permeability by creating micron-scale channels (micropores) that provide a drug delivery pathway. Adequate formation of the micropores is critical to the success of this unique drug delivery technique. The objective of these studies was to develop sensitive and reproducible impedance spectroscopy techniques to monitor micropore formation in animal models and human subjects. Hairless guinea pigs, a Yucatan miniature pig, and human volunteers were treated with 100 microneedle insertions per site following an overnight pre-hydration period. Repeated measurements were made pre- and post-microneedle treatment using dry and gel Ag/AgCl electrodes applied with light vs. direct pressure to hold the electrode to the skin surface. Impedance measurements dropped significantly post-microneedle application at all sites (p < 0.05, irrespective of electrode type or gel application), confirming micropore formation. In the Yucatan pig and human subjects, gel electrodes with direct pressure yielded the lowest variability (demonstrated by lower %RSD), whereas dry electrodes with direct pressure were superior in the guinea pigs. These studies confirm that impedance measurements are suitable for use in both clinical and animal research environments to monitor formation of new micropores that will allow for drug delivery through the impermeable skin layers.
Keywords: microneedle, impedance spectroscopy, micropore, transdermal, human
Introduction
Microneedle (MN) – assisted transdermal delivery represents a minimally invasive physical enhancement technique that selectively permeabilizes the stratum corneum (SC), the outermost skin layer (1, 2). This allows for various types of molecules to cross the otherwise impermeable outer layers of skin, including macromolecules, hormones, and hydrophilic compounds (3-6). MN delivery systems include hollow, coated, and dissolving MNs (1, 7). In one of the simplest MN techniques, a solid MN array is applied to the skin to create micropores in the SC; application of a drug solution or patch to the MN-treated area allows a drug to cross the skin into the systemic circulation (known as the “poke/press and patch” method).
The primary factors that influence drug delivery with the “poke/press and patch” technique are 1) adequate micropore formation, and 2) micropore lifetime in the SC. In order to expand the utilization of this novel drug delivery technique, it is important to develop appropriate in vivo models for evaluating micropore formation and lifetime following MN treatment in animals and human subjects. Two common methods to investigate skin barrier function include transepidermal water loss (TEWL) and impedance spectroscopy. The SC is the skin’s primary means of preventing water loss from the body, and also serves as the skin’s barrier to ion movement (i.e. flow of electrical current) (8-12). TEWL increases when the skin is damaged, while impedance decreases, rendering these as complementary techniques for assessing skin barrier integrity; both are reliable techniques for such evaluations (8, 10, 11).
Impedance measurements of the SC using various techniques have been described (8, 13) but limited publications depict this technique specifically for evaluating micropore formation following MN treatment in animals or humans (14, 15). The aim of this work was to develop sensitive and reproducible impedance techniques in two animal models commonly utilized in percutaneous drug studies (Yucatan miniature pig and hairless guinea pigs) and human subjects, to monitor micropore formation in the SC.
Materials and Methods
Microneedle arrays and application
Stainless steel arrays contained 50 MNs arranged in a 5 × 10 array configuration; each MN measured 800 μm in length and 200 μm in width at the base. Geometry and configuration of the MN arrays were designed by Mark Prausnitz’s lab, Georgia Institute of Technology. MN arrays were assembled into adhesive patches with ARcare® 7717 (Adhesives Research, Inc., Glen Rock, PA); the adhesive holds the MNs firmly against the skin by compensating for the mechanical mismatch between the flexible skin tissue and the rigid MN array. The MN patches were ethylene oxide sterilized before use.
MN insertion was achieved by placing the MN array on the skin and pressing gently for approximately 10 – 15 seconds followed by immediate removal. The array was rotated 45 degrees for a second insertion, to create 100 non-overlapping micropores. The same investigator performed all MN insertions, eliminating inter-investigator variability.
Impedance techniques
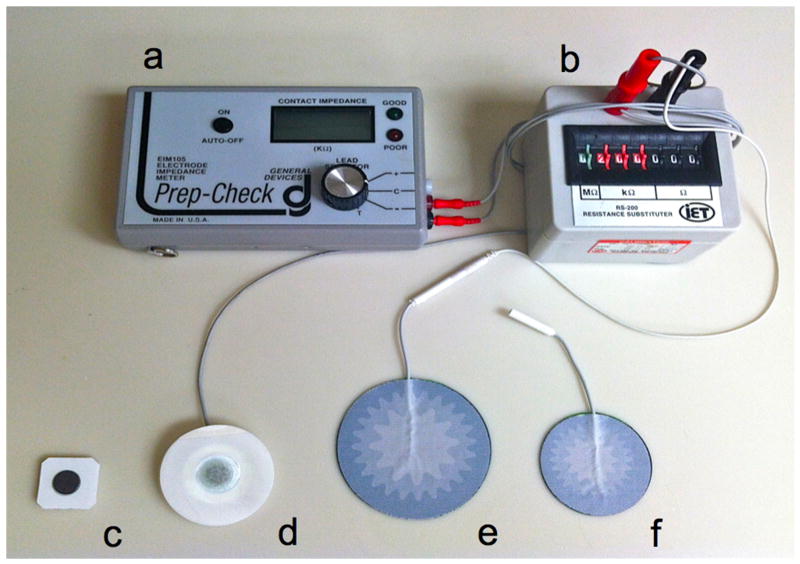
Two types of measurement Ag/AgCl electrodes were evaluated: one with a dry active electrode measurement surface (T-3403 electrodes, Thought Technology Ltd, Montreal, QC, Canada), while the other has foam, wet-gel active electrode measurement surface (Series 800 electrodes, S&W Healthcare Corporation, Brooksville, FL). A large electrode with a conductive gel surface served as the reference electrode (Superior Silver Electrode with PermaGel, Tyco Healthcare Uni-Patch, Wabasha, MN). The reference electrode was placed in the middle of the treatment area, equidistant from all treatment sites. Lead wires were connected to the measurement and reference electrodes, the opposite ends of the wires being connected to an impedance meter (Figure 1). This applies a low frequency alternating current modified with a 200 kΩ resistor in parallel.
Figure 1. Impedance setup used for all human and animal studies.
a: Prep-Check impedance meter; b: 200 kΩ resistor in parallel; c: dry Ag/AgCl measurement electrodes; d: gel Ag/AgCl measurement electrodes; e: Reference electrode for Yucatan miniature pig and human studies; f: reference electrode used for hairless guinea pig studies.
Animal study procedures
Studies were carried out in two animal models: Yucatan miniature pig (n = 1, weight of ~60 kg) and hairless guinea pigs (n = 3, mean (± SD) weight of 785 ± 51 g). All studies were approved by the University of Kentucky IACUC. Six sites (Yucatan pig) or four sites (guinea pigs) on the dorsal surface were treated with MNs. Half of the sites were evaluated with Ag/AgCl dry electrodes; the remaining sites were evaluated with gel Ag/AgCl electrodes. Repeated baseline (pre-MN) measurements were made at each site: 3 measurements using very light pressure to hold the electrode to the skin, and 3 measurements using more direct pressure, applied by the thumb of the investigator (similar pressure to that required to ring a doorbell). The same procedure was followed immediately post-MN treatment.
Impedance measurements were made on normal (non-hydrated) vs. hydrated skin. For the hydrated condition, treatment sites were covered overnight with blank occlusive patches secured to the skin with Bioclusive® waterproof tape, allowing for the skin to locally hydrate under the patches. The patches were removed one at a time and all measurements were made at one site before moving to the next.
Human study procedures
Healthy human volunteers between 18 – 45 years of age with no history of dermatologic disease were examined by a physician to determine appropriateness for the study. All procedures were approved by the Institutional Review Board and were completed according to the principles defined in the World Medical Association Declaration of Helsinki. All subjects provided written consent prior to beginning the study.
Measurements were made on hydrated skin (same pre-hydration process as described for the animals). Six sites on the upper arm were treated with MNs; three sites evaluated with Ag/AgCl dry electrodes, the other three with gel electrodes. The sites were unoccluded one at a time, and six measurements were made at each site: three measurements using light pressure and three using more direct pressure. MN arrays were applied to all sites, followed by six additional measurements (three with light pressure, three with direct pressure). All measurements took 30 seconds to obtain. Table 1 demonstrates the distribution of electrode types and pressure applications amongst the treatment sites.
Table 1.
Description of repeated measurements made at a total of 6 treatment sites on the upper arms of healthy human volunteers.
| Dry Ag/AgCl electrodes | Gel Ag/AgCl electrodes | |||
|---|---|---|---|---|
| Light pressure (n = 3 sites) | Direct pressure (n = 3 sites) | Light pressure (n = 3 sites) | Direct pressure (n = 3 sites) | |
| Pre-MN | 9 measurements | 9 measurements | 9 measurements | 9 measurements |
| One-time MN treatment (50 MN array applied twice) | One-time MN treatment (50 MN array applied twice) | |||
| Post-MN | 9 measurements | 9 measurements | 9 measurements | 9 measurements |
| Total1 | 36 total measurements per subject (18 measurements of intact skin baseline; 18 measurements of MN-treated skin) | 36 total measurements per subject (18 measurements of intact skin baseline; 18 measurements of MN-treated skin) | ||
All measurements were made on skin sites that had been pre-hydrated under blank occlusive patches overnight. The same measurement techniques were used in the Yucatan pig and the hairless guinea pigs, with additional measurements made on non pre-hydrated skin in the animal models.
Calculation of micropore impedance
Three parallel and independent pathways for electrical current are distinguishable: resistor box (Zbox), intact skin (Zskin, pre-MN baseline), and micropores (Zpores). The measurements yield a total impedance value (Ztotal) that is a function of the three pathways. The Ztotal, Zbox and Zskin are known; therefore, micropore impedance can be calculated according to the following equation (employing the assumption that the micropores occupy approximately 2% of the total measurement area):
According to this equation, Zpores can be calculated and the “upper limit” of impedance at the 2% area of the micropores can be estimated, allowing for comparison of the impedance difference from pre- to post-MN treatment at the same small skin area affected by the MN treatment. This method completely removes the effect of the relatively large area of intact skin surrounding the micropores that is also measured under the electrode surface.
Transepidermal water loss
In the guinea pigs, TEWL was measured pre- and post-MN as a complementary technique, using an evaporimeter (cyberDERM, Inc., Broomall, PA). Measurements were made by placing the probe on the skin surface until the reading stabilized (TEWL is calculated by the device in units of g•m-2•h-1). Each measurement took approximately 30 – 60 seconds to obtain.
Staining techniques
Skin staining was utilized in both animal models to visually confirm the presence of micropores. Gentian violet stains microporated skin, making this a quick and effective means of visualizing the micropores (17); in the presence of micropores, a grid can be clearly visualized. The dye was applied to MN treated skin for approximately one minute, followed by removal of excess dye with isopropanol alcohol wipes. A non-treated control site was also stained.
Data analysis
Student’s t-tests were used to compare calculated Zpores and TEWL values pre- vs. post-MN. p < 0.05 was considered significant (GraphPad Prism® software, version 5.04).
Results
Hairless guinea pigs
Male and female guinea pigs were treated with MNs at four independent sites on the dorsal surface; studies were performed on non pre-hydrated and pre-hydrated skin. Impedance dropped significantly from baseline to post-MN (p < 0.05, Student’s t-test) at all sites, irrespective of skin hydration status, electrode type, or pressure applied. The lowest variability was observed with dry electrodes applied with direct pressure. On non pre-hydrated skin, mean (± SD) %RSD on pre- and post-MN skin were 22.4 ± 11.1% (range 3.2 – 35.4%) and 5.9 ± 2.5% (3.9 – 9.8%), respectively, compared to pre-MN values of 10.8 ± 7.1% (3.4 – 23.5%) and post-MN values of 19.9 ± 10.0% (6.0 – 29.5%) for gel electrodes under the same conditions. All %RSD values for measurements made with dry electrodes and direct pressure can be seen in Table 2.
Table 2.
Mean (± SD) %RSD values for the conditions that generated the least variability in 2 animal models and 10 human subjects.
| Non pre-hydrated skin | Pre-hydrated skin | ||
|---|---|---|---|
| Yucatan pig1(gel electrodes, direct pressure) | Pre-MN | 13.9 ± 2.63 (10.8 – 15.3) | 8.01 ± 2.99 (5.09 – 11.06) |
| Post-MN | 4.14 ± 1.68 (2.27 – 5.52) | 8.77 ± 2.87 (6.74 – 10.80) | |
| Guinea pigs2(dry electrodes, direct pressure) | Pre-MN | 22.4 ± 11.1 (3.2 – 35.4) | 5.99 ± 1.61 (3.11 – 7.41) |
| Post-MN | 5.9 ± 2.5 (3.9 – 9.8) | 16.29 ± 9.92 (5.95 – 33.23) | |
| Human subjects3(gel electrodes, direct pressure) | Pre-MN | NA | 9.69 ± 6.13 (0.84 – 24.07) |
| Post-MN | NA | 2.36 ± 3.01 (0 – 11.80) |
In the Yucatan pig, values were calculated from measurements made at 3 sites.
For the guinea pigs, values were calculated from measurements made at 9 sites in 3 guinea pigs.
For the human subjects, the values were calculated across 30 treatment sites in 10 subjects.
Baseline TEWL values were low regardless of skin hydration, confirming intact barrier function. Mean (± SD) baseline TEWL values were 1.4 ± 0.6 g•m-2•h-1 (non pre-hydrated) and 21.6 ± 5.6 g•m-2•h-1 (pre-hydrated). TEWL significantly increased after MN treatment at all sites (p < 0.05, Student’s t-test), with post-MN measurements on non pre-hydrated and pre-hydrated skin of 44.5 ± 17.7 g•m-2•h-1 and 48.2 ± 15.4 g•m-2•h-1, respectively.
In order to visualize the micropores using a third independent means of evaluating MN insertion, one guinea pig was treated on non pre-hydrated skin at an additional site with one application of a 50 MN array, followed by gentian violet staining. A grid in the SC could clearly be visualized, demonstrating the presence of 50 non-overlapping micropores (Figure 3). No staining was observed at the intact skin control site.
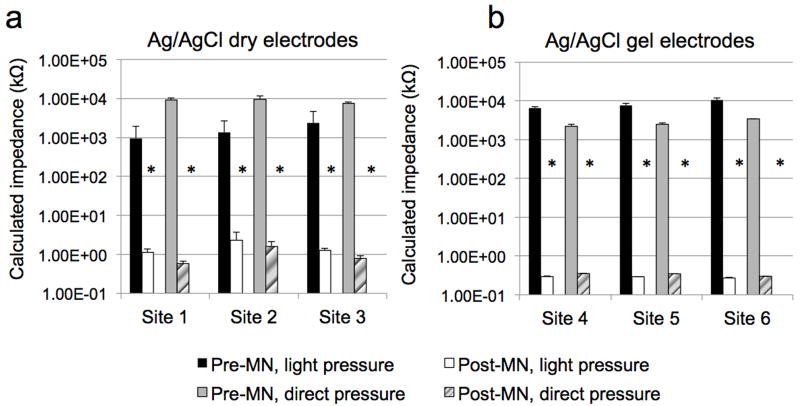
Figure 3. Representative impedance profiles at six treatment sites on one human subject; all measurements were made on the hairless upper arm following an overnight pre-hydration period.
Each bar represents the mean ± SD (error bars) of 3 measurements. a: measurements made at 3 independent with dry Ag/AgCl electrodes. b: measurements made at 3 independent with gel Ag/AgCl electrodes. Mean (± SD) %RSD with gel electrodes under direct application for pre-MN was 7.8 ± 6.5% (range 0.8 – 13.6%), whereas there was no deviation between repeated measurements under post-MN conditions (at each site, the 3 measurements were identical). Under the same conditions (direct pressure) for the dry electrodes, the mean %RSD pre-MN was 14.6 ± 8.9% (range 6.8 – 24.3%) and 23.0 ± 9.2% (range 17.6 – 33.7%) for post-MN.
Yucatan miniature pig
One male Yucatan miniature pig was treated with MN arrays at six independent sites on the dorsal surface and repeated impedance measurements were made pre- and post-MN treatment on pre-hydrated vs. non pre-hydrated skin. A total of 72 measurements were made on pre-hydrated skin; the same number of measurements were made on non pre-hydrated skin; one measurement was thrown out as an outlier (> 20 kΩ difference from other measurements at the same site). Similar to the guinea pigs, impedance decreased significantly following MN treatment at all sites (p < 0.05, Student’s t-test) irrespective of skin hydration status, electrode type, or pressure applied. In the Yucatan pig, the lowest overall variability under both hydration conditions was observed with gel electrodes applied with direct pressure. For non pre-hydrated skin, mean (± SD) %RSD on pre-MN and post-MN skin were 13.9 ± 2.6 (range 10.8 – 15.3%) and 4.1 ± 1.8% (2.3 – 5.5%), respectively, compared to pre-MN values of 15.1 ± 9.0% (7.0 – 24.8%) and post-MN 4.7 ± 5.3% (1.0 – 10.8%) for dry electrodes under the same conditions. Table 2 displays all %RSD values from measurements made with gel electrodes applied with direct pressure.
One additional (non pre-hydrated) site was treated with a MN array to create 50 micropores (rather than the 100 micropores used for the impedance measurements) and stained with gentian violet dye. All 50 micropores could be clearly seen in the skin, confirming formation of 50 independent micropores (image not shown). The non-MN treated control site did not display any staining patterns.
Human studies
Four males and six females completed the protocol, with a mean (± SD) age of 27 ± 4.1 years (seven subjects were Caucasian, three were Asian). Six pre-hydrated sites were treated on the upper arm of each subject, and a total of 360 measurements were made for each electrode type: 180 baseline pre-MN measurements (90 measurements with light pressure; 90 measurements with direct pressure) and 180 post-MN measurements, also divided equally between light and direct pressure techniques. A total of 331 measurements were analyzed across the 10 subjects (four sites total excluded from three subjects due to inadequate insertion of the MN array into the skin (no significant decrease in impedance observed post-MN), and any individual measurements that differed by ≥ 20 kΩ from the other measurements at the same site were also excluded). All MN applications were well tolerated and no infection or irritation was seen at any treatment sites. Some subjects experienced mild irritation from the Bioclusive® tape that secured the occlusive patches to the skin during the overnight hydration period. The irritation resolved completely following a short course of topical steroid treatment. Mean impedance values dropped significantly from baseline to post-MN for all treatment sites, irrespective of pressure or electrode type (p <0.05, Student’s t-test). Overall variability was lowest with gel electrodes, particularly for the calculated impedance of the micropores. In several subjects there was no variation at all between the 3 measurements made post-MN. Mean ± SD pre-MN and post-MN %RSD was 9.7 ± 6.1% (0.8 – 24.1%) and 2.4 ± 3.0% (0.00 – 11.8%) respectively, across all 10 subjects. A representative profile from one subject is shown in Figure 3.
Discussion
Microneedles offer a wide range of flexibility as a drug delivery technique. Several parameters can be modified to meet specific therapeutic needs, including the delivery method, MN geometry, drug formulation, and healing rates of the micropores. For the “poke/press and patch” technique, adequate micropore formation is critical to the success of drug delivery. A variety of methods are available for evaluating micropore formation in the SC, including optical coherence tomography, confocal microscopy, infrared spectroscopy, impedance spectroscopy, and TEWL. Most of these techniques require equipment and software that are less convenient and user-friendly than what is desired in a clinical research environment. The impedance setup described in these studies is portable, requires very little training to use, and has no need for software to obtain measurements, making it ideal for a range of clinical and research environments. Our current work provides complementary results to previous studies in the field, and we have demonstrated that small adjustments in electrode type or pressure applied during measurements can further optimize this valuable technique for MN studies.
Differences between impedance measurement techniques
A previous study by Gupta et al. has also examined impedance spectroscopy in the setting of micropore formation, using dry Ag/AgCl electrodes on hydrated and non-hydrated skin in human subjects (9). Due to slight differences in study design and data analysis, our results cannot be compared directly to the previous work, but several trends are similar and lend support to the conclusions of the present studies. In both animal models and in human subjects in the current work, measurements were noisy and somewhat erratic on intact skin (regardless of hydration status). This trend was also observed in the previous work (9) and likely represents the skin’s effective barrier in preventing movement of current. Furthermore, it was postulated that inconsistent contact between dry electrodes and dry skin may have created a measurement artifact, and this would have added to the already erratic measurements. This argument would also support our results which showed that 1) gel electrodes produce less variability between measurements in human subjects (potentially due to increased contact with the skin at the electrode/skin interface), and 2) direct pressure applied to the electrode also reduced variability (again, directly related to more consistent contact with the skin). Post-MN measurements were less variable and noisy due to the creation of an un-impeded pathway for the movement of current, which was also observed by Gupta and colleagues (9). These authors suggested that skin with a breached barrier (and therefore low impedance) is overall less sensitive to even small changes in hydration of the barrier status, due to the reciprocal relationship between impedance (Z) and permeability (P) as follows: P ~ 1/Z (9). This supports our current results that measurements were less noisy on MN-treated skin, and further confirms that impedance is highly suitable to monitor micropore formation due to its ability to handle small changes in the skin without introducing additional variability. Last, an overall trend was observed that measurements were generally lower when direct pressure was applied to the electrode (regardless of electrode type or pre- vs. post-MN). This is likely due to enhanced contact between the electrode surface and the skin, providing a less tortuous pathway for the current to travel.
Measurements with gel electrodes were generally more stable compared to dry electrodes (both pressure techniques), which was most notable on MN-treated skin. This could be the case for a variety of reasons. First, the gel provides a pathway of lesser resistance compared to the dry electrodes, providing an aqueous pathway for the current to move across. Second, the gel surface likely enhances contact between the electrode and the micropores, resulting in lower impedance measurements due to the higher number of micropores in a small area. This would also explain why measurements were consistently lower with direct pressure vs. light pressure techniques with the same electrode type. Direct pressure allows for better contact with the whole grid of 100 micropores, whereas lighter pressure might not provide complete contact between all of the micropores under the electrode surface. Thus, the optimal situation would be to make measurements with the gel electrodes while applying direct pressure to maintain close contact between the electrode and the skin. In fact, this is consistent with our observation that the lowest variability was observed in human subjects and the Yucatan pig when this technique was used. The results in hairless guinea pigs followed a slightly different trend in that dry electrodes (direct pressure) produced the least amount of variability, rather than the gel electrodes that were superior in the Yucatan pig and human subjects. This finding is somewhat challenging to objectively explain. Less agreement exists between in vitro results comparing human skin to hairless guinea pig skin, as opposed to good agreement between pig vs. human skin. While considered an acceptable model for in vitro studies, guinea pig skin is typically more permeable to chemicals or drugs compared to human skin, and significant differences are present when evaluating permeation lag time (18). Despite these differences for in vitro results, the structure of the skin is similar between guinea pig, porcine, and human skin (18). While the current work was not performed in vitro, the previous literature would suggest that differences between guinea pig skin and porcine or human skin are expected, which we did note in our studies. Therefore, our findings that a different electrode surface (dry vs. gel) is superior for reducing variability in hairless guinea pigs vs. pig or humans is not surprising, but yet provides useful information for future MN animal studies.
Due to typical inter-subject variation, some subjects have lower baseline impedance measurements (regardless of electrode type). These situations can make the difference between baseline and post-MN treatment somewhat more difficult to interpret from the raw impedance values (Ztotal). Despite this potential challenge it is not likely be clinically significant, as it is not a uniform concern across all subjects, and the calculation of Zpores clearly distinguishes that the SC has been sufficiently breached, despite a seemingly small difference in the raw values pre-and post-MN.
Skin hydration
The nature of transdermal patches is such that the skin remains occluded underneath the patch for a timeframe of hours to days, resulting in a local increase in skin hydration. This can create a substantial problem with TEWL readings when the micropores in the SC begin to heal and subtle changes are difficult to discern from the effects of the hydration alone. While impedance measurements are also somewhat affected by hydration status, the technique is sensitive enough to detect changes in the micropores under both hydrated and non-hydrated conditions, as the technique is less sensitive overall to hydration effects (9). We used TEWL and impedance spectroscopy as complementary techniques in hairless guinea pigs to compare the two methods under similar conditions. TEWL values followed expected trends, and significant increases in TEWL were observed after MN treatment, regardless of skin hydration. Despite this trend, however, variability was notably higher when compared to the impedance techniques. Mean ± %RSD values for TEWL measurements post-MN were 35.1 ± 19.9% (range 11.1 – 56.6%) for non pre-hydrated skin, and 32.6 ± 16.9% (range 8.6 – 48.2%) on hydrated skin. Conversely, impedance measurements with dry electrodes were superior and produced mean RSD values less than half of those observed with TEWL (all impedance %RSD seen in Table 2). While variability is both expected and unavoidable with in vivo and biological systems, the variability presented by the TEWL data under both skin conditions presented here may be inappropriate for evaluating changes in the skin at the micrometer scale produced by MN insertion.
Anytime the skin is occluded after MN treatment (the typical situation for the “poke/press and patch” method), impedance measurements for the resulting treatment period would be made on hydrated skin. While MNs would not likely be applied to pre-hydrated skin in clinical practice, in a controlled research setting the pre-hydration removes an additional variable in the impedance setup (dry vs. hydrated skin, a discrepancy that would arise if the baseline measurements were made on dry skin but all other measurements were made on skin that had been occluded). As demonstrated by the results in the animal models, the skin’s hydration status does not affect the efficiency of MN insertion into the skin or the ability of the impedance setup to detect a barrier breach. The primary difference observed between the hydration states was that the baseline measurements were somewhat more erratic on non pre-hydrated skin. However, the absolute values of the baseline measurements are not as critical because the MN treatment produces such a clear effect on the impedance measurements. Furthermore, in a situation when micropore impedance would be followed over time (during which time the skin would hydrate under a patch), a non-MN treated control site under the same conditions would be used in the equation for calculating Zpores, removing the need for the initial baseline value after the first measurement. For these reasons, it would be suitable to use impedance methods for measuring micropore formation in a typical clinical scenario, when human subjects would be treated on dry baseline skin.
Assessing micropore closure kinetics
One novel application of impedance measurements is monitoring micropore lifetime after one application of a MN array by following the Zpores value over time. To be used in this setting, however, it was necessary to develop a measurement setup that minimizes variability between measurements, to accurately characterize the kinetics of micropore closure. As the micropores begin to close the measurements would likely become more erratic as the biological processes in the skin begin to restore the intact barrier baseline. In fact, this is consistent with previous reports by Gupta et al. In human subjects treated with MNs of varying geometries (using an identical setup with dry Ag/AgCl electrodes), a significant amount of experimental noise was observed for measurements made on intact skin but not on microporated skin, irrespective of the MN geometry (9). As MN-treated skin began to reseal, measurements again become noisy. For this reason it is imperative to have a measurement technique that introduces the least amount of variability, such that the variations observed are directly reflective of the skin healing, rather than the measurement technique itself.
In the current study, Zpores was specifically calculated. By calculating the upper limit of the impedance values expected at the 2% area occupied by the micropores, Zpores can be followed over time until it reaches the upper limit, when the micropores would be considered “closed”. While this provides a clear numerical target for evaluating when the micropores have closed, it could be perceived to falsely exaggerate the difference between pre- and post-MN impedance values. Thus, it is important to evaluate the formation of the micropores with an alternative method to ensure the applicability of the current calculations. An additional method is to calculate the permeable area (Apermeable) of all micropores created by the application of an array, according to the following equation provided by Gupta et al. (9):
where ρ represents the electrical resistivity of interstitial fluid present in the skin (~78 Ω-cm), L estimates the SC thickness (~15 μm over most of the body), and Z is the absolute impedance measured. Under the technique that generated the least amount of variability in human subjects (gel electrodes applied with direct pressure), this analysis results in a range of Apermeable spanning from 4.65 × 10-4 mm2 to 2.81 × 10-3 mm2 (less than the cross-section of a human hair). Furthermore, the radii of each individual micropore can be calculated (assuming a circular cross section and that each micropore contributes 1/100 of the total Apermeable). According to these assumptions, the mean (± SD) radii of the individual micropores was 1.97 ± 0.51 μm, which is remarkably consistent with previous estimates of an effective radius of ~2 μm (9, 16). In a similar manner, a limit of the Apermeable can be estimated based on pre-MN measurements, which would also provide a numerical target for evaluating that the micropores have closed. Thus, our method demonstrated adequate formation of micropores in the SC based on the Zpores, and suitability of this method is confirmed by the similarity of our data with previous reports, based on the alternative calculation of the Apermeable.
Limitations
There are some limitations to this work. Exploring the effect of varying MN designs (different lengths or numbers of needles) would have further reinforced our results. However, we only used one MN geometry in all studies to maintain consistency and create micropores of the same dimensions at all treated sites, in order to compare the same conditions for all experimental settings. Furthermore, other studies have demonstrated that differences in MN geometry do not significantly affect the initial decrease in impedance post-MN insertion; rather, the geometry is more of a concern when evaluating healing time of the micropores under occluded conditions (9), which was beyond the scope of our study. Additionally, we did not perform skin permeation studies with marker compounds to validate that the observed barrier disruption correlates with drug diffusion through the skin. However, it has been well established in the literature that MNs of the length used in this study allow clinically relevant amounts of drug diffusion through the micropores (3). Previous reports with experimental modeling have also confirmed that the permeable area created by the micropores correlates predictably with experimental drug diffusion data (3, 9). Therefore, we did not repeat drug permeability studies with the current study, in order to maintain the non-invasive nature of the experiments.
Conclusions
This is the first methods development study to explore multiple impedance spectroscopy conditions to minimize experimental variability when monitoring micropore formation in animal models and human subjects. The impedance setup allows for great flexibility that can be tailored to the conditions of MN treatment (varying hydration status, different animal models, different electrode types and pressure applications) and is highly appropriate for a clinical research setting. Additionally, the impedance of the micropores can be specifically calculated or the permeable area of microporated skin can be determined, allowing for various means to compare the effectiveness of treatment and monitoring the closure of the micropores over time.
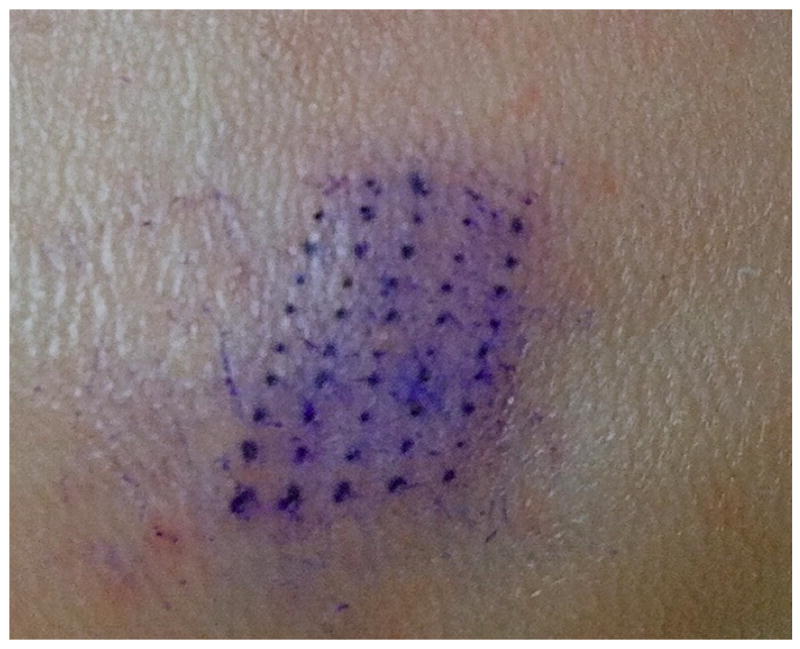
Figure 2. A micropore grid on the dorsal surface of a hairless guinea pig treated once with a 50 MN array.
The 50 individual micropores can be clearly visualized in the skin, demonstrating adequate penetration of the SC with the MN array.
Acknowledgments
We would like to acknowledge Dr. Mark Prausnitz and Dr. Vladimir Zarnitsyn (Georgia Institute of Technology) for their expert advice and assistance with the MN arrays, and Dr. Bruce Walcott (University of Kentucky) for his contributions to the impedance setup. This work was funded by the following NIH grants: CTSA 1UL1RR033173-01, R01DA13425, R42DA32191, F31DA029374, and the Clinical Loan Repayment Program. Additional funding sources included the University of Kentucky Center for Clinical and Translational Science Pilot Research Program and the University of Kentucky Center for Clinical and Translational Science Seed Grant.
Abbreviations used
- MN
Microneedle
- SC
Stratum corneum
- TEWL
Transepidermal water loss
Footnotes
Conflict of interest
Audra L. Stinchcomb is a significant shareholder in and Chief Scientific Officer of AllTranz Inc., a Lexington, KY specialty pharmaceutical company involved in the development of transdermal formulations for microneedle delivery.
All work was performed at University of Kentucky, Lexington, KY, USA.
References
- 1.Prausnitz MR. Microneedles for transdermal drug delivery. Adv drug del rev. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Prausnitz MR, Langer R. Transdermal drug delivery. Nat biotech. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, Stinchcomb AL. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105(6):2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharm. 2008;364(2):227–236. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 6.Lee JW, Choi SO, Felner EI, Prausnitz MR. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small. 2011;7(4):531–539. doi: 10.1002/smll.201001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv drug del rev. 2012 doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curdy C, Naik A, Kalia YN, Alberti I, Guy RH. Non-invasive assessment of the effect of formulation excipients on stratum corneum barrier function in vivo. Int J Pharm. 2004;271(1-2):251–256. doi: 10.1016/j.ijpharm.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalia YN, Guy RH. The electrical characteristics of human skin in vivo. Pharm Res. 1995;12(11):1605–1613. doi: 10.1023/a:1016228730522. [DOI] [PubMed] [Google Scholar]
- 11.Kawai E, Nakanishi J, Kumazawa N, Ozawa K, Denda M. Skin surface electric potential as an indicator of skin condition: a new, non-invasive method to evaluate epidermal condition. Exp Dermatol. 2008;17(8):688–692. doi: 10.1111/j.1600-0625.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Yamamoto Y. Electrical properties of the epidermal stratum corneum. Med Biol Eng. 1976;14(2):151–158. doi: 10.1007/BF02478741. [DOI] [PubMed] [Google Scholar]
- 13.Puurtinen MM, Komulainen SM, Kauppinen PK, Malmivuo JA, Hyttinen JA. Measurement of noise and impedance of dry and wet textile electrodes, and textile electrodes with hydrogel. Conf Proc IEEE Eng Med Biol Soc. 2006;1:6012–6015. doi: 10.1109/IEMBS.2006.260155. [DOI] [PubMed] [Google Scholar]
- 14.Brogden NK, Milewski M, Ghosh P, Hardi L, Crofford LJ, Stinchcomb AL. Diclofenac delays micropore closure following microneedle treatment in human subjects. J Control Release. 2012;163(2):220–229. doi: 10.1016/j.jconrel.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100(24):13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 18.Barbero AM, Frasch HF. Pig and guinea pig skin as surrogates for human in vitro penetration studies: a quantitative review. Toxicol In Vitro. 2009;23(1):1–13. doi: 10.1016/j.tiv.2008.10.008. [DOI] [PubMed] [Google Scholar]