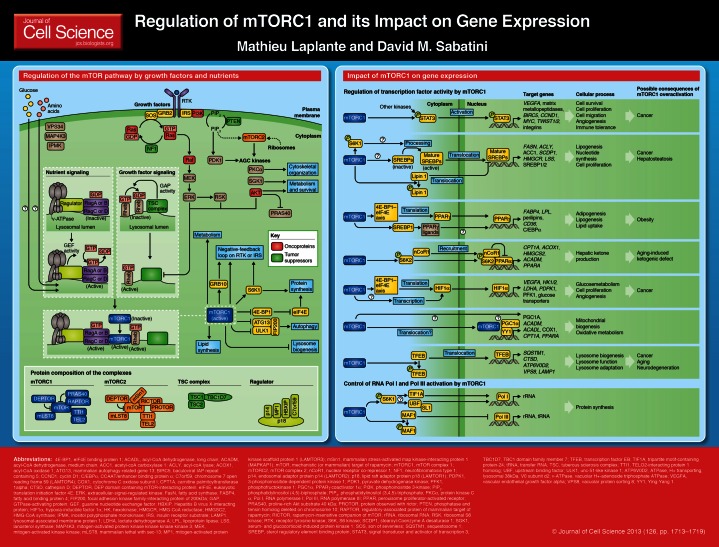
Summary
The mechanistic (or mammalian) target of rapamycin (mTOR) is a kinase that regulates key cellular functions linked to the promotion of cell growth and metabolism. This kinase, which is part of two protein complexes termed mTOR complex 1 (mTORC1) and 2 (mTORC2), has a fundamental role in coordinating anabolic and catabolic processes in response to growth factors and nutrients. Of the two mTOR complexes, mTORC1 is by far the best characterized. When active, mTORC1 triggers cell growth and proliferation by promoting protein synthesis, lipid biogenesis, and metabolism, and by reducing autophagy. The fact that mTORC1 deregulation is associated with several human diseases, such as type 2 diabetes, cancer, obesity and neurodegeneration, highlights its importance in the maintenance of cellular homeostasis. Over the last years, several groups observed that mTORC1 inhibition, in addition to reducing protein synthesis, deeply affects gene transcription. Here, we review the connections between mTORC1 and gene transcription by focusing on its impact in regulating the activation of specific transcription factors including including STAT3, SREBPs, PPARγ, PPARα, HIF1α, YY1–PGC1α and TFEB. We also discuss the importance of these transcription factors in mediating the effects of mTORC1 on various cellular processes in physiological and pathological contexts.
Introduction
The mechanistic (or mammalian) target of rapamycin (mTOR) has emerged over the last decade as a central regulator of cell growth and metabolism. Deregulation of the mTOR signaling pathway has now been linked to aging and to the development of human diseases, including cancer, obesity, type 2 diabetes and neurodegeneration (reviewed by Laplante and Sabatini, 2012). The connection between mTOR dysfunction and these pathologies highlights the importance of this signaling pathway in the maintenance of cellular homeostasis. These observations also raise the possibility that strategies to modulate mTOR function could serve as new avenues to slow aging and to treat many human diseases (reviewed by Laplante and Sabatini, 2012).
The mTOR kinase nucleates two distinct protein complexes termed mTOR complex 1 (mTORC1) and complex 2 (mTORC2). The protein composition of mTORC1 and mTORC2 is illustrated on the poster that accompanies this article. Growth factors and nutrients are the best-characterized cellular inputs contributing to mTORC1 activation. The molecular mechanisms through which these signals activate mTORC1 are described in Boxes 1 and 2. Other factors including oxygen, energy, inflammation, Wnt signaling and phosphatidic acid have also been identified as regulators of mTORC1 (reviewed by Laplante and Sabatini, 2009). When active, mTORC1 phosphorylates the translational regulator eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1), which, in turn, promote protein synthesis (Blommaart et al., 1995; Hara et al., 1998). Through the phosphorylation of several other effectors, mTORC1 promotes lipid biogenesis and metabolism and suppresses autophagy (reviewed by Laplante and Sabatini, 2009). The activity of mTORC1 towards certain substrates is very sensitive to the macrolide rapamycin. When bound to the 12 kDa FK506-binding protein (FKBP12), rapamycin physically interacts with and suppresses mTORC1 kinase activity (Brown et al., 1994; Sabatini et al., 1994; Sabers et al., 1995).
Box 1. Regulation of mTOR signaling by growth factors
Growth factors, such as insulin or insulin-like growth factor 1, exert strong anabolic effects and play crucial roles in promoting cell survival, growth and metabolism. Over the last decades, several groups have demonstrated the importance of the mTOR pathway in regulating many of the cellular response to growth factors. Activation of receptor tyrosine kinases (RTKs) by growth factors promotes phosphatidylinositol (3,4,5)-triphosphate [PtdIns(3,4,5)P3] production through phosphoinositide 3-kinase (PI3K), an event that is required for the activation of AKT (also known as PKB) (reviewed by Manning and Cantley, 2007). Active AKT promotes mTORC1 action by two means: (1) reducing the interaction of proline-rich AKT substrate 40 kDa (PRAS40) with mTORC1 (Sancak et al., 2007; Thedieck et al., 2007; Vander Haar et al., 2007; Wang et al., 2007), and (2) phosphorylating and inactivating the tuberous sclerosis complex (TSC), a protein complex composed of TSC1 (also known as hamartin), TSC2 (also known as tuberin) and TBC1D7 (Dibble et al., 2012; Inoki et al., 2002; Manning et al., 2002; Potter et al., 2002; Roux et al., 2004). TSC1 and TSC2 are key upstream regulators of mTORC1 and function as a GTPase-activating protein (GAP) for the Rheb GTPase. The GTP-bound form of Rheb directly interacts with mTORC1 and strongly stimulates its kinase activity. As a Rheb GAP, TSC1 and 2 impair mTORC1 by converting Rheb into its inactive GDP-bound state (Dibble et al., 2012; Inoki et al., 2003; Tee et al., 2003). The extracellular-signal-regulated kinase 1/2 (ERK1/2) and ribosomal S6 kinase (RSK1), which are both activated by the Ras pathway downstream of RTKs, also promote mTORC1 activity by phosphorylating and inhibiting TSC1 and TSC2 (Ma et al., 2005; Roux et al., 2004). Sustained activation of mTORC1 ultimately leads to the retro-inhibition of RTK signaling. This negative-feedback loop is amplified by many components of the mTOR signaling pathway, including growth factor receptor-bound protein 10 (GRB10) (Hsu et al., 2011; Yu et al., 2011), S6K1 (Um et al., 2004) and mTORC1 itself (Tzatsos and Kandror, 2006). Similar to mTORC1, mTORC2 action is also induced by growth factors, but the exact mechanisms involved are still poorly understood. One potential mechanism suggests a role for ribosomes, as ribosomes are needed for mTORC2 activation and mTORC2 binds to lysosomes in a PI3K-dependent fashion (Zinzalla et al., 2011).
Box 2. Regulation of mTORC1 activity by nutrients
Amino acids are required for the activation of mTORC1, but not mTORC2. Despite the fact that it had been known for some time that amino acids had a crucial role in regulating the action of mTORC1, the exact molecular mechanism remained unknown for many years. New findings now indicate that amino acid signaling initiates within the lysosomal lumen (Zoncu et al., 2011). The authors proposed an ‘inside-out’ model of amino acid sensing, in which amino acids accumulate in the lysosomal lumen and initiate signaling through a mechanism that requires the vacuolar H+-adenoside triphosphate ATPase (v-ATPase). At the surface of the lysosome, the v-ATPase directly interacts with the Ragulator, a pentameric protein complex that is essential for amino acid regulation of mTORC1 (Bar-Peled et al., 2012; Sancak et al., 2010). The Ragulator serves as a key scaffold protein complex and possesses guanine nucleotide exchange factor (GEF) activity towards the Rag GTPases (Bar-Peled et al., 2012), a group of GTPases that can recruit and activate mTORC1 at the lysosomal surface (Sancak et al., 2008). Four Rag proteins have been identified, RagA to RagD, which form obligate heterodimers of either RagA or RagB with either RagC or RagD. The two members of the heterodimer have opposing nucleotide-loading states, so that when RagA or RagB is bound to GTP, RagC or RagD is bound to GDP and vice versa (Kim et al., 2008; Sancak et al., 2008). Upon amino acid stimulation, the GEF activity of the Ragulator promotes the loading of RagA or B with GTP in a v-ATPase-dependent manner, which enables RagA or B to interact with the RAPTOR component of mTORC1 (Sancak et al., 2008). This interaction results in the recruitment of mTORC1 from a poorly characterized cytoplasmic location to the lysosomal surface, where its endogenous activator Rheb resides. Glucose has also been demonstrated to promote mTORC1 recruitment to the lysosome through the Rag GTPase (Efeyan et al., 2013). Importantly, the lysosomal localization of mTORC1 that is induced by nutrients is a prerequisite for the activation of mTORC1 by growth factors.
Compared with mTORC1, less is known about mTORC2; it is insensitive to amino acids, but responds to growth factors through a poorly defined mechanism (see Box 1). When active, mTORC2 regulates cell survival, metabolism and cytoskeletal organization through the phosphorylation of several members of the AGC kinase subfamily (reviewed by Laplante and Sabatini, 2012). Because the activity of mTORC2 is not blocked by acute treatment with rapamycin, this complex was originally described as the rapamycin-insensitive mTOR complex (Jacinto et al., 2004; Sarbassov et al., 2004). This simple model was later challenged by reports showing that chronic treatment with rapamycin disrupts mTORC2 integrity and action in some, but not all, cell types (Sarbassov et al., 2006).
mTORC1 inhibition, in addition to impairing protein synthesis, deeply affects gene transcription. The modulation of transcription by mTORC1 was originally characterized in yeast, where the nuclear localization and the activity of several transcription factors are affected by rapamycin (reviewed by Crespo and Hall, 2002). Most of the transcription factors that are regulated by mTORC1 in yeast elicit changes in the expression levels of enzymes involved in metabolic pathways, which is in line with the established nutrient-sensing role of mTORC1. Interestingly, mTORC1 inhibition also affects an important set of genes in mammalian cells (Cunningham et al., 2007; Düvel et al., 2010; Jimenez et al., 2010; Peng et al., 2002; Wang et al., 2011). Here, we review the impact of mTORC1 on gene expression by focusing on its impact on the activity of transcription factors including STAT3, SREBPs, PPARγ, PPARα, HIF1α, YY1–PGC1α and TFEB.
Regulation of STAT3 activity by mTORC1
In response to cytokines or growth factors, the signal transducer and activator of transcription 3 (STAT3) is phosphorylated at Tyr705 by the Janus kinase (JAK) or by receptor tyrosine kinases (RTKs), which facilitates STAT3 dimerization, nuclear translocation and DNA binding. Additionally, STAT3 can be phosphorylated by numerous kinases on Ser727, a site that promotes its transcriptional activity (reviewed by Decker and Kovarik, 2000). When active, STAT3 regulates various cellular processes, such as growth, survival and proliferation.
Yokogami et al. have observed that rapamycin blocks ciliary neurotrophic factor (CNTF)-induced STAT3 phosphorylation at Ser727 (Yokogami et al., 2000). The authors identified mTOR as one of the kinases that can phosphorylate STAT3 at Ser727, and found that rapamycin reduces STAT3 transcriptional activity, an effect that was confirmed by others (Kim et al., 2009). Similarly, loss of TSC1 or TSC2, which strongly activates mTORC1, was in many studies linked to the elevation of STAT3 phosphorylation (Goncharova et al., 2009; Ma et al., 2010; Onda et al., 2002; Weichhart et al., 2008). Some of these reports showed that mTORC1 not only promotes the phosphorylation of STAT3 at Ser727 but also induces its phosphorylation at Tyr705 through a mechanism that remains to be clarified.
STAT3 activity is elevated in many human cancers (reviewed by Kortylewski et al., 2005). STAT3 activation induces tumorigenesis at least in part by promoting the expression of genes that regulate cell survival, proliferation, angiogenesis, metastasis and immune evasion (reviewed by Kortylewski et al., 2005). Importantly, STAT3 is required to promote the proliferation and survival of cells in 1which mTORC1 is constitutively active (Goncharova et al., 2009). Consistent with this study, activation of the mTORC1–STAT3 pathway is required for the viability and the maintenance of breast cancer stem-like cells (Zhou et al., 2007). Taken together, these observations suggest that the mTORC1–STAT3 axis might have a significant role in promoting tumorigenesis and that this pathway could potentially be targeted to treat cancers.
mTORC1 promotes lipid biosynthesis through SREBPs
In addition to protein synthesis, actively growing cells require a substantial amount of lipids to support membrane biogenesis (reviewed by Menendez and Lupu, 2007). Over the last few years, several reports showed that mTORC1 plays a fundamental role in promoting lipid biogenesis by regulating the expression of many lipogenic genes.
One important group of transcription factors that are involved in lipid synthesis are the sterol-regulatory-element-binding proteins (SREBPs). SREBPs are basic helix-loop-helix (bHLH) transcription factors that regulate lipid homeostasis by controlling the expression of lipogenic genes (reviewed by Horton et al., 2002). Three members of the SREBP family have been described in mammals, SREBP1a and SREBP1c (hereafter referred to as SREBP1) and SREBP2. SREBP1 is involved in insulin-mediated fatty acid synthesis, whereas SREBP2 mainly controls cholesterol biosynthesis (reviewed by Horton et al., 2002). Insulin increases SREBP1 expression and cleavage, which allows the release of a mature form of SREBP1 that translocates into the nuclei to regulate gene expression. On the other hand, cholesterol depletion induces the expression of SREBP2 and its subsequent cleavage, thus promoting its activity.
mTORC1 positively regulates the activation of SREBPs through several mechanisms (reviewed by Bakan and Laplante, 2012). Blocking of mTOR signaling reduces the mRNA and protein levels of SREBPs in several experimental models (Li et al., 2010; Li et al., 2011; Owen et al., 2012; Wang et al., 2011; Yecies et al., 2011). Although differences exist between cell types, some of these studies have reported that mTORC1 regulates transcription of SREBPs through a mechanism that is independent of the mTORC1 substrate S6K1. In addition to promoting expression of SREBPs, mTORC1 induces the processing and the nuclear accumulation of the mature and active form of these transcription factors (Düvel et al., 2010; Owen et al., 2012; Porstmann et al., 2008; Wang et al., 2011; Yecies et al., 2011). Studies have revealed that S6K1 plays a crucial in promoting processing of SREBPs downstream of mTORC1 but the exact mechanism involved is still unknown. Finally, it has been shown that mTORC1 promotes the activation of SREBPs by inducing their nuclear accumulation through a mechanism that requires Lipin 1, a phosphatidic acid phosphatase that also serves as a transcriptional coactivator (Peterson et al., 2011). When active, mTORC1 phosphorylates Lipin 1, which results in its exclusion from the nucleus. Upon mTORC1 inhibition, Lipin 1 accumulates in the nucleus, which promotes the association of SREBPs to the nuclear matrix and impairs their ability to bind target genes (Peterson et al., 2011). The fact that mTORC1 regulates activation of SREBPs at multiple levels suggests that the control of lipid synthesis must be intimately coupled to nutrient and growth factor signaling to maintain cellular homeostasis.
An increase in lipogenesis is a hallmark of proliferating cancer cells (reviewed by Menendez and Lupu, 2007). As mTORC1 is often hyperactivated in cancers, it is possible that it could have a role in driving tumorigenesis by promoting lipid synthesis through the activation of SREBPs. In support of this idea, Manning’s group recently reported that depletion of SREBPs blocks proliferation in cells with constitutively active mTORC1 (Düvel et al., 2010). The mTORC1–SREBPs axis might also play a role in the development of nonalcoholic fatty liver disease (NAFLD), a condition that is characterized by excessive accumulation of lipids in the liver that can lead to cirrhosis and liver cancer. Obesity and nutrient overload, which are linked to NAFLD, exacerbate mTORC1 activity in the liver (Khamzina et al., 2005; Tremblay et al., 2007), which, in turn, might promote NAFLD by activating SREBP1. Consistent with this idea, liver-specific deletion of mTORC1 impairs SREBP1 function and makes mice resistant to western-diet-induced NAFLD (Peterson et al., 2011).
mTORC1 promotes PPARγ action and adipogenesis
Because mTORC1 senses growth factors and nutrients, which are the main factors driving adipose tissue accumulation in mammals, several groups have tested the role of mTORC1 in regulating fat cell formation. mTORC1 inhibition severely impairs adipogenesis and adipose cell maintenance in vitro (Cho et al., 2004; Gagnon et al., 2001; Kim and Chen, 2004; Polak et al., 2008; Yu et al., 2008; Zhang et al., 2009). mTORC1 affects these processes by modulating the expression and the activity of peroxisome proliferator-activated receptor γ (PPARγ), a nuclear receptor that controls the expression of genes that are required for fatty acid synthesis, uptake and esterification (reviewed by Rosen and MacDougald, 2006).
The mechanism by which mTORC1 activates PPARγ is still not fully elucidated. Le Bacquer et al. have observed that the mTORC1–4E-BP axis regulates the translation of PPARγ and that of other factors that are required for the activation of the adipogenic cascade, namely CCAAT/enhancer-binding protein α (C/EBPα) and C/EBPδ (Le Bacquer et al., 2007). Another report also indicates that mTORC1 promotes the transactivation capability of PPARγ, but the mechanism involved was not identified (Kim and Chen, 2004). It is possible that mTORC1 could activate PPARγ through its effect on SREBP1, which has been shown to promote the production of endogenous PPARγ ligands (Kim et al., 1998).
Despite the established role of mTORC1 in activating PPARγ and adipogenesis, we recently observed that overexpression of DEPTOR, an endogenous and partial inhibitor of mTOR signaling, does not inhibit but instead increases adipogenesis in vitro and adipose tissue accumulation in vivo (Laplante et al., 2012). In that study, we showed that DEPTOR promotes adipogenesis by dampening the negative effect of mTORC1 on insulin signaling, which activates the pro-adipogenic functions of Akt (see Box 1). These results indicate that, although mTORC1 is a key regulator of PPARγ, its activity must be tightly controlled to execute the adipogenic cascade within a physiological context.
mTORC1 regulates PPARα activity and hepatic ketogenesis
The liver controls systemic glucose and lipid homeostasis in response to the nutritional status. Recently, it has been observed that mTORC1 regulates hepatic ketone body production in response to fasting (Sengupta et al., 2010). mTORC1 activity is low during fasting, and mice with constitutively active mTORC1 in the liver are unable to turn on ketone body production when they are fasted. This work revealed that mTORC1 blocks hepatic ketogenesis by inhibiting the activity of PPARα, a nuclear receptor that controls the expression of genes required for fatty acid oxidation and ketone body synthesis (reviewed by Lefebvre et al., 2006). mTORC1 impairs PPARα activity by promoting the nuclear accumulation of nuclear receptor corepressor 1 (nCoR1), a negative regulator of several nuclear receptors. A more recent study suggests that the effect of mTORC1 on the nCoR1–PPARα axis is mediated by S6K2, which acts downstream of mTORC1 (Kim et al., 2012). Indeed, deletion of S6K2 in mice promotes ketone body production, and S6K2-null hepatocytes show high PPARα activity. The authors found that S6K2 blocks PPARα by interacting with and promoting nCoR1 nuclear localization (Kim et al., 2012).
It has been found that mTORC1 activity is elevated in the livers of old mice (Sengupta et al., 2010). This is interesting, as aging has been linked to reduced PPARα activity and hepatic ketogenesis (Okuda et al., 1987; Sanguino et al., 2004; Sastre et al., 1996). The observation that mTORC1 inhibition is sufficient to prevent aging-induced defects in PPARα activation and ketone body production supports the idea that there is a key role for mTORC1 in these effects (Sengupta et al., 2010) and also that a deregulation of the mTORC1–PPARα axis upon aging could contribute to the deterioration of systemic glucose and lipid homeostasis by impairing the metabolic flexibility of the liver (Petersen et al., 2003).
Modulation of metabolism by the mTORC1–HIF1α axis
Hypoxia-inducible factor 1α (HIF1α) controls a transcriptional program that allows cells to cope with oxygen deprivation (reviewed by Majmundar et al., 2010). HIF1α activation promotes the expression of genes that regulate glucose transport and glycolysis and provides a means to maintain energy production when cellular respiration is reduced. HIF1α also helps to resolve hypoxic stress by promoting angiogenesis (reviewed by Majmundar et al., 2010).
Duvel et al. found that the DNA-binding motif of HIFα is overrepresented in the promoter of genes whose expression is modulated by mTORC1 (Düvel et al., 2010). Consistent with this observation, several reports have shown that mTORC1 is a positive regulator of HIF1α (Brugarolas et al., 2003; Düvel et al., 2010; Hudson et al., 2002; Laughner et al., 2001). mTORC1 increases HIFα protein levels by promoting its cap-dependent translation through the 4E-BP1–eIF4 axis. There are also indications that mTORC1 can induce the transcription of HIF1α, but the underlying mechanism involved has not has not been further defined (Düvel et al., 2010).
The interplay between mTORC1 and HIF1α is also interesting in the context of cancer development and progression. As is the case for mTORC1, HIF1α activity is also frequently elevated in cancer. The increase in HIF1α activity, which results from oncogenic activation and/or intratumoral hypoxia, has been associated with an increased patient mortality in several types of cancer (reviewed by Keith et al., 2012). HIF1α promotes tumorigenesis by conferring adaptive, proliferative and survival advantages to the cancer cell through the modulation of energy metabolism and tumor oxygenation. The identification of a link between mTORC1 and the expression of HIF1α supports the possibility that this connection could be targeted for the treatment of cancers.
mTORC1 promotes mitochondrial biogenesis through YY1 and PGC1α
Cell growth and proliferation, which are both linked to mTORC1 activation, consume a substantial amount of energy, and it has been shown that mTORC1 controls mitochondrial biogenesis and function. For instance, mTORC1 activation increases the number of copies of mitochondrial DNA and the expression of genes that encode proteins regulating mitochondrial metabolism (Cunningham et al., 2007; Koyanagi et al., 2011). Consequently, the genetic ablation of mTORC1 in mouse heart or skeletal muscle reduces the number of mitochondria and the expression of oxidative genes (Bentzinger et al., 2008; Romanino et al., 2011; Shende et al., 2011). A report indicates that nuclear mTORC1 controls the transcriptional activity of PPARγ coactivator-1 (PGC1α), a nuclear cofactor that regulates mitochondrial biogenesis and metabolism, by altering its physical interaction with another transcription factor, yin-yang 1 (YY1) (Cunningham et al., 2007). Although this is an interesting possible mechanism, it is difficult to reconcile it with other published reports, which show that mTORC1 is active at the surface of the lysosome and that little to no endogenous mTORC1 is found in the nucleus. Additional studies are needed to validate the role of the contribution of YY1 and PGC1α in mediating the effect of mTORC1 on mitochondrial biogenesis.
Importantly, the role of mTORC1 in mitochondrial function and oxidative metabolism might differ between tissues. As discussed above, mTORC1 impairs PPARα activity and the expression of genes regulating lipid oxidation in the liver (Sengupta et al., 2010), whereas opposing effects have been observed in the heart and in skeletal muscles (Romanino et al., 2011; Shende et al., 2011). The underlying reasons for these differences remain to be identified.
Control of lysosome biogenesis by the mTORC1–TFEB axis
Lysosomes are organelles that contribute to cellular homeostasis by regulating a plethora of physiological processes, including cellular clearance, lipid homeostasis, energy metabolism and pathogen defense (reviewed by Singh and Cuervo, 2011). In addition, as described in Box 2, lysosomes are also an important signaling hub that is essential for the activation of mTORC1 by nutrients.
A factor that regulates lysosome biogenesis and function, and their adaptation to environmental cues is the bHLH leucine zipper transcription factor EB (TFEB). In response to starvation or lysosomal dysfunction, TFEB positively regulates the expression of lysosomal hydrolases, lysosomal membrane proteins and components of the v-ATPase complex (Palmieri et al., 2011). TFEB also promotes autophagosome formation and their fusion with the lysosome (Settembre et al., 2011). Together, these processes help cells to cope with stressful conditions by increasing their ability produce energy from the degradation of cellular components. Recently, mTORC1 has been identified as a key regulator of TFEB function (Martina et al., 2012; Roczniak-Ferguson et al., 2012; Settembre et al., 2012). In the presence of nutrients, mTORC1 phosphorylates TFEB at the lysosome surface, which promotes the binding of TFEB to 14-3-3 proteins and inhibits its transport into the nucleus. Conversely, conditions that impair mTORC1 reduce TFEB phosphorylation and its binding to 14-3-3 proteins, and so rapidly increase the accumulation of TFEB in the nucleus, where it orchestrates the expansion of lysosomal and autophagic compartments.
Lysosomal dysfunction can lead to health problems by reducing the ability of cells to clear protein aggregates, debris and organelles. The efficiency of the lysosome in degrading cellular components has been shown to decline over time, an effect that has been linked to the development of aging and age-related diseases, such as neurodegeneration and cancer (reviewed by Rubinsztein et al., 2011). Because mTORC1 inhibition promotes lysosomal biogenesis and autophagy, strategies aimed at blocking mTORC1 are considered as potential therapeutic avenues to reduce aging and related diseases. As a proof-of-concept, a reduction in mTORC1 activity has been shown to promote life extension in mice (Harrison et al., 2009; Lamming et al., 2012; Miller et al., 2011) and to reduce the severity of neurodegeneration in several models (reviewed by Sarkar and Rubinsztein, 2008). Despite the hope and excitement generated by these results, more studies are needed to determine the risks and benefits associated with chronic mTORC1 inhibition (reviewed by Laplante and Sabatini, 2012).
Regulation of RNA polymerases I and III by mTORC1
Although this review focuses on the role of mTORC1 in regulating the expression of genes transcribed by RNA polymerase II, it is important to mention that mTORC1 also affects the expression of ribosomal RNA (rRNA) and transfer RNA (tRNA) through the modulation of the activity of RNA polymerase (Pol) I and III. One study showed that the mTOR–S6K1 pathway activates the regulatory element tripartite motif-containing protein-24 (TIF1A, also known as TRIM24), which promotes its interaction with Pol I and the expression of rRNA (Mayer et al., 2004). By contrast, the mTOR–S6K1 axis has also been shown to control rRNA expression by promoting the interaction of upstream binding factor (UBF) with SL1, an event that facilitates Pol I activation (Hannan et al., 2003). Finally, mTORC1 phosphorylates and inhibits MAF1, a Pol III repressor, and thereby induces the transcription of 5S rRNA and tRNA (Kantidakis et al., 2010; Shor et al., 2010). These effects of mTORC1 on Pol I and III contribute to cell growth by promoting protein synthesis.
Conclusions
The mTORC1 pathway, which previously has been tightly linked to the control of mRNA translation, is now also emerging as a key regulator of gene transcription. Considering the intense interest in mTOR, it is likely that the list of transcriptional regulators whose activity is modulated by this signaling pathway will expand in the near future. Beyond its ability to regulate the activity of specific transcription factors, it is important to note that mTORC1 might also regulate gene expression through alternative processes, such as epigenetic mechanisms or by affecting directly RNA stability or degradation. In the years to come, our understanding of the molecular mechanisms through which mTORC1 controls gene transcription will undoubtedly expand, which might lead to the identification of new transcription factors that could be targeted to treat diseases linked to mTORC1 deregulation.
Footnotes
Funding
The research of M.L. is funded by the Canadian Institute of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Fonds de Recherche du Québec-Santé [grant number 24726], the Canadian Liver Foundation and the Fondation de l’Institut universitaire de cardiologie et de pneumologie de Québec. The research of D.M.S. is supported by the National Institutes of Health (NIH) [grant numbers CA103866, CA129105, AI47389]. D.M.S. is also an investigator of the Howard Hughes Medical Institute. Deposited in PMC for release after 12 months.
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.125773/-/DC1.
References
- Bakan I., Laplante M. (2012). Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 23, 226–234 10.1097/MOL.0b013e328352dd03 [DOI] [PubMed] [Google Scholar]
- Bar-Peled L., Schweitzer L. D., Zoncu R., Sabatini D. M. (2012). Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208 10.1016/j.cell.2012.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger C. F., Romanino K., Cloëtta D., Lin S., Mascarenhas J. B., Oliveri F., Xia J., Casanova E., Costa C. F., Brink M. et al. (2008). Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 8, 411–424 10.1016/j.cmet.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Blommaart E. F., Luiken J. J., Blommaart P. J., van Woerkom G. M., Meijer A. J. (1995). Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J. Biol. Chem. 270, 2320–2326 10.1074/jbc.270.5.2320 [DOI] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. (1994). A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369, 756–758 10.1038/369756a0 [DOI] [PubMed] [Google Scholar]
- Brugarolas J. B., Vazquez F., Reddy A., Sellers W. R., Kaelin W. G., Jr (2003). TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4, 147–158 10.1016/S1535-6108(03)00187-9 [DOI] [PubMed] [Google Scholar]
- Cho H. J., Park J., Lee H. W., Lee Y. S., Kim J. B. (2004). Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem. Biophys. Res. Commun. 321, 942–948 10.1016/j.bbrc.2004.07.050 [DOI] [PubMed] [Google Scholar]
- Crespo J. L., Hall M. N. (2002). Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 66, 579–591 10.1128/MMBR.66.4.579-591.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J. T., Rodgers J. T., Arlow D. H., Vazquez F., Mootha V. K., Puigserver P. (2007). mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450, 736–740 10.1038/nature06322 [DOI] [PubMed] [Google Scholar]
- Decker T., Kovarik P. (2000). Serine phosphorylation of STATs. Oncogene 19, 2628–2637 10.1038/sj.onc.1203481 [DOI] [PubMed] [Google Scholar]
- Dibble C. C., Elis W., Menon S., Qin W., Klekota J., Asara J. M., Finan P. M., Kwiatkowski D. J., Murphy L. O., Manning B. D. (2012). TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol. Cell 47, 535–546 10.1016/j.molcel.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düvel K., Yecies J. L., Menon S., Raman P., Lipovsky A. I., Souza A. L., Triantafellow E., Ma Q., Gorski R., Cleaver S. et al. (2010). Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 10.1016/j.molcel.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A., Zoncu R., Chang S., Gumper I., Snitkin H., Wolfson R. L., Kirak O., Sabatini D. D., Sabatini D. M. (2013). Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493, 679–683 10.1038/nature11745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon A., Lau S., Sorisky A. (2001). Rapamycin-sensitive phase of 3T3-L1 preadipocyte differentiation after clonal expansion. J. Cell. Physiol. 189, 14–22 10.1002/jcp.1132 [DOI] [PubMed] [Google Scholar]
- Goncharova E. A., Goncharov D. A., Damera G., Tliba O., Amrani Y., Panettieri R. A., Jr, Krymskaya V. P. (2009). Signal transducer and activator of transcription 3 is required for abnormal proliferation and survival of TSC2-deficient cells: relevance to pulmonary lymphangioleiomyomatosis. Mol. Pharmacol. 76, 766–777 10.1124/mol.109.057042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan K. M., Brandenburger Y., Jenkins A., Sharkey K., Cavanaugh A., Rothblum L., Moss T., Poortinga G., McArthur G. A., Pearson R. B. et al. (2003). mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol. Cell. Biol. 23, 8862–8877 10.1128/MCB.23.23.8862-8877.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K., Yonezawa K., Weng Q. P., Kozlowski M. T., Belham C., Avruch J. (1998). Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 273, 14484–14494 10.1074/jbc.273.23.14484 [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S. et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. D., Goldstein J. L., Brown M. S. (2002). SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. P., Kang S. A., Rameseder J., Zhang Y., Ottina K. A., Lim D., Peterson T. R., Choi Y., Gray N. S., Yaffe M. B. et al. (2011). The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332, 1317–1322 10.1126/science.1199498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C. C., Liu M., Chiang G. G., Otterness D. M., Loomis D. C., Kaper F., Giaccia A. J., Abraham R. T. (2002). Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 10.1128/MCB.22.20.7004-7014.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- Inoki K., Li Y., Xu T., Guan K. L. (2003). Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17, 1829–1834 10.1101/gad.1110003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004). Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 10.1038/ncb1183 [DOI] [PubMed] [Google Scholar]
- Jimenez R. H., Lee J. S., Francesconi M., Castellani G., Neretti N., Sanders J. A., Sedivy J., Gruppuso P. A. (2010). Regulation of gene expression in hepatic cells by the mammalian Target of Rapamycin (mTOR). PLoS ONE 5, e9084 10.1371/journal.pone.0009084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantidakis T., Ramsbottom B. A., Birch J. L., Dowding S. N., White R. J. (2010). mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc. Natl. Acad. Sci. USA 107, 11823–11828 10.1073/pnas.1005188107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B., Johnson R. S., Simon M. C. (2012). HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamzina L., Veilleux A., Bergeron S., Marette A. (2005). Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146, 1473–1481 10.1210/en.2004-0921 [DOI] [PubMed] [Google Scholar]
- Kim J. E., Chen J. (2004). regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 53, 2748–2756 10.2337/diabetes.53.11.2748 [DOI] [PubMed] [Google Scholar]
- Kim J. B., Wright H. M., Wright M., Spiegelman B. M. (1998). ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 95, 4333–4337 10.1073/pnas.95.8.4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. (2008). Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 10, 935–945 10.1038/ncb1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Yoon M. S., Chen J. (2009). Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J. Biol. Chem. 284, 35425–35432 10.1074/jbc.M109.051516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Pyo S., Um S. H. (2012). S6 kinase 2 deficiency enhances ketone body production and increases peroxisome proliferator-activated receptor alpha activity in the liver. Hepatology 55, 1727–1737 10.1002/hep.25537 [DOI] [PubMed] [Google Scholar]
- Kortylewski M., Jove R., Yu H. (2005). Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 24, 315–327 10.1007/s10555-005-1580-1 [DOI] [PubMed] [Google Scholar]
- Koyanagi M., Asahara S., Matsuda T., Hashimoto N., Shigeyama Y., Shibutani Y., Kanno A., Fuchita M., Mikami T., Hosooka T. et al. (2011). Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1. PLoS ONE 6, e23238 10.1371/journal.pone.0023238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D. W., Ye L., Katajisto P., Goncalves M. D., Saitoh M., Stevens D. M., Davis J. G., Salmon A. B., Richardson A., Ahima R. S. et al. (2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D. M. (2009). mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 10.1242/jcs.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D. M. (2012). mTOR signaling in growth control and disease. Cell 149, 274–293 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Horvat S., Festuccia W. T., Birsoy K., Prevorsek Z., Efeyan A., Sabatini D. M. (2012). DEPTOR cell-autonomously promotes adipogenesis, and its expression is associated with obesity. Cell Metab. 16, 202–212 10.1016/j.cmet.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughner E., Taghavi P., Chiles K., Mahon P. C., Semenza G. L. (2001). HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 21, 3995–4004 10.1128/MCB.21.12.3995-4004.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bacquer O., Petroulakis E., Paglialunga S., Poulin F., Richard D., Cianflone K., Sonenberg N. (2007). Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J. Clin. Invest. 117, 387–396 10.1172/JCI29528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P., Chinetti G., Fruchart J. C., Staels B. (2006). Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Invest. 116, 571–580 10.1172/JCI27989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Brown M. S., Goldstein J. L. (2010). Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 107, 3441–3446 10.1073/pnas.0914798107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ogawa W., Emi A., Hayashi K., Senga Y., Nomura K., Hara K., Yu D., Kasuga M. (2011). Role of S6K1 in regulation of SREBP1c expression in the liver. Biochem. Biophys. Res. Commun. 412, 197–202 10.1016/j.bbrc.2011.07.038 [DOI] [PubMed] [Google Scholar]
- Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P. P. (2005). Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121, 179–193 10.1016/j.cell.2005.02.031 [DOI] [PubMed] [Google Scholar]
- Ma J., Meng Y., Kwiatkowski D. J., Chen X., Peng H., Sun Q., Zha X., Wang F., Wang Y., Jing Y. et al. (2010). Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J. Clin. Invest. 120, 103–114 10.1172/JCI37964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar A. J., Wong W. J., Simon M. C. (2010). Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B. D., Cantley L. C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B. D., Tee A. R., Logsdon M. N., Blenis J., Cantley L. C. (2002). Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10, 151–162 10.1016/S1097-2765(02)00568-3 [DOI] [PubMed] [Google Scholar]
- Martina J. A., Chen Y., Gucek M., Puertollano R. (2012). MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 10.4161/auto.19653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C., Zhao J., Yuan X., Grummt I. (2004). mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 18, 423–434 10.1101/gad.285504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J. A., Lupu R. (2007). Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 7, 763–777 10.1038/nrc2222 [DOI] [PubMed] [Google Scholar]
- Miller R. A., Harrison D. E., Astle C. M., Baur J. A., Boyd A. R., de Cabo R., Fernandez E., Flurkey K., Javors M. A., Nelson J. F. et al. (2011). Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. 66A, 191–201 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y., Kawai K., Yamashita K. (1987). Age-related change in ketone body metabolism: diminished glucagon effect on ketogenesis in adult rats. Endocrinology 120, 2152–2157 10.1210/endo-120-5-2152 [DOI] [PubMed] [Google Scholar]
- Onda H., Crino P. B., Zhang H., Murphey R. D., Rastelli L., Gould Rothberg B. E., Kwiatkowski D. J. (2002). Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol. Cell. Neurosci. 21, 561–574 10.1006/mcne.2002.1184 [DOI] [PubMed] [Google Scholar]
- Owen J. L., Zhang Y., Bae S. H., Farooqi M. S., Liang G., Hammer R. E., Goldstein J. L., Brown M. S. (2012). Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc. Natl. Acad. Sci. USA 109, 16184–16189 10.1073/pnas.1213343109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri M., Impey S., Kang H., di Ronza A., Pelz C., Sardiello M., Ballabio A. (2011). Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 10.1093/hmg/ddr306 [DOI] [PubMed] [Google Scholar]
- Peng T., Golub T. R., Sabatini D. M. (2002). The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol. Cell. Biol. 22, 5575–5584 10.1128/MCB.22.15.5575-5584.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K. F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D. L., DiPietro L., Cline G. W., Shulman G. I. (2003). Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 10.1126/science.1082889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T. R., Sengupta S. S., Harris T. E., Carmack A. E., Kang S. A., Balderas E., Guertin D. A., Madden K. L., Carpenter A. E., Finck B. N. et al. (2011). mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420 10.1016/j.cell.2011.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak P., Cybulski N., Feige J. N., Auwerx J., Rüegg M. A., Hall M. N. (2008). Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 8, 399–410 10.1016/j.cmet.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Porstmann T., Santos C. R., Griffiths B., Cully M., Wu M., Leevers S., Griffiths J. R., Chung Y. L., Schulze A. (2008). SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 10.1016/j.cmet.2008.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. J., Pedraza L. G., Xu T. (2002). Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 4, 658–665 10.1038/ncb840 [DOI] [PubMed] [Google Scholar]
- Roczniak-Ferguson A., Petit C. S., Froehlich F., Qian S., Ky J., Angarola B., Walther T. C., Ferguson S. M. (2012). The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42 10.1126/scisignal.2002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanino K., Mazelin L., Albert V., Conjard-Duplany A., Lin S., Bentzinger C. F., Handschin C., Puigserver P., Zorzato F., Schaeffer L. et al. (2011). Myopathy caused by mammalian target of rapamycin complex 1 (mTORC1) inactivation is not reversed by restoring mitochondrial function. Proc. Natl. Acad. Sci. USA 108, 20808–20813 10.1073/pnas.1111448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E. D., MacDougald O. A. (2006). Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 10.1038/nrm2066 [DOI] [PubMed] [Google Scholar]
- Roux P. P., Ballif B. A., Anjum R., Gygi S. P., Blenis J. (2004). Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA 101, 13489–13494 10.1073/pnas.0405659101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D. C., Mariño G., Kroemer G. (2011). Autophagy and aging. Cell 146, 682–695 10.1016/j.cell.2011.07.030 [DOI] [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. (1994). RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78, 35–43 10.1016/0092-8674(94)90570-3 [DOI] [PubMed] [Google Scholar]
- Sabers C. J., Martin M. M., Brunn G. J., Williams J. M., Dumont F. J., Wiederrecht G., Abraham R. T. (1995). Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J. Biol. Chem. 270, 815–822 10.1074/jbc.270.2.815 [DOI] [PubMed] [Google Scholar]
- Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., Sabatini D. M. (2007). PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 25, 903–915 10.1016/j.molcel.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. (2008). The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 10.1126/science.1157535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y., Bar-Peled L., Zoncu R., Markhard A. L., Nada S., Sabatini D. M. (2010). Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 10.1016/j.cell.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguino E., Ramón M., Michalik L., Wahli W., Alegret M., Sánchez R. M., Vázquez-Carrera M., Laguna J. C. (2004). Lack of hypotriglyceridemic effect of gemfibrozil as a consequence of age-related changes in rat liver PPARalpha. Biochem. Pharmacol. 67, 157–166 10.1016/j.bcp.2003.08.034 [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006). Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 10.1016/j.molcel.2006.03.029 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Rubinsztein D. C. (2008). Small molecule enhancers of autophagy for neurodegenerative diseases. Mol. Biosyst. 4, 895–901 10.1039/b804606a [DOI] [PubMed] [Google Scholar]
- Sastre J., Pallardó F. V., Plá R., Pellín A., Juan G., O’Connor J. E., Estrela J. M., Miquel J., Viña J. (1996). Aging of the liver: age-associated mitochondrial damage in intact hepatocytes. Hepatology 24, 1199–1205 10.1002/hep.510240536 [DOI] [PubMed] [Google Scholar]
- Sengupta S., Peterson T. R., Laplante M., Oh S., Sabatini D. M. (2010). mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468, 1100–1104 10.1038/nature09584 [DOI] [PubMed] [Google Scholar]
- Settembre C., Di Malta C., Polito V. A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S. U., Huynh T., Medina D., Colella P. et al. (2011). TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C., Zoncu R., Medina D. L., Vetrini F., Erdin S., Erdin S., Huynh T., Ferron M., Karsenty G., Vellard M. C. et al. (2012). A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende P., Plaisance I., Morandi C., Pellieux C., Berthonneche C., Zorzato F., Krishnan J., Lerch R., Hall M. N., Rüegg M. A. et al. (2011). Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation 123, 1073–1082 10.1161/CIRCULATIONAHA.110.977066 [DOI] [PubMed] [Google Scholar]
- Shor B., Wu J., Shakey Q., Toral-Barza L., Shi C., Follettie M., Yu K. (2010). Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells. J. Biol. Chem. 285, 15380–15392 10.1074/jbc.M109.071639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Cuervo A. M. (2011). Autophagy in the cellular energetic balance. Cell Metab. 13, 495–504 10.1016/j.cmet.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee A. R., Manning B. D., Roux P. P., Cantley L. C., Blenis J. (2003). Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13, 1259–1268 10.1016/S0960-9822(03)00506-2 [DOI] [PubMed] [Google Scholar]
- Thedieck K., Polak P., Kim M. L., Molle K. D., Cohen A., Jenö P., Arrieumerlou C., Hall M. N. (2007). PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE 2, e1217 10.1371/journal.pone.0001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F., Brûlé S., Hee Um S., Li Y., Masuda K., Roden M., Sun X. J., Krebs M., Polakiewicz R. D., Thomas G. et al. (2007). Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 104, 14056–14061 10.1073/pnas.0706517104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatsos A., Kandror K. V. (2006). Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol. Cell. Biol. 26, 63–76 10.1128/MCB.26.1.63-76.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um S. H., Frigerio F., Watanabe M., Picard F., Joaquin M., Sticker M., Fumagalli S., Allegrini P. R., Kozma S. C., Auwerx J. et al. (2004). Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 10.1038/nature02866 [DOI] [PubMed] [Google Scholar]
- Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., Kim D. H. (2007). Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9, 316–323 10.1038/ncb1547 [DOI] [PubMed] [Google Scholar]
- Wang L., Harris T. E., Roth R. A., Lawrence J. C., Jr (2007). PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J. Biol. Chem. 282, 20036–20044 10.1074/jbc.M702376200 [DOI] [PubMed] [Google Scholar]
- Wang B. T., Ducker G. S., Barczak A. J., Barbeau R., Erle D. J., Shokat K. M. (2011). The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc. Natl. Acad. Sci. USA 108, 15201–15206 10.1073/pnas.1103746108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T., Costantino G., Poglitsch M., Rosner M., Zeyda M., Stuhlmeier K. M., Kolbe T., Stulnig T. M., Hörl W. H., Hengstschläger M. et al. (2008). The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–577 10.1016/j.immuni.2008.08.012 [DOI] [PubMed] [Google Scholar]
- Yecies J. L., Zhang H. H., Menon S., Liu S., Yecies D., Lipovsky A. I., Gorgun C., Kwiatkowski D. J., Hotamisligil G. S., Lee C. H. et al. (2011). Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14, 21–32 10.1016/j.cmet.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogami K., Wakisaka S., Avruch J., Reeves S. A. (2000). Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr. Biol. 10, 47–50 10.1016/S0960-9822(99)00268-7 [DOI] [PubMed] [Google Scholar]
- Yu W., Chen Z., Zhang J., Zhang L., Ke H., Huang L., Peng Y., Zhang X., Li S., Lahn B. T. et al. (2008). Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol. Cell. Biochem. 310, 11–18 10.1007/s11010-007-9661-9 [DOI] [PubMed] [Google Scholar]
- Yu Y., Yoon S. O., Poulogiannis G., Yang Q., Ma X. M., Villén J., Kubica N., Hoffman G. R., Cantley L. C., Gygi S. P. et al. (2011). Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332, 1322–1326 10.1126/science.1199484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. H., Huang J., Düvel K., Boback B., Wu S., Squillace R. M., Wu C. L., Manning B. D. (2009). Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS ONE 4, e6189 10.1371/journal.pone.0006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wulfkuhle J., Zhang H., Gu P., Yang Y., Deng J., Margolick J. B., Liotta L. A., Petricoin E., 3rd, Zhang Y. (2007). Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. USA 104, 16158–16163 10.1073/pnas.0702596104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzalla V., Stracka D., Oppliger W., Hall M. N. (2011). Activation of mTORC2 by association with the ribosome. Cell 144, 757–768 10.1016/j.cell.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., Sabatini D. M. (2011). mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334, 678–683 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]