Summary
A link between elevated luteinizing hormone (LH) levels, GATA-4 and LH receptor (LHCGR) expression and gonadotropin-dependent adrenocortical tumorigenesis in humans and mice has been shown. To assess the mechanistic tumorigenic interrelationships between these factors, we transgenically expressed Gata4 under the 21-hydroxylase promoter (Cyp21a1, 21-OH) in C57Bl/6N mice. There was a gradual age-dependent increase of GATA-4 expression only in 21-OH-GATA-4 (TG) female adrenals, in association with slowly progressing neoplasia of non-steroidogenic spindle-shaped A cells in the subcapsular cortex. Gonadectomy (GDX), apparently through direct action of elevated serum LH, markedly enhanced the adrenocortical neoplasia, which now also appeared in GDX TG males. The neoplastic areas of the post-GDX TG adrenals contained, besides A cells, larger lipid-laden, steroidogenically active and LHCGR-positive B cells. Prolonged (>10 months) exposure to elevated post-GDX LH levels resulted in formation of adrenocortical adenomas in the TG mice. Intact and GDX TG mouse adrenals displayed elevated FOG-2 and decreased GATA-6 expression. Additionally, increased expression/activation of components of the Inhbb–Acvr2a–Acvr1c–Smad2/3 signaling system was observed in 12-month-old GDX TG adrenals. Our findings show that two distinct GATA-4-dependent populations of neoplastic adrenocortical cells form: non-steroidogenic LH-independent A cells and steroidogenic LH-dependent B cells.
Key words: GATA-4, Adrenal, Tumor, Luteinizing hormone, LH, LHCGR, LHR, Gonadectomy
Introduction
Two members of the GATA zinc finger transcription factors family, GATA-4 and GATA-6, are expressed in the murine and human adrenal cortex with distinct developmental profiles (Kiiveri et al., 2002a). Fetal adrenals express both GATA factors but adults only GATA-6 (Kiiveri et al., 2002b; Kiiveri et al., 2002a). During adrenocortical tumorigenesis in mice we found re-emergence of abundant GATA-4 expression, whereas GATA-6 was downregulated (Kiiveri et al., 1999). GATA-6 is functional throughout adrenal development from fetal to adult age, whereas GATA-4, with a similar expression pattern with CYP17a1, may serve a role only in the fetal adrenal gene regulation, but is not essential for subsequent adrenocortical differentiation (Keeney et al., 1995; Kiiveri et al., 2002a). Transcriptional activity of the GATA family members is regulated by FOG-2 (Friend of GATA-2), encoded by the Zpfm2 gene (also known as Fog2), which interacts with their N-terminal zinc finger and is highly co-expressed with GATA factors in the heart and gonads (Robert et al., 2002). Depending on the cellular context, FOG-2 may act either as a transcriptional repressor or activator for the GATA factors (Lu et al., 1999).
In previous studies, we showed an apparent positive and reciprocal feed-forward amplification link between GATA-4 and luteinizing hormone/human chorionic gonadotropin receptor (LHCGR/LHR) expression during adrenocortical tumorigenesis in humans and mice (Kananen et al., 1996; Bielinska et al., 2003; Rahman et al., 2004). However, it remains unknown how GATA-4 and LHCGR convene their activities in this process. In humans, LHCGR is expressed in zona reticularis and inner zona fasciculata of the adrenal cortex (Pabon et al., 1996). Wild-type (WT) murine adrenal glands do not express detectable levels of LHCGR, whereas exceptional conditions such as chronically elevated serum LH levels (e.g. GDX) can induce its expression (Rilianawati et al., 1998; Kero et al., 2000; Rahman et al., 2004). Aberrant LHCGR expression in the adrenal cortex has been reported in women with transient Cushing syndrome during pregnancy and persistent Cushing syndrome after menopause, both associated with elevated serum levels of hCG or LH (Guilhaume et al., 1992; Pabon et al., 1996; Lacroix et al., 1999; Mijnhout et al., 2004). Abundant LHCGR expression at protein and/or mRNA levels has also been found in human adrenocortical hyperplasia, adenomas, carcinomas and macronodular adrenal hyperplasia (Bugalho et al., 2000; Miyamura et al., 2002; Wy et al., 2002; Feelders et al., 2003; Goodarzi et al., 2003; Miyamura et al., 2003; Dall’Asta et al., 2004).
In murine models for adrenocortical tumorigenesis, neoplasms may develop in response to chronically elevated serum LH, caused either by GDX, or stimulation from xenografted gonadotropin secreting tumor cells (Risma et al., 1995; Bielinska et al., 2003; Bielinska et al., 2005). DBA/2J, C3H and CE mouse strains have been found to be genetically susceptible to develop gonadotropin-dependent adrenocortical hyperplasia or tumors, whereas such responses do not occur in non-susceptible C57BL/6 or FVB/N mice (Bielinska et al., 2003; Vuorenoja et al., 2007; Bernichtein et al., 2008). DBA/2J and C3H adrenocortical tumors are composed of spindle-shaped A cells that proliferate in the subcapsular region (Hofmann et al., 1960; Murthy et al., 1970), and steroidogenically active lipid-laden B cells that appear later within patches of A cells in adrenal cortex (Rosner et al., 1966). In several transgenic models of adrenocortical tumorigenesis, such as inhibin alpha-subunit promoter/simian virus 40 T-antigen (inhα/Tag) mice, or inhibin α knockout mice (inhα−/−), one of the driving forces for tumorigenesis seems to be the elevated serum LH (Risma et al., 1995; Kero et al., 2000; Mikola et al., 2003; Looyenga et al., 2004). In inhα/Tag mice, GATA-4 and LHCGR appeared simultaneously at 3 months post-GDX along with the adrenocortical tumorigenesis, suggesting them as potential biomarkers for these neoplasms (Bielinska et al., 2003; Rahman et al., 2004; Bielinska et al., 2005). It has also been suggested that both GATA-4 and LH could be important factors in inducing stem cell transformation to tumor cells (Bielinska et al., 2003; Mitani et al., 2003).
In the present study, we transgenically expressed GATA-4 in C57Bl/6N mouse adrenals using the 21-hydroxylase (Cyp21a1, also known as 21-OH) promoter. GDX-induced adrenal tumorigenesis was further studied in this genetically non-susceptible mouse strain. Our goal was to identify the molecular mechanisms underlying the consequences of ectopic GATA-4 expression on putative adrenocortical neoplasia formation and its relationship to elevated LH concentration.
Results
Expression of GATA-4 in adrenals of the 21-OH-GATA-4 TG mice
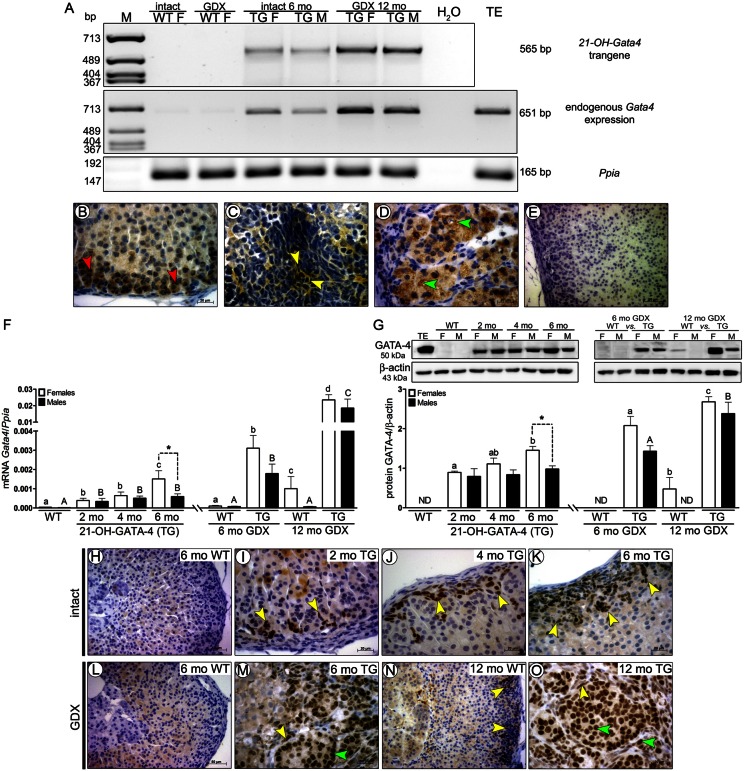
Expression of the 21-OH-Gata4 transgene in TG adrenals was found only in intact and GDX TG adrenals by RT-PCR at mRNA level (Fig. 1A). Additionally, endogenous Gata4 was shown in TG adrenals and at very low levels in WT adrenals (Fig. 1A). Immunohistochemical staining of anti-DsRed (type of RFP) at protein level (Fig. 1B–E) showed DsRed localization to cytoplasma of zona glomerulosa cells (Fig. 1B), and two different types of neoplastic cells of intact (A cells) and GDX (A and B cells) TG adrenals (Fig. 1C,D) but never in WT adrenals (Fig. 1E).
Fig. 1.
Spatiotemporal expression of transgenic DsRed and GATA-4 mRNA and protein in intact and GDX WT and 21-OH-GATA-4 mouse adrenals. (A–E) Analysis of 21-OH-Gata4 transgene and endogenous Gata4 mRNA expression by RT-PCR (A), and by immunohistochemical localization of DsRed in 6-month-old intact (B,C) and 12-month-old GDX TG (D) and WT (E) adrenals. DsRed was localized in normal glomerulosa cells (B, red arrows) and neoplastic cells of 6-month-old intact (C, yellow arrows) and 12-month-old GDX (D, green arrows) TG adrenals but not in WT adrenals (E). (F) Quantification of total Gata4 mRNA by qPCR. Each bar represents the mean±s.e.m. (n = 5) relative to Ppia. (G) Quantification of GATA-4 protein by western blot in 2-, 4- and 6-month-old intact and 6- and 12-month-old GDX TG and WT mice. Different letters above the bars indicate significant differences between groups (small letters and white bars for females, capitals and black bars for males). Asterisks indicate differences between females and males (*P<0.05; ***P<0.001). The upper panels show representative western blots of GATA-4 and β-actin. Murine WT testis (TE) was used as a positive control. The lower panels show densitometric quantification of the bands. Each bar represents the mean±s.e.m. (n = 5) relative to β-actin. ND, non-detectable; TG, transgenic 21-OH-GATA-4 mice; F, females; M, males; GDX, gonadectomy/gonadectomized. (H–O) Immunohistochemical localization of GATA-4 in adrenal glands of 2-, 4- and 6-month-old intact TG and WT females (H–K), and 6- or 2-month-old GDX TG and WT females (L–O). Nuclear staining of GATA-4-positive cells is indicated by yellow (A cells) and green (B cells) arrowheads. Adrenal glands of intact (H) and 6-month-old GDX WT mice (L) were GATA-4 negative. Rare small foci of GATA-4-positive A cells (yellow arrowheads) were observed in WT females at 11 months post GDX (N).
GATA-4 expression was analyzed by qPCR, Western blot and immunohistochemistry in adrenals of intact (2-, 4- and 6-months old) and GDX (6- and 12-months old) WT and 21-OH-GATA-4 TG female and males. As there were no differences between ages of 2–6 months in GATA-4, GATA-6, FOG-2 and LHCGR expression, adrenal weights or histology in intact WT mice (data not shown), we used only 6-month-old animals as controls. The minimum time after prepubertal GDX for the appearance of adrenal LHCGR expression in WT mice has been found to be 5 months (6 months of age) (Kero et al., 2000; Bernichtein et al., 2008). We therefore analyzed the post-GDX WT and TG mice at 6 and 12 months of age. Total GATA-4 mRNA and protein levels in the TG adrenals were significantly higher than in WT adrenals where GATA-4 expression was negligible (intact WT), low (GDX WT) (mRNA; Fig. 1F) or non-detectable (protein; Fig. 1G). Total GATA-4 mRNA and protein levels of 6-month-old TG mouse adrenals were significantly higher in females than males. GATA-4 protein expression was significantly upregulated in 6-month-old TG females, compared with 2-month-old mice (Fig. 1G). There were no significant differences of total GATA-4 at mRNA and protein levels between the different age groups of TG males (Fig. 1F,G). Total GATA-4 mRNA and protein levels were significantly higher in 6- and 12-month-old GDX TG mice than in age-matched control WT littermates (Fig. 1F,G).
In 2- and 4-month-old TG adrenals, single or small patches of nuclear stained GATA-4-positive cells were found mainly in the subcapsular region of zona glomerulosa (Fig. 1I,J). At 6 months, GATA-4-positive cell clusters co-localized with histopathological neoplastic changes observed in the peripheral region of the female but not in male adrenal cortex (Fig. 1K). GDX amplified GATA-4 expression in adrenals of females and males and enhanced the presence of neoplastic cells with nuclear GATA-4 staining (Fig. 1M,O). In WT controls, only 12-month-old GDX WT females displayed GATA-4-positive cells in subcapsular region of the adrenal cortex (Fig. 1L,N).
Ectopic GATA-4 expression participates in the induction of adrenocortical neoplasia
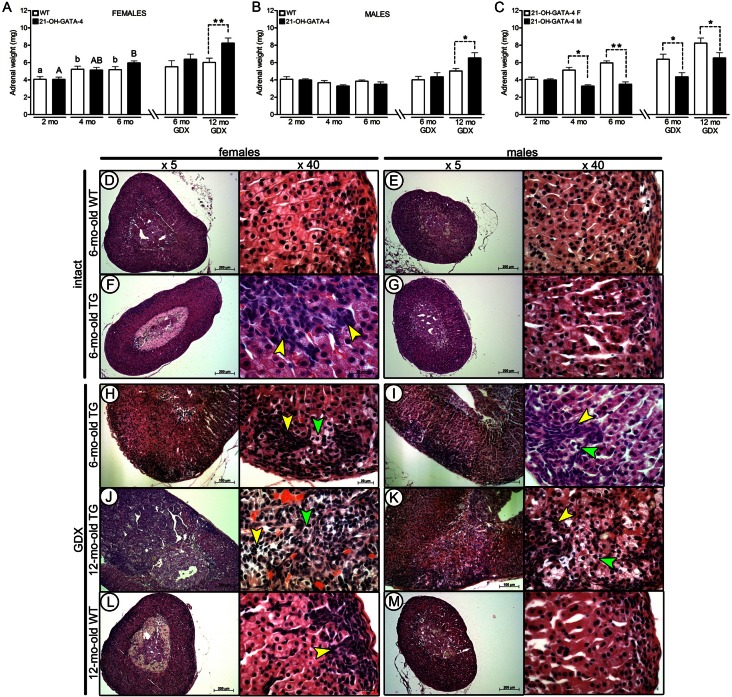
We analyzed total body and adrenal weights and histopathology (HE staining) of 2- (n = 16/8; weight/histology), 4- (n = 16/8) and 6-month-old (n = 16/8) intact and 6- and 12-month-old GDX TG mice (n = 8/8 each) of both sexes. No significant differences in total body weights were observed between WT and TG mice (supplementary material Fig. S1A–C). Only 12-month-old GDX TG females and males had significantly higher adrenal weights compared with GDX WT mice (Fig. 2A,B). Intact WT and TG female adrenals showed age-dependent weight gain that became significant at the age of 6 months compared to 2 months (Fig. 2A). Adrenals of 4- and 6-month-old intact and GDX TG females were significantly heavier than in age-matched males (Fig. 2C).
Fig. 2.
Weights and morphological characteristics of intact and GDX WT and 21-OH-GATA-4 adrenals. (A–C) Total adrenal weights (mean±s.e.m.) of WT and TG female and male mice (n = 16, 12 and 8 for the TG, WT and GDX groups, respectively). Different letters above the bars indicate significant differences between groups (small letters and white bars for females, capitals and black bars for males). Asterisks indicate differences between designated groups (*P<0.05; **P<0.01). ND, non-detectable; TG; transgenic 21-OH-GATA-4 mice; GDX, gonadectomy/gonadectomized; WT, wild type. (D–M) Histopathology of intact 6-month-old TG female (F), male (G) and control littermate (D,E) adrenals. In adrenal cortex of 6-month-old TG females, intensively stained spindle-shaped (A-type, yellow arrowheads) neoplastic cells originating from the subcapsular region are seen (F). Both 6-month-old GDX TG females (H) and males (I) developed multiple hyperplastic foci of invasive A cells by the age of 6 months (small with spindle-shaped nucleus; yellow arrowheads) and large single B cells (larger, with pale cytoplasm; green arrowheads). Post-GDX adenomas were induced in 12-month-old TG adrenals (J,K) that were not observed in control littermates (L,M).
No histopathological differences were observed in between adrenals of 2- and 4-month-old TG females and males (supplementary material Fig. S2A–D), compared with WT mice (Fig. 2D,E). Accumulation of intensively dark-stained A cells with spindle-shaped cell nuclei, originating from the subcapsular cortex, was observed at 6 months in intact TG females (Fig. 2F), but not in TG males (Fig. 2G) or age-matched WT littermates (Fig. 2D,E). A larger number of foci of A cells was observed in older (≥12 months) intact TG females, but not in TG males (supplementary material Fig. S2E–F). TG males developed adrenocortical neoplasia only after GDX (Fig. 2I). In GDX TG females, neoplasia appeared at around 4 months and became more marked by the age of 6 months (Fig. 2H). Both 6-month-old GDX TG female and male adrenals displayed areas of spindle-shaped A cells (Fig. 2H,I; yellow arrowheads), as well as single large, lipid-laden B cells (green arrowheads), also found in the subcapsular region between A cells, that were not observed in intact TG adrenals (Fig. 2F,G). Moreover, individual A cells invaded the cortex from the subcapsular area and towards the zona fasciculata/reticularis region. Adrenocortical adenomas were observed in TG females and males 11 months post-GDX (12-month-old) (Fig. 2J,K). Rarely, small foci of A cells, but never B cells, were observed in 12-month-old intact (supplementary material Fig. S2G,H) and GDX WT females (Fig. 2L,M).
Immunohistochemistry of proliferation marker Ki-67 was used to monitor neoplastic progression (supplementary material Fig. S3A–G). Intact and GDX 6-month-old WT and TG mice showed similar distribution of occasionally proliferating cells throughout the adrenal cortex. The number of proliferating cells was only significantly increased in GDX 12-month-old TG female and male adrenals (supplementary material Fig. S3A,F,G).
GATA-4 is involved in the regulation of GATA-6 and FOG-2 expression
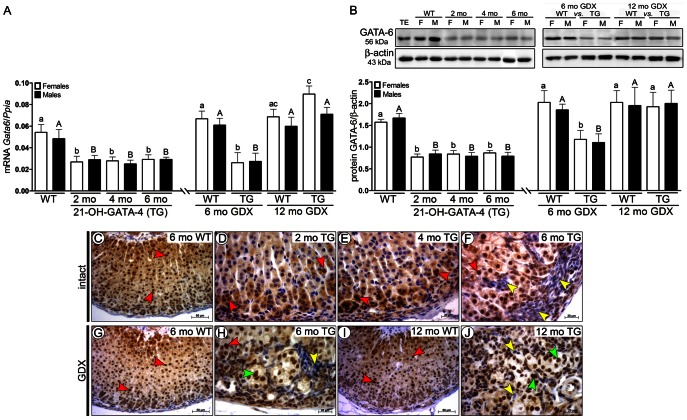
We next analyzed the effects of ectopically expressed GATA-4 on spatiotemporal expression of GATA-6 and FOG-2. GATA-6 at both mRNA and protein levels was significantly downregulated in intact and 6-month-old GDX TG mice of both sexes as compared with WT mice (Fig. 3A,B). Downregulated GATA-6 expression became normalized at 11 months post-GDX (Fig. 3A,B). The GATA-4-positive A cells (Fig. 1H–O) found in serial sections of intact and GDX TG adrenals were GATA-6 negative (Fig. 3F,H,J), while B cells observed only after GDX were GATA-6 positive (Fig. 3H–J).
Fig. 3.
GATA-4 regulated spatiotemporal expression of GATA-6 in adrenals of 21-OH-GATA-4 mice. (A) Quantification of Gata6 mRNA by qPCR. Each bar represents the mean±s.e.m. (n = 5) relative to Ppia. (B) Western blot of GATA-6 protein in 2-, 4- and 6-month-old intact and 6- and 12-month-old GDX TG and WT mice. The upper panels show representative western blots of GATA-6 and β-actin. Murine WT testis (TE) was used as a positive control. The lower panels show densitometric quantification of the bands. Each bar represents the mean±s.e.m. (n = 5) relative to β-actin. Different letters above the bars indicate significant differences between groups (small letters and white bars for females, capitals and black bars for males). ND, non-detectable; TG, transgenic 21-OH-GATA-4 mice; F, females; M, males; GDX, gonadectomy/gonadectomized; WT, wild type; TE, testis. (C–J) Immunohistochemistry of GATA-6 in adrenal glands of 2-, 4- and 6-month-old intact TG and WT females (C–F), and in 6- or 12-month-old GDX TG and WT females (G–J). Clear lack of nuclear immunoreactivity for GATA-6 was observed in neoplastic A cells (yellow arrowheads) of intact (F) and GDX (H,J) TG adrenals in comparison with normal adrenocortical cells (C,D; red arrowheads). In 6- and 12-month-old GDX TG female adrenals, expression of GATA-6 was observed, throughout the histologically normal adrenal cortex and in B cells (F,J; green arrowheads).
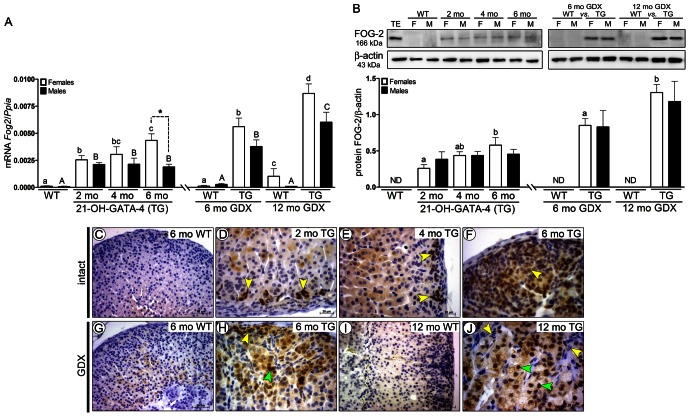
High FOG-2 expression was found in intact and GDX TG adrenals, in contrast to traceable mRNA and non-detectable protein levels in WT mice (Fig. 4A,B). GDX significantly increased FOG-2 expression at 6 and 12 months in TG mice (Fig. 4A,B). There was an increase of FOG-2 expression at 6 months in intact TG female adrenals compared with at 2 months (Fig. 4B). FOG-2 was localized in neoplastic A and B cells of the intact (Fig. 4D–F) and GDX (Fig. 4H,J) TG, but not in WT adrenals (Fig. 4C,G,I).
Fig. 4.
GATA-4 regulated spatiotemporal expression of FOG-2 in adrenals of 21-OH-GATA-4 mice. (A) Quantification of Fog2 mRNA by qPCR. Each bar represents the mean±s.e.m. (n = 5) relative to Ppia. (B) Western blot of FOG-2 protein in 2-, 4- and 6-month-old intact and 6- and 12-month-old GDX TG and WT mice. The upper panels show representative western blots of FOG-2 and β-actin. Murine WT testis (TE) was used as a positive control. The lower panels show densitometric quantification of the bands. Each bar represents the mean±s.e.m. (n = 5) relative to β-actin. Different letters above the bars indicate significant differences between groups (small letters and white bars for females, capitals and black bars for males). 1. ND, non-detectable; TG, transgenic 21-OH-GATA-4 mice; F, females; M, males; GDX, gonadectomy/gonadectomized; WT, wild type; TE, testis. (C–J) Immunohistochemistry of FOG-2 in adrenal glands of 2-, 4- and 6-month-old intact TG and WT females (C–F) and 6- and 12-month-old GDX TG and WT females (G–J). Positive nuclear immunoreaction for FOG-2 was observed in subcapsularly located A cells (yellow arrowheads) of intact (D–F) and GDX TG mice (H,J) and in B cells (green arrowheads) of GDX TG (H,J) adrenals, but not in the control littermates (C,G,I).
GATA-4 regulates activin B signaling in GDX 21-OH-GATA-4 murine adrenals
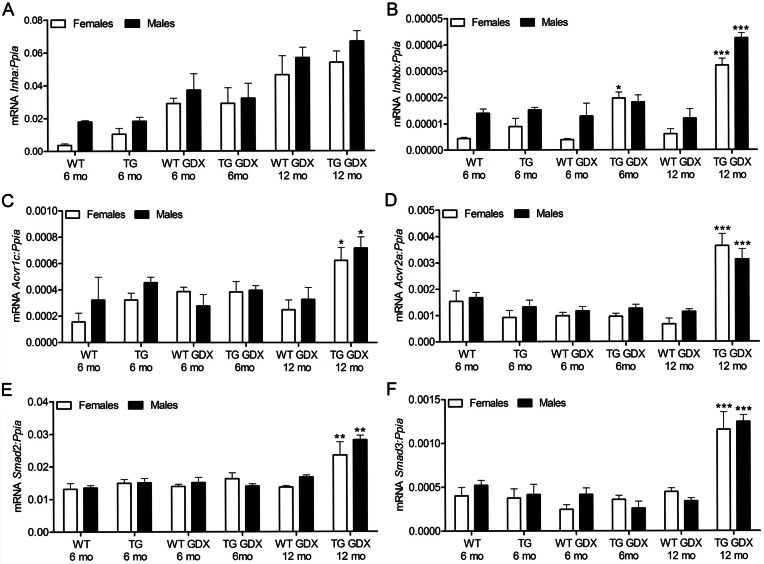
GATA-4 has been demonstrated to selectively upregulate the transcription of the inhibin/activin β-B subunit gene, but not that of α-subunit (Feng et al., 2000), and to be sufficient to activate gonadotropin independent expression of ovary-specific genes in the mouse Y1 adrenocortical cell line (Looyenga and Hammer, 2006). To check the influence of ectopic GATA-4 expression on inhibin/activin signaling, we analyzed Inha, Inhab, Inhbb and the activin transmembrane serine/threonine kinase receptors Acvr1, Acvr1b, Acvr1c, Acvr2a, Acvr2, as well as the two downstream signaling genes Smad2 and Smad,3 at mRNA levels in the adrenals of intact and GDX WT and TG mice. Inha expression was similar in TG and WT adrenals (Fig. 5A). A significant increase of Inhbb expression was observed in 6- (females) and 12-month-old (females and males) GDX TG adrenals compared to WT GDX control-littermates (Fig. 5B). Expression of activin type I receptor Acvr1c (Fig. 5C), type II receptor Acvr2a (Fig. 5D), Smad2 and Smad3 (Fig. 5E,F) was significantly higher in TG adrenals at 11 months post-GDX compared to any other group. Expression of Inhab was higher only in 6-month-old WT male adrenals in comparison with age-matched TG animals (supplementary material Fig. S4A). In turn, the expression of Acvr1 was elevated in 6-month-old TG females (supplementary material Fig. S4B). Both, female and male 6-month-old WT adrenals displayed higher expression of Acvr1b than age-matched TG adrenals (supplementary material Fig. S4C). There were no statistically significant differences in Acvr2b expression among the groups (supplementary material Fig. S4D).
Fig. 5.
Expression of inhibin/activin and Smad genes in 21-OH-GATA-4 adrenals. qPCR analysis of Inha (A), Inhbb (B), Acvr1c (C), Acvr2a (D), Smad2 (E) and Smad3 (F) mRNA expression in adrenal glands of intact (6-month-old) and GDX (6- and 12-month-old) TG and WT mice. Each bar represents the mean±s.e.m. relative to Ppia expression (n = 5 per group). Asterisks indicate differences between age-matched WT and TG mice (*P<0.05; ***P<0.001). TG, transgenic 21-OH-GATA-4 mice; GDX, gonadectomized; WT, wild type.
Steroidogenic profile of the 21-OH-GATA-4 adrenals
We characterized the steroidogenic profile of the intact and GDX TG adrenals by immunohistochemical localization of StAR, SF-1, DAX-1, CYP21A1a, CYP11A1a, CYP11B2, CYP17A1 and CYP19A markers (Table 1). Within the neoplastic areas, B cells expressed all analyzed proteins (except for CYP11B2 and CYP21A1a) (mostly at 11 months post-GDX), but none was expressed in A cells (Table 1; supplementary material Fig. S5A,B). As expected, positive cytoplasmic immunoreaction of StAR and nuclear localization of SF-1 and DAX-1 were found throughout the histologically normal adrenal cortex of intact or GDX TG and WT adrenals (Table 1; supplementary material Fig. S5A). Cytoplasmic expression of CYP11A1a was mainly found in zona fasciculata and reticularis. In turn, CYP11B2 and CYP21A1a were expressed only in the cytoplasm of zona glomerulosa cells (Table 1; supplementary material Fig. S5B). Gonad-specific CYP17A1 and CYP19A1 were found in the adrenal cortex of both TG females and males only after GDX (Table 1; supplementary material Fig. S5B). Intact 6-month-old males did not differ from WT males in the adrenal distribution of steroidogenic markers. For StAR, SF-1, DAX-1, CYP11A1a, CYP11B2 and CYP21A1a localization there were no differences between normal adrenocortical areas of TG and intact and GDX WT mice at any age studied. The small foci of A cells in 12-month GDX WT females remained steroidogenically inactive (Table 1; supplementary material Fig. S5A,B).
Table 1.
Steroidogenic profile of 21-OH-GATA-4 transgenic mouse adrenal cortex
| Mice | Cell type | StAR | SF-1 | DAX-1 | CYP21 | CYP11A1 | CYP11B2 | CYP17A1 | CYP19A1 | ||||||||
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ||
| 21-OH-GATA-4(6 months) | N | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| A | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| B | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | |
| 21-OH-GATA-4GDX (6 and 12 months) | N | + | + | + | + | + | + | + | + | + | + | + | + | +/− | +/− | +/− | +/− |
| A | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| B | + | + | + | + | + | + | − | − | + | + | − | − | + | + | + | + | |
| WT(6 months) | N | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| A | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | |
| B | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | |
| WT GDX(12 months) | N | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| A | − | NP | − | NP | − | NP | − | NP | − | NP | − | NP | − | NP | − | NP | |
| B | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | |
N, normal adrenocortical cells; A, A cells; B, B cells; NP, cell type not present; +, strong positive staining; +/−, week positive staining; −, no staining.
We did not find any evidence of functionally significant sex steroid production by the neoplastic adrenocortical tissue. There were no significant differences between GDX WT and TG female uterine weights (9.2±1.8 mg versus 9.7±1.5 mg, respectively; intact WT uterus − 107±9,2 mg) or in their uterine macroscopic morphology (supplementary material Fig. S6) and histology (data not shown).
Ectopic LHCGR expression in 21-OH-GATA-4 murine adrenals
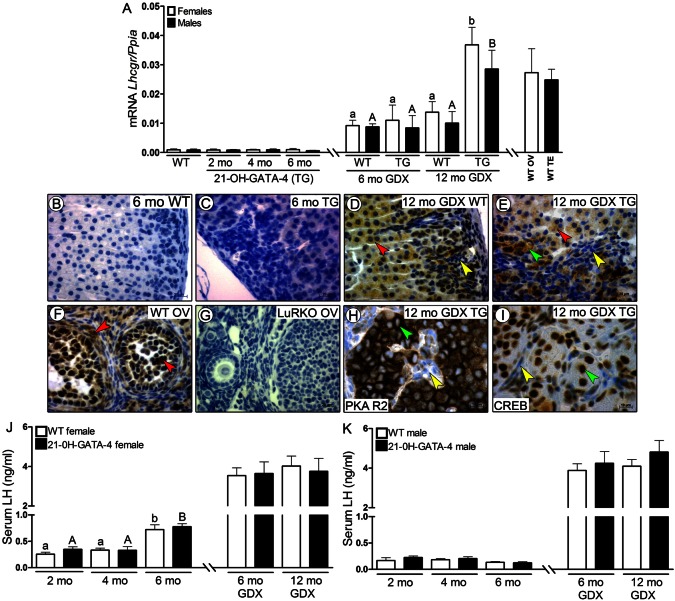
The concomitant appearance of GATA-4 and LHCGR in adrenal tumors have been previously reported (Rahman et al., 2004). As the WT C57Bl/6 mouse adrenals has no detectable LHCGR (Kananen et al., 1996; Rahman et al., 2004; Bernichtein et al., 2008), we wanted to study further whether the ectopic GATA-4 expression could be associated with the appearance of LHCGR in the adrenal cortex. In line with our former report (Bernichtein et al., 2008), where GDX induced LHCGR expression in the adrenal cortex of WT mice, we also observed upregulated LHCGR expression in GDX WT and TG mice at 6 and 12 months compared with intact WT and TG animals (Fig. 6A) (a trace amount at mRNA levels, but non-detectable at protein level). However, only 12-month-old GDX TG females and males displayed significantly higher Lhcgr expression in comparison with GDX age-related control WT littermates; this level of Lhcgr expression was comparable to those observed in WT murine ovary and testis (Fig. 6A).
Fig. 6.
Expression of LHCGR and activation of PKA R2 and CREB in 21-OH-GATA-4 adrenals, as well as serum LH concentration in 21-OH-GATA-4 mice. (A) qPCR analysis of Lhcgr mRNA expression in adrenal glands of intact (2-, 4- and 6-month-old) and GDX (6- and 12-month-old) TG and WT mice. Each bar represents the mean±s.e.m. relative to Ppia expression (n = 5 per group). Different letters above the bars indicate significant differences between groups (small letters and white bars for females, capitals and black bars for males). TG, transgenic 21-OH-GATA-4 mice; GDX, gonadectomized, WT, wild type; OV, ovary; TE, testis. (B–I) Immunohistochemistry of LHCGR in adrenal glands of 6-month-old intact and 12-month-old GDX TG females (C,E) and their control littermates (B,D). WT and LuRKO (luteinizing hormone receptor knockout mice) ovaries were used as positive and negative controls, respectively, for the LHCGR antibody (F,G). Adrenal glands of intact TG and WT mice were LHCGR negative (B,C). Positive staining for LHCGR was observed in normal cortex (red arrowheads) and in B cells (green arrowheads) but not in A cells (yellow arrowheads) of GDX WT and TG mice (D,E). LHCGR in WT ovary was localized to granulosa, theca and luteal cells (F, red arrowheads), whereas all structures of the LuRKO ovary were LHCGR negative (G). LHCGR-positive B cells, but not A cells, were PKA R2 (H) and CREB positive (I) in 12-month-old GDX TG adrenals. (J,K) Serum LH concentration in 2-, 4- and 6-month-old intact, and 6- and 12-month-old GDX TG and WT mice. Each bar represents the mean LH concentration (±s.e.m.) of at least eight individuals in each group.
We did not observe any LHCGR staining in WT and intact TG adrenals (Fig. 6B,C) but the GDX TG animals expressed LHCGR in normal cortex (as WT GDX adrenals) and in B cells, but not the A cells (Fig. 6D,E). WT ovary used as a positive control displayed specific staining for LHCGR in the follicles, theca cells and corpora lutea (Fig. 6F). LHCGR knockout (LuRKO) (Zhang et al., 2001) ovary used as negative control showed no immunoreaction for LHCGR (Fig. 6G). Additionally, we checked whether the neoplastic cells were cAMP responsive by the immunolocalization of activated Protein Kinase A R2 (PKA R2) phosphorylated on serine 96 and cAMP response element-binding (CREB) phosporylated on serine 133. Both PKA R2 (detected as strong brown rings around cell nuclei) and CREB (nuclear staining) in morphologically altered areas of the adrenal cortex were found only in B cells of 12-month-old GDX TG adrenals (Fig. 6H,I).
In order to detect a putative link between elevated serum LH and adrenocortical hyperplasia in TG mice, we also measured serum LH levels. There was no significant difference in serum LH levels between TG females, males and the age-matched control WT littermates at any age (Fig. 6J,K). Both WT and TG females at 6 mo had significantly higher serum LH levels than at 2 and 4 mo. As expected, serum LH levels in all GDX animals were significantly higher than in the non-GDX controls.
Discussion
The phenotype of the 21-OH-GATA-4 mice was a consequence of ectopic GATA-4 expression, in contrast to earlier studies of murine models for adrenocortical tumorigenesis (Vuorenoja et al., 2007), where GATA-4 was used only as a marker of the neoplastic transformation. In TG adrenals, GATA-4-positive cells were observed in the subcapsular region, owing to promoter activity in zona glomerulosa cells (Forest, 2004). However, in contrast to Morley et al., we did not observe transgene expression under Cyp21a1 promoter in zona fasciculata and reticularis (Morley et al., 1996a). Interestingly, we also found that endogenous Gata4 expression was activated during adrenocortical neoplasia in 21-OH-GATA-4 mice.
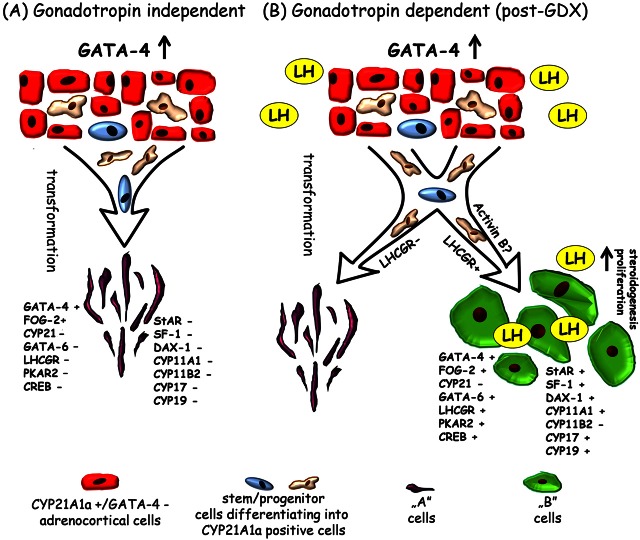
Considering (1) transgene localization, (2) the lack of GATA-4 expression in normal zona glomerulosa cells, (3) the absence of CYP21A1a-positive neoplastic cells and (4) the very low number of GATA-4-positive neoplastic cells in TG adrenals before the age of 4 mo, we suggest that the neoplastic cells were derived from the sparse subcapsular stem/progenitor cells (differentiating into CYP21A1a-positive adrenocortical cells), and not from the normal CYP21A1a-positive zona glomerulosa cells. Most likely, during differentiation of the stem/progenitor cells into CYP21A1a-positive adrenocortical cells in TG adrenals the 21-OH promoter induces transgenic GATA-4 expression that thereafter activates neoplastic transformation of the stem/progenitor cells (summarized in Fig. 7). Stem/progenitor cells have been proposed to be the putative origin of neoplastic differentiation during adrenocortical tumorigenesis (Simon and Hammer, 2012).
Fig. 7.
Possible mechanisms of gonadotropin-independent and/or gonadotropin-dependent (LH) induction of adrenocortical neoplasia in conjunction with transgenically expressed GATA-4 in C57Bl/6N murine adrenals. In the adrenal cortex of intact TG mice (A), sparse stem/progenitor cells (differentiating into normal CYP21 positive adrenocortical cells) under transgene 21-OH-Gata4 influence transform into steroidogenically inactive neoplastic A cells. After gonadectomy (GDX) (B), chronically elevated serum LH might induce LHCGR expression to facilitate the stem/progenitor cells transformation to steroidogenically active neoplastic B cells. The population of LHCGR-negative stem/progenitor cells in GDX TG adrenals, analogous to intact TG adrenals, transforms into A cells. LH-dependent transformation of stem/progenitor cells into B cells is possibly modulated by activin B signalling.
The adrenal glands of intact 6-month-old TG females displayed neoplasia with A cells, while the neoplastic areas of GDX TG animals additionally contained B cells. In light of these observations, it appears that ectopic GATA-4 expression and elevated gonadotropin levels (especially LH) could provide a microenvironment in the adrenal gland that favours the transformation of stem/progenitor cells (differentiating into CYP21-positive adrenocortical cells) into B cells. Recently, GATA-4 was shown to be a key modifier of GDX-induced adrenocortical neoplasia, post-ovariectomy obesity, and sex steroidogenic cell differentiation in DBA/2J genetic background mice (Krachulec et al., 2012). In this study, they also showed that mutations in Gata4 inhibited GDX-induced adrenocortical tumorigenesis in DBA/2J mice (Krachulec et al., 2012). In our study, as GATA-4 expression alone was able to trigger only A cells formation (Fig. 7), it seems that the absence of B cells in intact TG females could be due to the low serum concentrations of LH and possible presence of other gonadal factors such as inhibin. According to previous reports, post-GDX A cells appear first in the adrenal cortex, and later under prolonged LH stimulation the LHCGR-positive lipid-laden B cells (Rosner et al., 1966; Murthy et al., 1970; Bielinska et al., 2003). It has been suggested that the process of A and B cell formation represents metaplasia of stem/progenitor cells in the adrenal cortex, which under the influence of unopposed gonadotropins transform into tissue resembling gonadal stroma (Dunn, 1970; Russfield, 1975; Bielinska et al., 2003). Since chronically elevated LH stimulation drives the expression of its own receptor (LHCGR) (Kero et al., 2000), and as suggested by other authors, it is likely, that the adrenal stem/progenitor cells exposed to high LH concentration may start to express LHCGR and become LH responsive (Looyenga and Hammer, 2006). Furthermore, as the LHCGR expressing B cells develop and proliferate, LH could be the key factor maintaining neoplastic cell proliferation and their progression to adenomas in TG mice.
It was suggested that TGFβ2 signaling, but not activins, is responsible for the adrenocortical tumorigenesis in Inhα−/− mice (Looyenga et al., 2010). In normal physiologic conditions, inhibin antagonizes TGFβ2 signaling in the adrenal cortex by binding to the betaglycan co-receptor, required for efficient TGFβ2 actions (Looyenga et al., 2010; Simon and Hammer, 2012). In Inhα−/− adrenals, due to the absence of Inhα, TGFβ2 could induce gonad-like transformation of adrenocortical progenitor cells (Looyenga et al., 2010; Simon and Hammer, 2012). However, in contrast to Inhα−/− mice, there is a gradual increase of Inhα expression in our GDX TG mice, suggesting that other factor(s) than TGFβ2 trigger the phenotype. It is likely that the combination of GATA-4 and elevated serum LH levels determine the phenotype of the GDX 21-OH-GATA-4 mice. Our findings suggest that activin B could be the novel additional factor involved in the GATA-4/GDX-induced cellular differentiation in the TG model. In support to this, an earlier in vitro study showed that GATA-4 selectively upregulates activin/inhibin β-B-subunit gene transcription (Feng et al., 2000). Moreover, activin B has been suggested to play a role in mesoderm induction (Nakamura et al., 1992), late fetal development and female fecundity (Schmelzer et al., 1990; Schrewe et al., 1994) that could explain the fate switch of the stem/progenitor cells towards gonadal lineages. It has also been shown that the combination of ACVR2A and ACVR1C receptors is specifically utilized by activin B, eventually activin AB, but not by activin A (Tsuchida et al., 2004; Tsuchida et al., 2009). Finally, elevated Smad2 and/or Smad3 expression is associated with adrenocortical (Looyenga and Hammer, 2007) and gonadal (Li et al., 2007) tumorigenesis. Further studies are still needed to establish the details of the Inhbb–Acvr2a–Acvr1c–Smad2/3 signaling mechanism in adrenal tumorigenesis.
GATA-6, in contrast to GATA-4, is abundantly expressed in fetal and adult murine and human adrenal cortex throughout development (Kiiveri et al., 2002b; Kiiveri et al., 2004). However, during adrenocortical tumorigenesis in mice, when GATA-4 expression is highly increased, GATA-6 expression is reciprocally suppressed (Kiiveri et al., 2002b; Kiiveri et al., 2004; Vuorenoja et al., 2007). Additionally, the GATA-4/FOG-2-positive neoplastic areas of A cells (but not B cells) in intact and GDX TG mice were GATA-6 negative. Therefore, the same reciprocity between GATA-4 and GATA-6 expression prevails in TG mice as upon adrenocortical tumorigenesis, when very high GATA-4 expression accompanies low GATA-6 expression (Vuorenoja et al., 2007). The expression of GATA-4 and GATA-6 in B cells found in the GDX TG adrenals is consistent with previous reports in humans (Kiiveri et al., 2004). GATA-6 is highly expressed in aldosterone- and cortisol-producing adenomas and ACTH-independent Cushing's syndrome (Bassett et al., 2005; Kiiveri et al., 2005).
Similar expression pattern of GATA-4 and FOG-2 (in contrast to GATA-6) in adrenals of TG mice supports the role of the latter as an important regulator of GATA-4 transcriptional activity (Robert et al., 2002). Furthermore, co-localization of FOG-2 and GATA-4 was found in adrenocortical neoplastic lesions of GDX or/and hCG-treated NU/J nude mice (Bielinska et al., 2005). However, the role of FOG-2 in the normal adrenal cortex still remains open. It is not known whether FOG-2 represses or enhances GATA-4 mediated transcription in the adrenals, as contradictory results of its action exist in gonads, brain and heart (Lu et al., 1999; Svensson et al., 2000; Tevosian et al., 2000; Robert et al., 2002; Anttonen et al., 2003).
Previous reports indicated that in GDX-induced subcapsular neoplasia (in susceptible strains CE, DBA/2J, and NU/J), A cells express GATA-4 and Anti-Müllerian Hormone (AMH) type 2 receptor (AMHR2), but none of the steroidogenic markers (Bielinska et al., 2003; Bielinska et al., 2005). In turn, B cells express GATA-4, SF-1, LHCGR, inhibin α, AMH, ERα, CYP17A1, and CYP19A1 (Bielinska et al., 2003; Bielinska et al., 2005). Immunohistochemical analysis of steroidogenic markers in the adrenals of intact and GDX TG mice confirmed that only B cells are steroidogenically active.
The non-steroidogenic character of A cells in the TG adrenals could be also related to the downregulated expression of GATA-6 and cellular origin of these cells. Based on its cellular distribution in murine and human adrenals, GATA-6 could be a key player in adrenocortical steroidogenesis (Kiiveri et al., 2002a; Kiiveri et al., 2005). GATA-6 has been shown to act in synergy with SF-1 and other factors to enhance the transcription of many enzymes such as CYP11A1, CYP17A1, CYB5 (cytochrome b5), SULT2A1 (sulfotransferase) and HSD3B2 (3β-dydroxysteroid dehydrogenase) (Jimenez et al., 2003; Flück and Miller, 2004; Kiiveri et al., 2005). Moreover, when non-steroidogenic cells were co-transfected with GATA-6 and SF-1, it was found that these two factors synergistically activated steroidogenesis (Jimenez et al., 2003). Diminished or absent GATA-6 expression is mainly found in non-functional adrenocortical carcinomas in Conn and Cushing syndromes, but not in virilising carcinomas or normal adrenal cortex (Kiiveri et al., 2004; Vuorenoja et al., 2007). On the basis of our present and other earlier studies (Looyenga and Hammer, 2006), it seems that GATA-6 downregulation could be linked with the lack of steroidogenic activity of A cells, however, its co-expression with GATA-4 is required for the gonadal cellular identity.
The simultaneous upregulation and co-localization of GATA-4 and LHCGR has been shown in adrenocortical tumors of humans and mice (Bielinska et al., 2003; Feelders et al., 2003; Rahman et al., 2004; Vuorenoja et al., 2007). However, it is not clear which one of these two genes is expressed first and whether there is a causal link between their expression and adrenocortical tumorigenesis. Our present study showed that GATA-4 overexpression was not able to induce LHCGR (for this, GDX was indispensable), although GATA-4 could induce adrenocortical neoplasia. Ectopic LHCGR expressing cells in adrenals responsive to chronically elevated serum LH levels in human may lead to ACTH-independent macronodular adrenocortical hyperplasia and Cushingoid symptoms (Lacroix et al., 1999; Feelders et al., 2003; Goodarzi et al., 2003; Miyamura et al., 2003). In turn, in susceptible inbred mouse strains (e.g. DBA/2J and CE/J) or murine models for adrenocortical tumorigenesis, [inhα/Tag, inh−/− or inh−/− mice crossbred with LH overexpressing mice (LH-CTP)], GDX triggered adrenocortical tumorigenesis (Bielinska et al., 2003; Mikola et al., 2003; Looyenga et al., 2004). Aberrant LHCGR-expressing cells were reported to develop hyperplasia and Cushing syndrome features in a xenotransplantation model (Mazzuco et al., 2006). However, our present and previous studies have shown that even GDX-induced chronically elevated serum LH, ectopic expression of LHCGR and lack of gonadal inhibin in C57BL/6 mice are not sufficient to induce adrenocortical neoplasia (Kero et al., 2000; Looyenga et al., 2004; Bernichtein et al., 2008).
Taken together, our data showed that ectopically expressed GATA-4 in adrenal glands induced neoplastic A cells in the subcapsular region and modulated formation of neoplastic B cells in GDX TG mice. Chronically elevated serum LH levels, LHCGR and changes in Inhbb–Acvr2a–Acvr1c–Smad2/Smad3 expression in the GDX TG mice could be responsible for the transformation of stem/progenitor cells into B cells. A reciprocal relationship between GATA-4 and GATA-6 was found in the TG adrenals and neoplastic A cells. Moreover, ectopic expression of GATA-4 induced FOG-2 in neoplastic cells. Functional phenotypic characterization of the 21-OH-GATA-4 TG mice does not only explain the complex molecular mechanisms underlying the ectopic GATA-4 induced adrenocortical neoplasia and GATA-4 interactions with GATA-6, FOG-2, LHCGR, activin B and steroidogenic genes, but also makes this model a useful tool to study further the aberrations of adrenocortical growth and function.
Materials and Methods
Experimental animals
In order to direct the expression of GATA-4 to the adrenal cortex, the 6.4 kb murine 21-hydroxylase (21-OH, Cyp21a1) gene promoter was cloned in front of the murine 2.0 kb GATA-4 cDNA (Morley et al., 1996b). A bicistronic IRES-DsRed (Clonetech, Saint-Germain, France) was later cloned into the 21-OH-Gata4 plasmid by Red/ET recombination, as previously reported (Zhang et al., 2003; Rivero-Müller et al., 2007). All modified areas were sequenced in order to ensure correctness. PCR amplification for recombineering was performed using the TripleMaster polymerase mix and buffers (Eppendorf, Horsholm, Denmark). The plasmid was propagated in bacteria by standard procedures and purified using a Maxiprep kit (Qiagen, Helsinki, Finland), linearized, gel-purified and injected into pronuclei of fertilized mouse oocytes of the C57BL/6N strain using standard procedures.
Six transgene-positive (4 females and 2 males) out of fifteen founder mice (9 females and 6 males), genotyped from ear lobe biopsies, were mated with C57BL/6N mice to create the TG lines. Similar phenotype has been observed in two positive founder female lines. One of them named 21-OH-GATA-4 TG line, was selected for future characterization. Phenotype of positive founder males as well as males from any generation of two positive founder females was much weaker than in age-matched TG females. After weaning at the age of 21 days, mice were housed two to four per cage in a room of controlled light (12 h light and 12 h darkness) and temperature (21±1°C). Mice were fed with commercial chow SDS RM-3 (Whitham, Essex, UK) and tap water ad libitum, kept in a specific pathogen-free surrounding and routinely screened for common mouse pathogens. Prepubertal (between 21 and 25 days of life) gonadectomy was performed under isoflurane anesthesia (Baxter, Deerfield, IL, USA). Buprenorphine (Schering-Plough, Brussels, Belgium) was administered as postoperative analgesia. Blood samples were obtained at the time of autopsy. Dissected adrenals for Western blot and RT-PCR analysis were snap-frozen in liquid nitrogen, and stored at −80°C. For immunohistochemical and histological staining, tissues were fixed in 4% paraformaldehyde. Ten intact and ten GDX TG mice per age group (intact: 2-, 4- and 6-month-old; GDX: 6- and 12-month-old) were used for the experiments. Age-matched WT control littermates were used as controls (n = 6 per group). The Ethics Committee for animal experimentation of the University of Turku and the State Provincial Office of Southern Finland approved all animal experiments.
RT-PCR and Real Time RT-PCR
Total RNA was extracted from adrenals by NucleoSpin RNA/Protein Kit (Marcherey-Nagel, Düren, Germany). The quantity and quality of isolated RNA was determined by NanoDrop (Thermo Scientific, Wilmington, DE) and gel electrophoresis. Before the RT reaction, a constant amount of 1 µg of total RNA was treated with DNase I (Invitrogene, Carlsbad, CA). The RT reaction was performed with DyNAmo™ cDNA Synthesis Kit (Finnzymes, Espoo, Finland) at 37°C for 1 h in 20 µl.
Expression of the 21-OH-Gata4 transgene and endogenous Gata4 was analysed by RT-PCR. Primers for the transgene (sense 5′-AGATTCTCCAAGGCTGATGG-3′; antisense 5′-GCCCTGCAGGTAGGACAG-3′, 565 bp) targeted fragment of 21-OH promoter and cloned exon 2 of Gata4 sequence. For endogenous Gata4 expression primers were targeting exon 1 (full exon 1 and part of exon 2, as 5′UTR of Gata4 were not cloned into the plasmid) and fragment of translated exon 2 of Gata4 (sense 5′-GGTTTTCTGGGAAACTGGAG-3′; antisense 5′-CGGAGTGGGCACGTAGAC-3′; 651 bp). The PCR conditions were as follows: 5 min at 95°C, 1 min/50 s at 57/58°C and 1 min at 72°C for 37 cycles. Samples were then subjected to electrophoresis on a 1% agarose gel containing ethidium bromide.
Quantification of Gata4, Gata6, Fog2, Lhcgr, Inha, Inhab, Inhbb, Acvr1, Acvr1b, Acvr1c, Acvr2a and Acvr2b mRNA was performed with a Bio-Rad CFX96 real-time PCR detection system using DyNAmo SYBR Green qPCR kit (Finnzymes, Espoo, Finland). Serial dilutions of the appropriate cDNA products were used as standard curves for DNA quantification. The cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 40 amplification cycles at 95°C for 15 s, 54–60°C at 15 s and 70°C at 5 min. After each PCR reaction, melting curves were obtained by stepwise increases in the temperature from 60 to 95°C to ensure single product amplification. Expression levels for investigated factors and receptors were normalized to the housekeeping gene peptidylprolyl isomerase A (Ppia). ForqPCR analysis following primers were used (primer sequence, expected product sizes, annealing temperatures and EMBL/references, respectively): Gata4 – sense 5′-GGGATTCAAACCAGAAAACG-3′ and antisense 5′-GCTGTGCCCATAGTGAGATG-3′, 198 bp, 58°C, NM_008092.3; Gata6 – sense 5′-CAAAAGCTTGCTCCGGTAAC-3′ and antisense 5′-TGTAGAGGCCGTCTTGACCT-3′, 207 bp, 54°C, NM_010258.3; Fog2 – sense 5′-TGGTCCTAAATGGCTTCTGG-3′ and antisense 5′-GGTACATCCCTTCGGTGAGA-3′, 203 bp, 60°C, NM_011766.5; Lhcgr – sense 5′-CAATGGGACGACGCTAATCT-3′ and antisense 5′-CTGGAGGGCAGAGTTTTCAG-3′, 204 bp, 56°C (Vuorenoja et al., 2009); Inha – sense 5′-GTCTCCCAGGCTATCCTTTT-3′ and antisense 5′-GGCCGGAATACATAAGTGAA-3′, 113 bp, 57°C, NM_010564.4; Inhab – sense 5′-GAGGGCCGAAATGAATGAAC-3′ and antisense 5′-GGCCGGAATACATAAGTGAA-3′, 182 bp, 60°C, NM_008380.1; Inhbb – sense 5′-AACATCACGCACGCTGTC-3′ and antisense 5′-TGTCTCTGCAAAGCTGATGAT-3′, 171 bp, 59°C, NM_008381.3; Acvr1 – sense 5′-CACCTCTTTTCATGCCGTTT-3′ and antisense 5′-GGGTTTCTGGTGGGATGTTA-3′, 159 bp, 59°C, NM_001110204.1; Acvr1b – sense 5′-AAAGCCCTTCTACTGCCTGA-3′ and antisense 5′-GATGATGCCGACCAGCTC-3′, 157 bp, 57°C, NM_007395.3; Acvr1c – sense 5′-GGCTGTGAAGCACGATTCTA-3′ and antisense 5′-TCCAACTGAACACCTTCGAG-3′, 193, 58°C, NM_001111030.1; Acvr2a – sense 5′-CGAGAACTTCCTACGGCTTC-3′ and antisense 5′-TGGTTGGTTCTGTCTCTTTCC-3′, 168 bp, 58°C, NM_007396.4; Acvr2b – sense 5′-GTGGGAGCTCGTCTCTCG-3′ and antisense 5′-GGTGTTTCAGCCAGTGATCC-3′, 164 bp, 58°C, NM_007397.2; Smad2 – sense 5′-TGGTGCCAAGTGCATAAAAA-3′ and antisense 5′-TCCATCCCAGAAGTCTCTTCA-3′, 154, 56°C, NM_001252481.1; Smad3 – sense 5′-GCACAGCCACCATGAATTAC-3′ and antisense 5′-GGGAATGGAATGGCTGTAGT-3′, 193 bp, 58°C, NM_016769.4, Ppia – sense 5′-CATCCTAAAGCATACAGGTCCTG-3′ and antisense 5′-TCCATGGCTTCCACAATGTT-3′, 165 bp, 57°C, NM_008907.1.
Immunoblotting analysis
Total protein was extracted from whole snap-frozen adrenals with NucleoSpin RNA/Protein Kit (Marcherey-Nagel, Düren, Germany). The protein level was measured spectrophotometrically using Protein Quantification Assay (Marcherey-Nagel). Equal amounts (25 µg) of protein were separated using 10% SDS-PAGE gel, followed by transfer into Hybond-P PVDF Membranes (GE Healthcare, Uppsala, Sweden), blocking with 5% non-fat dry milk and immunoblotting with specific primary antibodies as follows: GATA-4 [sc-25310; Santa Cruz Biotechnology (sc), Inc., Santa Cruz, CA; 1∶250], GATA-6 [ab32390, Abcam (ab), Cambridge, UK, 1∶600] and FOG-2 (sc-10755, 1∶500). After incubation with secondary antibodies, immunoreactions were visualized by Amersham ECL Plus Western Blotting Detection System (GE Healthcare) and recorded with Fujifilm LAS-4000 chemiluminometer (Fujifilm, Tokyo, Japan). Dual Colour Precision Plus Protein Standards (Bio-Rad, Hercules, CA) were used as standards. The intensity of specific bands was quantified with the ImageJ 1.42q visual quantitative system (Wayne Rasband, National Institutes of Health, USA; htpp://rsb.info.nih.gov/ij) (Collins, 2007).
Immunohistochemistry
Paraformaldehyde-fixed paraffin sections (5 µm) of intact, GDX 21-OH-GATA-4 and WT adrenal glands were deparaffinized, hydrated and boiled in 10 mM citric acid (pH 6.0) for 15 min for antigen retrieval. Endogenous peroxidase activity was blocked by incubating tissue sections in methanol with 3% H2O2 for 5 min at room temperature (RT). Tissue sections were then incubated with blocking solutions [10% normal goat serum (NGS) with 3% BSA or only 3% BSA] PBS with 0.1% Triton X-100 for 1.5 h at RT in order to reduce non-specific background staining. Thereafter, sections were incubated overnight at 4°C with the following primary antibodies for GATA-4 (sc-25310, 1∶250), DsRed (RFP; ab34771, 1∶100) Ki-67 (M7249, Dako, 1∶500), GATA-6 (ab32390, 1∶600), FOG-2 (sc-10755, 1∶250), StAR (sc-25806, 1∶500), SF-1 (sc-28740, 1∶400), DAX-1 (ab60144, 1∶400), CYP11A1a (Sigma, 1∶300), CYP11B2 (LS-C6353; LifeSpan BioSciences, Inc.; 1∶100), CYP21 (orb13362, Biobryt, 1∶300), CYP17A1 (Proteintech Group, 1∶400), CYP19A1 (Serotec, MCA2077S, 1∶400), LHCGR (kindly provided by Dr Fazleabbas; 1∶700), PKA R2 (ab32390, 1∶500) and CREB (ab32096, 1∶500). Primary antibodies were linked with the respective secondary IgG biotin-conjugated secondary antibodies (Vector Laboratories Inc.) (GATA-4, GATA-6, FOG-2, DAX-1, SF-1) for 1 h at RT or with polymer (Dako) (DsRed, CYP21, Ki-67, StAR, CYP11A1a, CYP11B2, CYP17A1, CYP19A1, LHCGR, PKA R2 and CREB) for 30 min at room temperature. The avidin–biotin immunoperoxidase system was used (when biotin conjugated secondary antibodies were used) to visualize bound antibody (Vectastain Ellite ABC Kit, Vector Laboratories, Inc.) with 3′3-diaminobenzidine as a substrate (Sigma-Aldrich, St. Louis, MO). Finally, the sections were covered with DPX mounting medium (Park Scientific Ltd, Northampton, UK). Two types of controls were performed to determine the specificity of immunohistochemical staining: (1) the primary antibody was omitted during the immunostaining procedure; (2) the primary antibody was substituted with nonspecific IgG. Murine WT and LuRKO (LHCGR knockout mice) (Zhang et al., 2001) ovaries were used for the LHCGR antibody as additional positive and negative controls, respectively.
Quantification of cell proliferation
Ki-67-positive cells were counted on the entire surface of adrenal sections using cell counter (ImageJ). The number of Ki-67-positive cells was then expressed relative to section surface (expressed in pixels). Counting was performed separately on 4–6 individuals (three sections from each) for each group.
Hormone measurement
Serum levels of LH were measured by immunofluorometric assay (Delfia; Perkin-Elmer-Wallac, Turku, Finland) as described previously (Haavisto et al., 1993). The approximate assay sensitivity for LH was 0.0075 µg/l. The intra- and interassay coefficients of variations for these assays were below 10%.
Statistical Analysis
Statistical analysis was carried out by one-way ANOVA with the post-hoc Bonferroni test and two-tailed Student's t-test using GraphPad PRISM v. 5.0 (GraphPad Software, Inc., San Diego, CA). All numerical data are presented as mean±s.e.m. The differences were considered to be significant at P<0.05.
Supplementary Material
Acknowledgments
We thank Dr David B. Wilson for the murine 21-OH promoter and the cDNA of murine Gata4 and Dr A. Fazleabbas for the kind donation of the LHCGR antibody.
Footnotes
Author contributions
N.R. designed the study concept; M.C., S.V. and B.M. performed the experiments; M.C., N.R., A.R.M. and X.L. analyzed and interpreted the results; M.C., N.R., I.H. and J.T. drafted the manuscript.
Funding
This work was supported by grants from the Academy of Finland [grant numbers 254366 to N.R., 253341 to J.T., 137848 to I.H.]; the Wellcome Trust [802101 to I.H.]; Turku University Hospital [personal grant to J.T.]; the Sigrid Juselius Foundation [personal grants to I.H., J.T.]; and the Finnish Cultural Foundation Varsinais-Suomi Regional Fund [personal grants to M.C., N.R.]. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.119347/-/DC1
References
- Anttonen M., Ketola I., Parviainen H., Pusa A. K., Heikinheimo M. (2003). FOG-2 and GATA-4 Are coexpressed in the mouse ovary and can modulate mullerian-inhibiting substance expression. Biol. Reprod. 68, 1333–1340 10.1095/biolreprod.102.008599 [DOI] [PubMed] [Google Scholar]
- Bassett M. H., Mayhew B., Rehman K., White P. C., Mantero F., Arnaldi G., Stewart P. M., Bujalska I., Rainey W. E. (2005). Expression profiles for steroidogenic enzymes in adrenocortical disease. J. Clin. Endocrinol. Metab. 90, 5446–5455 10.1210/jc.2005-0836 [DOI] [PubMed] [Google Scholar]
- Bernichtein S., Petretto E., Jamieson S., Goel A., Aitman T. J., Mangion J. M., Huhtaniemi I. T. (2008). Adrenal gland tumorigenesis after gonadectomy in mice is a complex genetic trait driven by epistatic loci. Endocrinology 149, 651–661 10.1210/en.2007-0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M., Parviainen H., Porter-Tinge S. B., Kiiveri S., Genova E., Rahman N., Huhtaniemi I. T., Muglia L. J., Heikinheimo M., Wilson D. B. (2003). Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology 144, 4123–4133 10.1210/en.2003-0126 [DOI] [PubMed] [Google Scholar]
- Bielinska M., Genova E., Boime I., Parviainen H., Kiiveri S., Leppäluoto J., Rahman N., Heikinheimo M., Wilson D. B. (2005). Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology 146, 3975–3984 10.1210/en.2004-1643 [DOI] [PubMed] [Google Scholar]
- Bugalho M. J., Li X., Rao C. V., Soares J., Sobrinho L. G. (2000). Presence of a Gs alpha mutation in an adrenal tumor expressing LH/hCG receptors and clinically associated with Cushing’s syndrome. Gynecol. Endocrinol. 14, 50–54 10.3109/09513590009167660 [DOI] [PubMed] [Google Scholar]
- Collins T. J. (2007). ImageJ for microscopy. Biotechniques 43 Suppl. 1, 25–30 10.2144/000112517 [DOI] [PubMed] [Google Scholar]
- Dall’Asta C., Ballare E., Mantovani G., Ambrosi B., Spada A., Barbetta L., Colombo P., Travaglini P., Loli P., Beck-Peccoz P. (2004). Assessing the presence of abnormal regulation of cortisol secretion by membrane hormone receptors: in vivo and in vitro studies in patients with functioning and non-functioning adrenal adenoma. Horm. Metab. Res. 36, 578–583 [DOI] [PubMed] [Google Scholar]
- Dunn T. B. (1970). Normal and pathologic anatomy of the adrenal gland of the mouse, including neoplasms. J. Natl. Cancer Inst. 44, 1323–1389 [PubMed] [Google Scholar]
- Feelders R. A., Lamberts S. W., Hofland L. J., van Koetsveld P. M., Verhoef-Post M., Themmen A. P., de Jong F. H., Bonjer H. J., Clark A. J., van der Lely A. J. et al. (2003). Luteinizing hormone (LH)-responsive Cushing’s syndrome: the demonstration of LH receptor messenger ribonucleic acid in hyperplastic adrenal cells, which respond to chorionic gonadotropin and serotonin agonists in vitro. J. Clin. Endocrinol. Metab. 88, 230–237 10.1210/jc.2002-020621 [DOI] [PubMed] [Google Scholar]
- Feng Z. M., Wu A. Z., Zhang Z., Chen C. L. (2000). GATA-1 and GATA-4 transactivate inhibin/activin beta-B-subunit gene transcription in testicular cells. Mol. Endocrinol. 14, 1820–1835 10.1210/me.14.11.1820 [DOI] [PubMed] [Google Scholar]
- Flück C. E., Miller W. L. (2004). GATA-4 and GATA-6 modulate tissue-specific transcription of the human gene for P450c17 by direct interaction with Sp1. Mol. Endocrinol. 18, 1144–1157 10.1210/me.2003-0342 [DOI] [PubMed] [Google Scholar]
- Forest M. G. (2004). Recent advances in the diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum. Reprod. Update 10, 469–485 10.1093/humupd/dmh047 [DOI] [PubMed] [Google Scholar]
- Goodarzi M. O., Dawson D. W., Li X., Lei Z., Shintaku P., Rao C. V., Van Herle A. J. (2003). Virilization in bilateral macronodular adrenal hyperplasia controlled by luteinizing hormone. J. Clin. Endocrinol. Metab. 88, 73–77 10.1210/jc.2002-021292 [DOI] [PubMed] [Google Scholar]
- Guilhaume B., Sanson M. L., Billaud L., Bertagna X., Laudat M. H., Luton J. P. (1992). Cushing’s syndrome and pregnancy: aetiologies and prognosis in twenty-two patients. Eur. J. Med. 1, 83–89 [PubMed] [Google Scholar]
- Haavisto A. M., Pettersson K., Bergendahl M., Perheentupa A., Roser J. F., Huhtaniemi I. (1993). A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 132, 1687–1691 10.1210/en.132.4.1687 [DOI] [PubMed] [Google Scholar]
- Hofmann F. G., Dickie M. M., Christy N. P. (1960). Studies of gonadectomized mice bearing adrenal cortical tumours. Acta Endocrinol. (Copenh.) 34, 84–96 [DOI] [PubMed] [Google Scholar]
- Jimenez P., Saner K., Mayhew B., Rainey W. E. (2003). GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology 144, 4285–4288 10.1210/en.2003-0472 [DOI] [PubMed] [Google Scholar]
- Kananen K., Markkula M., Mikola M., Rainio E. M., McNeilly A., Huhtaniemi I. (1996). Gonadectomy permits adrenocortical tumorigenesis in mice transgenic for the mouse inhibin alpha-subunit promoter/simian virus 40 T-antigen fusion gene: evidence for negative autoregulation of the inhibin alpha-subunit gene. Mol. Endocrinol. 10, 1667–1677 10.1210/me.10.12.1667 [DOI] [PubMed] [Google Scholar]
- Keeney D. S., Jenkins C. M., Waterman M. R. (1995). Developmentally regulated expression of adrenal 17 alpha-hydroxylase cytochrome P450 in the mouse embryo. Endocrinology 136, 4872–4879 10.1210/en.136.11.4872 [DOI] [PubMed] [Google Scholar]
- Kero J., Poutanen M., Zhang F. P., Rahman N., McNicol A. M., Nilson J. H., Keri R. A., Huhtaniemi I. T. (2000). Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J. Clin. Invest. 105, 633–641 10.1172/JCI7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiveri S., Liu J., Westerholm-Ormio M., Narita N., Wilson D. B., Voutilainen R., Heikinheimo M. (2002a). Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue. Endocrinology 143, 3136–3143 10.1210/en.143.8.3136 [DOI] [PubMed] [Google Scholar]
- Kiiveri S., Liu J., Westerholm-Ormio M., Narita N., Wilson D. B., Voutilainen R., Heikinheimo M. (2002b). Transcription factors GATA-4 and GATA-6 during mouse and human adrenocortical development. Endocr. Res. 28, 647–650 10.1081/ERC-120016980 [DOI] [PubMed] [Google Scholar]
- Kiiveri S., Liu J., Heikkilä P., Arola J., Lehtonen E., Voutilainen R., Heikinheimo M. (2004). Transcription factors GATA-4 and GATA-6 in human adrenocortical tumors. Endocr. Res. 30, 919–923 10.1081/ERC-200044149 [DOI] [PubMed] [Google Scholar]
- Kiiveri S., Liu J., Arola J., Heikkilä P., Kuulasmaa T., Lehtonen E., Voutilainen R., Heikinheimo M. (2005). Transcription factors GATA-6, SF-1, and cell proliferation in human adrenocortical tumors. Mol. Cell. Endocrinol. 233, 47–56 10.1016/j.mce.2005.01.012 [DOI] [PubMed] [Google Scholar]
- Kiiveri S., Siltanen S., Rahman N., Bielinska M., Lehto V. P., Huhtaniemi I. T., Muglia L. J., Wilson D. B., Heikinheimo M. (1999). Reciprocal changes in the expression of transcription factors GATA-4 and GATA-6 accompany adrenocortical tumorigenesis in mice and humans. Mol. Med. 5, 490–501 [PMC free article] [PubMed] [Google Scholar]
- Krachulec J., Vetter M., Schrade A., Löbs A. K., Bielinska M., Cochran R., Kyrönlahti A., Pihlajoki M., Parviainen H., Jay P. Y. et al. (2012). GATA4 is a critical regulator of gonadectomy-induced adrenocortical tumorigenesis in mice. Endocrinology 153, 2599–2611 10.1210/en.2011-2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix A., Hamet P., Boutin J. M. (1999). Leuprolide acetate therapy in luteinizing hormone—dependent Cushing’s syndrome. N. Engl. J. Med. 341, 1577–1581 10.1056/NEJM199911183412104 [DOI] [PubMed] [Google Scholar]
- Li Q., Graff J. M., O’Connor A. E., Loveland K. L., Matzuk M. M. (2007). SMAD3 regulates gonadal tumorigenesis. Mol. Endocrinol. 21, 2472–2486 10.1210/me.2007-0147 [DOI] [PubMed] [Google Scholar]
- Looyenga B. D., Hammer G. D. (2006). Origin and identity of adrenocortical tumors in inhibin knockout mice: implications for cellular plasticity in the adrenal cortex. Mol. Endocrinol. 20, 2848–2863 10.1210/me.2006-0182 [DOI] [PubMed] [Google Scholar]
- Looyenga B. D., Hammer G. D. (2007). Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery. Mol. Endocrinol. 21, 2440–2457 10.1210/me.2006-0402 [DOI] [PubMed] [Google Scholar]
- Looyenga B., Beuschlein F., Nilson J., Hammer G. D. (2004). Mechanistic roles of inhibin as a tumor suppressor in the adrenal cortex. Endocr. Res. 30, 585–586 10.1081/ERC-200043745 [DOI] [PubMed] [Google Scholar]
- Looyenga B. D., Wiater E., Vale W., Hammer G. D. (2010). Inhibin-A antagonizes TGFbeta2 signaling by down-regulating cell surface expression of the TGFbeta coreceptor betaglycan. Mol. Endocrinol. 24, 608–620 10.1210/me.2008-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. R., McKinsey T. A., Xu H., Wang D. Z., Richardson J. A., Olson E. N. (1999). FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol. Cell. Biol. 19, 4495–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuco T. L., Chabre O., Feige J. J., Thomas M. (2006). Aberrant expression of human luteinizing hormone receptor by adrenocortical cells is sufficient to provoke both hyperplasia and Cushing’s syndrome features. J. Clin. Endocrinol. Metab. 91, 196–203 10.1210/jc.2005-1975 [DOI] [PubMed] [Google Scholar]
- Mijnhout G. S., Danner S. A., van de Goot F. R., van Dam E. W. (2004). Macronodular adrenocortical hyperplasia in a postmenopausal woman. Neth. J. Med. 62, 454–455 [PubMed] [Google Scholar]
- Mikola M., Kero J., Nilson J. H., Keri R. A., Poutanen M., Huhtaniemi I. (2003). High levels of luteinizing hormone analog stimulate gonadal and adrenal tumorigenesis in mice transgenic for the mouse inhibin-alpha-subunit promoter/Simian virus 40 T-antigen fusion gene. Oncogene 22, 3269–3278 10.1038/sj.onc.1206518 [DOI] [PubMed] [Google Scholar]
- Mitani F., Mukai K., Miyamoto H., Suematsu M., Ishimura Y. (2003). The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim. Biophys. Acta 1619, 317–324 10.1016/S0304-4165(02)00490-7 [DOI] [PubMed] [Google Scholar]
- Miyamura N., Taguchi T., Murata Y., Taketa K., Iwashita S., Matsumoto K., Nishikawa T., Toyonaga T., Sakakida M., Araki E. (2002). Inherited adrenocorticotropin-independent macronodular adrenal hyperplasia with abnormal cortisol secretion by vasopressin and catecholamines: detection of the aberrant hormone receptors on adrenal gland. Endocrine 19, 319–326 10.1385/ENDO:19:3:319 [DOI] [PubMed] [Google Scholar]
- Miyamura N., Tsutsumi A., Senokuchi H., Nakamaru K., Kawashima J., Sakai K., Taguchi T., Tokunaga H., Nishida K., Uehara M. et al. (2003). A case of ACTH-independent macronodular adrenal hyperplasia: simultaneous expression of several aberrant hormone receptors in the adrenal gland. Endocr. J. 50, 333–340 10.1507/endocrj.50.333 [DOI] [PubMed] [Google Scholar]
- Morley S. D., Viard I., Chung B. C., Ikeda Y., Parker K. L., Mullins J. J. (1996a). Variegated expression of a mouse steroid 21-hydroxylase/beta-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol. Endocrinol. 10, 585–598 10.1210/me.10.5.585 [DOI] [PubMed] [Google Scholar]
- Morley S. D., Viard I., Parker K. L., Mullins J. J. (1996b). Adrenocortical-specific transgene expression directed by steroid hydroxylase gene promoters. Endocr. Res. 22, 631–639 [DOI] [PubMed] [Google Scholar]
- Murthy A. S., Brezak M. A., Baez A. G. (1970). Postcastrational adrenal tumors in two strains of mice: morphologic, histochemical, and chromatographic studies. J. Natl. Cancer Inst. 45, 1211–1222 [PubMed] [Google Scholar]
- Nakamura T., Asashima M., Eto Y., Takio K., Uchiyama H., Moriya N., Ariizumi T., Yashiro T., Sugino K., Titani K. et al. (1992). Isolation and characterization of native activin B. J. Biol. Chem. 267, 16385–16389 [PubMed] [Google Scholar]
- Pabon J. E., Li X., Lei Z. M., Sanfilippo J. S., Yussman M. A., Rao C. V. (1996). Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J. Clin. Endocrinol. Metab. 81, 2397–2400 10.1210/jc.81.6.2397 [DOI] [PubMed] [Google Scholar]
- Rahman N. A., Kiiveri S., Rivero-Müller A., Levallet J., Vierre S., Kero J., Wilson D. B., Heikinheimo M., Huhtaniemi I. (2004). Adrenocortical tumorigenesis in transgenic mice expressing the inhibin alpha-subunit promoter/simian virus 40 T-antigen transgene: relationship between ectopic expression of luteinizing hormone receptor and transcription factor GATA-4. Mol. Endocrinol. 18, 2553–2569 10.1210/me.2002-0282 [DOI] [PubMed] [Google Scholar]
- Rilianawati, Paukku T., Kero J., Zhang F. P., Rahman N., Kananen K., Huhtaniemi I. (1998). Direct luteinizing hormone action triggers adrenocortical tumorigenesis in castrated mice transgenic for the murine inhibin alpha-subunit promoter/simian virus 40 T-antigen fusion gene. Mol. Endocrinol. 12, 801–809 10.1210/me.12.6.801 [DOI] [PubMed] [Google Scholar]
- Risma K. A., Clay C. M., Nett T. M., Wagner T., Yun J., Nilson J. H. (1995). Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc. Natl. Acad. Sci. USA 92, 1322–1326 10.1073/pnas.92.5.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Müller A., Lajić S., Huhtaniemi I. (2007). Assisted large fragment insertion by Red/ET-recombination (ALFIRE)—an alternative and enhanced method for large fragment recombineering. Nucleic Acids Res. 35, e78 10.1093/nar/gkm250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert N. M., Tremblay J. J., Viger R. S. (2002). Friend of GATA (FOG)-1 and FOG-2 differentially repress the GATA-dependent activity of multiple gonadal promoters. Endocrinology 143, 3963–3973 10.1210/en.2002-220280 [DOI] [PubMed] [Google Scholar]
- Rosner J. M., Charreau E., Houssay A. B., Epper C. (1966). Biosynthesis of sexual steroids by hyperplastic adrenal glands of castrated female C3H/Ep mice. Endocrinology 79, 681–686 10.1210/endo-79-4-681 [DOI] [PubMed] [Google Scholar]
- Russfield A. B. (1975). Experimental endocrinopathies. Methods Achiev. Exp. Pathol. 7, 132–148 [PubMed] [Google Scholar]
- Schmelzer C. H., Burton L. E., Tamony C. M., Schwall R. H., Mason A. J., Liegeois N. (1990). Purification and characterization of recombinant human activin B. Biochim. Biophys. Acta 1039, 135–141 10.1016/0167-4838(90)90178-I [DOI] [PubMed] [Google Scholar]
- Schrewe H., Gendron-Maguire M., Harbison M. L., Gridley T. (1994). Mice homozygous for a null mutation of activin beta B are viable and fertile. Mech. Dev. 47, 43–51 10.1016/0925-4773(94)90094-9 [DOI] [PubMed] [Google Scholar]
- Simon D. P., Hammer G. D. (2012). Adrenocortical stem and progenitor cells: implications for adrenocortical carcinoma. Mol. Cell. Endocrinol. 351, 2–11 10.1016/j.mce.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E. C., Huggins G. S., Dardik F. B., Polk C. E., Leiden J. M. (2000). A functionally conserved N-terminal domain of the friend of GATA-2 (FOG-2) protein represses GATA4-dependent transcription. J. Biol. Chem. 275, 20762–20769 10.1074/jbc.M001522200 [DOI] [PubMed] [Google Scholar]
- Tevosian S. G., Deconinck A. E., Tanaka M., Schinke M., Litovsky S. H., Izumo S., Fujiwara Y., Orkin S. H. (2000). FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell 101, 729–739 10.1016/S0092-8674(00)80885-5 [DOI] [PubMed] [Google Scholar]
- Tsuchida K., Nakatani M., Yamakawa N., Hashimoto O., Hasegawa Y., Sugino H. (2004). Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol. Cell. Endocrinol. 220, 59–65 10.1016/j.mce.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Tsuchida K., Nakatani M., Hitachi K., Uezumi A., Sunada Y., Ageta H., Inokuchi K. (2009). Activin signaling as an emerging target for therapeutic interventions. Cell Commun. Signal. 7, 15 10.1186/1478-811X-7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorenoja S., Rivero-Muller A., Kiiveri S., Bielinska M., Heikinheimo M., Wilson D. B., Huhtaniemi I. T., Rahman N. A. (2007). Adrenocortical tumorigenesis, luteinizing hormone receptor and transcription factors GATA-4 and GATA-6. Mol. Cell. Endocrinol. 269, 38–45 10.1016/j.mce.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Vuorenoja S., Mohanty B. P., Arola J., Huhtaniemi I., Toppari J., Rahman N. A. (2009). Hecate-CGbeta conjugate and gonadotropin suppression shows two distinct mechanisms of action in the treatment of adrenocortical tumors in transgenic mice expressing Simian Virus 40 T antigen under inhibin-alpha promoter. Endocr. Relat. Cancer 16, 549–564 10.1677/ERC-08-0232 [DOI] [PubMed] [Google Scholar]
- Wy L. A., Carlson H. E., Kane P., Li X., Lei Z. M., Rao C. V. (2002). Pregnancy-associated Cushing’s syndrome secondary to a luteinizing hormone/human chorionic gonadotropin receptor-positive adrenal carcinoma. Gynecol. Endocrinol. 16, 413–417 [PubMed] [Google Scholar]
- Zhang F. P., Poutanen M., Wilbertz J., Huhtaniemi I. (2001). Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol. Endocrinol. 15, 172–183 10.1210/me.15.1.172 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Muyrers J. P., Rientjes J., Stewart A. F. (2003). Phage annealing proteins promote oligonucleotide-directed mutagenesis in Escherichia coli and mouse ES cells. BMC Mol. Biol. 4, 1 10.1186/1471-2199-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.