Abstract
Functional plant traits are likely to adapt under the sustained pressure imposed by environmental changes through natural selection. Employing Brassica napus as a model, a multi-generational study was performed to investigate the potential trajectories of selection at elevated [CO2] in two different temperature regimes. To reveal phenotypic divergence at the manipulated [CO2] and temperature conditions, a full-factorial natural selection regime was established in a phytotron environment over the range of four generations. It is demonstrated that a directional response to selection at elevated [CO2] led to higher quantities of reproductive output over the range of investigated generations independent of the applied temperature regime. The increase in seed yield caused an increase in aboveground biomass. This suggests quantitative changes in the functions of carbon sequestration of plants subjected to increased levels of CO2 over the generational range investigated. The results of this study suggest that phenotypic divergence of plants selected under elevated atmospheric CO2 concentration may drive the future functions of plant productivity to be different from projections that do not incorporate selection responses of plants. This study accentuates the importance of phenotypic responses across multiple generations in relation to our understanding of biogeochemical dynamics of future ecosystems. Furthermore, the positive selection response of reproductive output under increased [CO2] may ameliorate depressions in plant reproductive fitness caused by higher temperatures in situations where both factors co-occur.
Keywords: Carbon dioxide, experimental evolution, global change, laboratory natural selection, oilseed rape, phenotypic divergence, plant-environment feedbacks
Introduction
Recent and projected future alterations of environmental features cause sustained pressures on global ecosystem functioning (Fischlin et al. 2007). Important environmental parameters are currently changing at an unprecedented pace as a consequence of human activities (Bell and Collins 2008). Where the plastic response potential of a population cannot fully compensate stressful changes in environmental conditions, only evolutionary adaptation can prevent wide-ranging declines in fitness and counter the increased risk of extinction (Jump and Peñuelas 2005). Especially for sessile organisms like plants, where migration likely fails to track the speed and magnitude of environmental change, adaptive responses will be of eminent importance.
The atmospheric carbon dioxide concentration – [CO2] – is one of the most prominent environmental parameters currently changing. By the end of the 21st century, [CO2] is likely to become more than doubled compared with pre-industrial concentrations (Swart et al. 2002). [CO2] is also inherently coupled to, and a strong driver of global temperature regimes (Forster et al. 2007). Hence, simultaneously to the increase in [CO2], global average temperature is projected to increase by 1.8 to 4.0°C compared to the values at the beginning of the century (Carter et al. 2007). Both [CO2] and temperature are documented as potent agents of directional selection in plant populations (e.g., Ward and Kelly 2004; Jump and Peñuelas 2005; Reusch and Wood 2007). Both factors affect the allocation of resources to components of plant fitness by response functions, which vary between individuals of a given population. These genotype-environment interactions are the basis for natural selection to act in intra-specific competition regimes and facilitate micro-evolutionary adaptation responses.
Adaption of phenotypes over a range of generations may lead to future biogeochemical dynamics and long-term trends of plant fitness, which are different from projections assuming evolutionary stasis (Bazzaz et al. 1995; Ward and Kelly 2004). In order to improve our understanding of future ecosystem structure, functioning, and biodiversity patterns, we need to evaluate the potential and nature of plant responses to natural selection in plants to the changes projected.
Within-generation estimates of adaptive evolution provide valuable information for species with a long generation time and/or selective processes under natural conditions. However, such estimates might lead to only incomplete and relatively limited conclusions with respect to evolutionary trajectories (Thomas and Jasienski 1996; Ward and Kelly 2004; Reusch and Wood 2007; Fuller et al. 2009). Selection experiments incorporating several generations provide a unique opportunity to evaluate and predict evolutionary responses of populations to projected changes in environments (Reusch and Wood 2007). Hence, multi-generational selection experiments will play a key role in understanding the many challenges that global environmental change will present to biodiversity and ecosystem functioning in the future (Bell and Gonzalez 2009). Until now, experimental evidence on the nature and direction of selection responses of phenotypic traits under simulated future conditions is very limited and rare. Exacerbating the limitations in our understanding of plant evolutionary responses to environmental change, the results of some of the very few studies conducted so far are biased by the applied methodology. The abundance of studies available applied artificial selection regimes to generate treatment-specific generational progress. A procedure like this may, however, interrupt the integrity of trade-offs between multiple fitness-related traits. Avoiding these methodological biases, natural selection experiments are proposed as “gold standard” to investigate the evolutionary trajectories under manipulated environmental conditions (“laboratory natural selection”, cf. Reusch and Wood 2007; Fuller et al. 2009). In natural selection experiments, populations freely adapt by fertility and mortality distributions, similar to natural conditions.
Here, we present the results of a natural selection experiment applying elevated [CO2] under two different temperature regimes. The response of biomass production and reproductive output to the selection regimes imposed by these environmental manipulations was investigated over a range of four generations. This study aimed to reveal if the manipulation of CO2 and temperature regimes is able to provide selection pressures, which are strong enough to drive phenotypic divergence over a short range of progressing generations. The experiment investigated Brassica napus as model species, because the species has demonstrated to complete its life cycle under laboratory and artificial light conditions within a comprehensible generation time. Explained by its crop identity, B. napus might experience less profound effects by the artificial growing conditions than more natural plant species, as it is already habituated to be partially detached from some ecological functions like, for example, a natural nutrient supply and interspecific interactions.
Higher plants, in their role as primary producers, crucially drive the budgets of ecologically imported cycles (e.g., carbon, nitrogen, and water). Hence, they are also major players in controlling and steering the global climate system by a multitude of feedback responses, among which the connections between carbon cycle and climate might amplify or dampen regional and global climate changes (Heimann and Reichstein 2008). It was investigated here, if phenotypic responses to the applied selective agents may point at potential uncertainties in our current assessments of future biogeochemical dynamics (e.g., carbon uptake) introduced by progressing phenotypic adaptation.
Methods
Environmental manipulations
Brassica napus L. plants were grown in four different environments varying in atmospheric [CO2] (ca. 390 vs. 650 ppm) and air temperature regimes (ca. 19/12 vs. 24/17°C) with these environmental manipulations imposed individually or in combination (Fig. 1, Table 1). Treatments were accommodated in four gastight chambers (width 6 m, depth 4 m, height 3 m) integrated in a phytotron facility at the Technical University of Denmark, DK (RERAF – Risø Environmental Risk Assessment Facility). The growth chamber system allowed individual control of air flow, light, temperature, humidity, [CO2], [O3], and watering. Twenty-eight high-pressure mercury and 14 halogen lamps (1000 W and 400 W, respectively) per chamber were used to generate a 16/8 hours day–night light regime. During daytime conditions, average photosynthetic active radiation (PAR) on top of the canopy was 520 μmolPhotons m−2 s−1 (measured with a Li-Cor LI-250A light meter, Li-Cor, USA). At the beginning of the daytime period, plants were watered by a drip irrigation system providing 4.4 L m−2 day−1 delivered to the individual pots. During early developmental stages, when water uptake was still low, water exceeding field capacity was allowed to drain from the soil phase. The amount of water delivered was decreased at the beginning of leaf senescence (approximately within the 3rd month after planting) in a stepwise fashion to facilitate and promote complete maturation and seed ripening. A relative humidity of 55/70% (day/night, measured with a HMP231 humidity and temperature transmitter, Vaisala, Finland) and a realistic background ozone concentration of 18.1 ppb (±15.5 ppb standard deviation, measured with the 400E ozone monitor model from Teledyne, USA) were established in the growth chambers. To prevent pathogen interference in the enclosed system (Long et al. 2006), a fungicide treatment (CANTUS®, BASF AG, Germany) was applied to all plants every second week during the first month of development. All of the manipulated environmental conditions are statistically summarized in Table 1.
Figure 1.
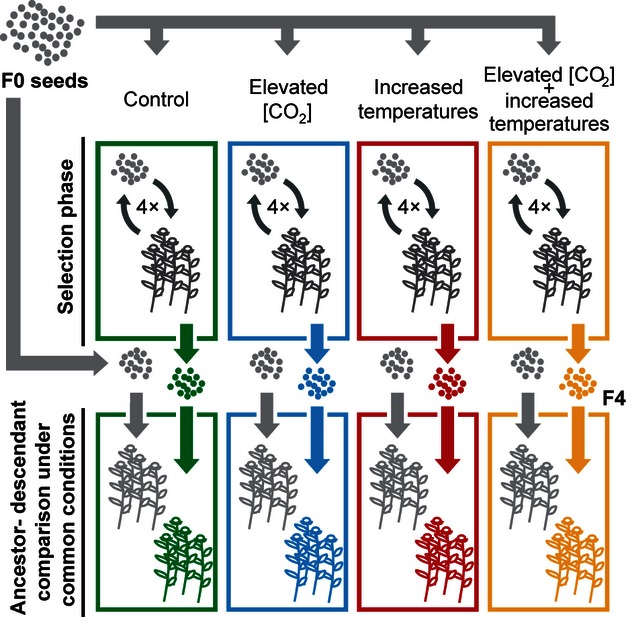
Schematic illustration of the selection experiment. Brassica napus populations were selected over four generations under manipulated experimental conditions, before descendent material was grown together with ancestral stock populations sharing a common environmental configuration. Experimental procedures are only represented for one of the four replicate selection linages (RSL) in each treatment.
Table 1.
Summary of manipulated environmental conditions for the four treatments and temporal delayed groups of cultivation in this study: daily mean values ± their standard deviation are summarized here. Filled symbols indicate the application of elevated carbon dioxide concentration ([CO2]) or higher temperature (Temp.) regime; open symbols indicate lower [CO2] and temperature conditions
| Group 1 | Group 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | [CO2] [ppm] | Temp. [°C] | [CO2] [ppm] | Temp. [°C] | |||||||||
| [CO2] | Temp. | Day | Day | Night | Day | Day | Night | ||||||
| □ | □ | 391.3 | ±20.1 | 18.5 | ±0.9 | 12.8 | ±1.0 | 391.8 | ±21.2 | 18.6 | ±0.7 | 13.0 | ±0.9 |
| ▪ | □ | 656.5 | ±41.5 | 18.4 | ±1.1 | 12.7 | ±0.8 | 660.2 | ±40.6 | 18.3 | ±1.0 | 12.8 | ±0.8 |
| □ | ▪ | 386.1 | ±11.3 | 23.4 | ±0.9 | 17.8 | ±0.8 | 389.9 | ±14.2 | 23.2 | ±0.9 | 18.0 | ±0.8 |
| ▪ | ▪ | 648.2 | ±54.9 | 23.5 | ±0.6 | 17.8 | ±0.8 | 650.5 | ±55.2 | 23.4 | ±0.6 | 18.0 | ±0.7 |
Plant material and generational progress
Four parallel selection lineages were initiated in each of the four treatments. Each linage was based on one of four different accessions of B. napus L. (Table 2, hereafter referred to as replicate selection linage – RSL). Each of the four RSLs in every treatment consisted of 36 individuals. At the beginning of the experiment, the first generation of plants (F0) was grown from seeds until maturity and harvested. In the absence of animal pollinators, B. napus plants of a given RSL were gently shaken during flowering to promote within-population cross-pollination by physical contact. The different RSLs in every treatment were kept spatially separated as they were grown on individual, moveable tables.
Table 2.
Origin, year of release, breeding organization, and distribution area of the four Brassica napus accessions founding the four replicate selection linages (RSL) of the experiment
| Accession | Origin | Year of release | Breeder | Distribution area |
|---|---|---|---|---|
| Bolero | Germany | 1997 | Raps GbR | Southern & eastern Europe |
| Mary | Denmark | 1978 | DLF Trifolium A/S | Denmark |
| Mozart | Germany | 1999 | NPZ/Lembke | Baltic countries |
| Tanto | France | 1990 | INRA/Serasem | No information available |
At the end of each cultivation cycle, the plant material was post-harvest processed as described below. Treatment-specific generational progress (F1, F2, F3) was established for each individual RSL-treatment combination (Fig. 1): Descendent populations of each RSL, F(g + 1), were based on seeds chosen randomly out of the pooled seed stock of its corresponding ancestor population – F(g), and were grown under the same environmental conditions as the parental population of a given RSL (Fig. 1).
During the final cycle of cultivation, the plants selected under treatment conditions for fourth generations, F4 plants of every RSL, were grown simultaneously with plants derived from the initial seed stock (F0) in every treatment. For this last cycle of cultivation, plants were grown in two batches, which were temporally displaced by 28 days (referred as groups within the description of statistical procedures). RSLs were randomly assigned to each of the two groups as a set of two – while the F0 population and F4 generation for each RSL were kept together, sharing identical environmental conditions during their development.
The control groups of this experiment were defined based on the propositions for the experimental design of laboratory natural selection experiments made by Fuller et al. (2009): (a) The control selection linages were maintained under the same demographic and environmental conditions as within the selective regimes, except for the environmental manipulations. (b) The evolved descendants were compared to the ancestral stock population. The original seed stock was maintained under long-term storage conditions throughout the duration of selection phase of the experiment (Fig. 1) implying evolutionary stasis. This procedure allowed separating directional changes unrelated to the selection regimes imposed by environmental manipulations form changes caused by the manipulated factor.
Conditions of cultivation
Plants were cultivated in 11 L pots each filled with 4 kg of a standard sphagnum substrate (Pindstrup Substrate No. 6, Pindstrup Mosebrug A/S, Denmark). The soil substrate in each pot was supplemented with 10 g NPK fertilizer (21-3-10, Kemira Denmark A/S). A competition regime for belowground resources, including water, nutrients, and space was created for sets of four plants sharing one pot. Plant density was established to be 64 individuals per m2 and equally distributed over the experimental populations. The experimental populations were restricted from growing over the defined spatial margins, by a white, 4-mm meshed polyethylene net of 30-cm height, thereby also reducing lateral irradiation-induced border effects at the edges of the population.
To avoid unilateral impacts, chamber-specific biases, and to minimize confounding effects of micro-environmental variation within the chambers, the tables holding the plants and the corresponding treatments were relocated among the different chambers of the phytotron facility every week together with their corresponding treatments. At this time, the relative position of a given population within a chamber was also changed.
Harvests
All plants were grown in the experimental environments until leaf senescence, silique, and seed ripening indicated life-cycle completion. Final harvests were performed at 109 and 116 days after planting for plants grown at elevated and lower temperature, respectively. At the final harvest, all individuals of the population were harvested as a pot-wise pooled sample, leading to nine replicate harvest samples for each experimental population. At that time, silique, stem, and leaf fractions were segregated. Siliques were dried in a force ventilated oven at 36°C (TU2, Heraeus, Germany). Leaf and stem material were first dried under ventilated room conditions before residual moisture was removed during an oven incubation at 72°C. After drying, seed and silique material were separated. Subsequently, dry weights were determined for all aboveground biomass fractions. The weight of a 100 seeds sub-sample, as enumerated by an automatic seed counter (Numigral, Sinar Technologies, UK), was determined in order to derive total seed number (Seed No.). Subsequently, mean per plant values were calculated for all response variables.
Data analysis and statistical procedures
All statistical procedures and calculations were performed in R (version 2.11.1; R Development Core Team, 2010). Individual linear mixed-effects models were fitted for each response parameter (Pinheiro and Bates 2000). The concentration of CO2 and temperature regime in the experimental environments, the number of generations, and all possible interactions among those three categorical, explanatory variables were modeled as fixed effects for the initial model. Group, treatment and RSL, with a hierarchical grouping structure according to the order they are mentioned here, defined the random intercept of the initial mixed-effects model. Treatment was included as grouping variable in order to nest error within the plants sharing a particular treatment and to prevent elevation of Type I error for the effects of environmental manipulations. To elucidate the general, species-level responses across the different RSL independent from effects imposed by their distinct genetic identity, all effects were analyzed as nested into RSL.
Linear mixed-effects models were simplified in a stepwise fashion: The initial maximal model was first subjected to a backward fitting algorithm for the fixed effects. In reverse order to the degree of interactions, terms were removed in the model at a threshold of 0.05 for its lower bound P-value and the P-value for the log-likelihood ratio test between the complex and the simplified model version (cf. Tremblay 2012). Non-significant terms involved in significant higher order interactions always remained as part of the model structure. Subsequently, random slopes for the fixed effects, which remained in the most parsimonious model structure, were tested for their significance and included into the random effects definition of the model if the P-values for the log-likelihood ratio test between the complex and the simpler model was smaller than 0.05. The model definition finally achieved by this procedure was confirmed by a visually inspection of residual and quantile distribution according to the criteria for parametric statistical tests. The model summaries for each response variable are given in Table A1.
Results
All measured biomass fractions responded to the applied environmental manipulations. Final aboveground dry weight (AG DW) of B. napus plants was strongly affected by the CO2 and temperature regimes. However, the different plant organs responded differentially to the individual combinations of environmental manipulations (Fig. 2). Brassica napus plants increased AG DW accumulation in response to elevated [CO2] in the present experiment (P < 0.001, Fig. 2A). For the F0 generation, the [CO2]-related increase in AG DW was 22% and 14% in average at ambient and elevated temperature, respectively. Concurrent with the responses of total AG DW to [CO2], vegetative fractions of leaf and stem material increased under high [CO2] growing conditions (leaves: P = 0.0063, stems: P = 0.003, Fig. 2B and C). The seed fraction of B. napus did reveal no significant response to the applied [CO2] conditions.
Figure 2.
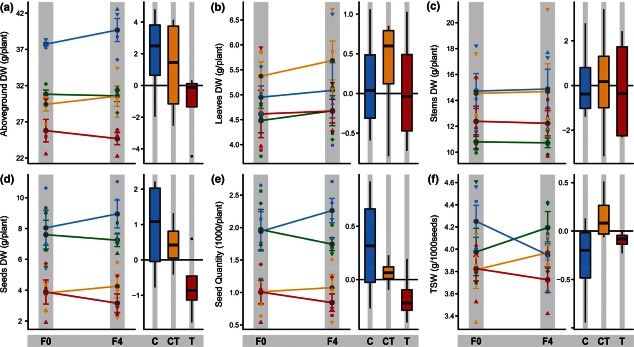
Mean values (±standard error) of biomasses fractions at final harvest determined for the four replicate selection linages of Brassica napus grown in a phytotron environment at the four possible combinations of two temperatures and [CO2] regimes (Table 1) in two generations: (a) aboveground DW, (b) Leaves DW, (c) Stems DW, (d) Seeds DW, (e) Seed Quantity, (f) thousand seed weight (TSW) F0 – ancestral stock population, F4 – fourth descendant population selected in the corresponding environments – gray-filled, round symbols connected by a line. Treatments: green – Ctrl (control), blue – elevated [CO2] (C), red – increased temperatures (T), orange – elevated [CO2] and increased temperatures (CT). Point characters of the same shape represent RSLs with the same identity. Boxplots show the difference in slopes between the trans-generational response in the given treatments and the control scenario for the four RSL: (trait value meanF4 – trait value mean F0)C, CT, T – (trait value meanF4 – trait value mean F0)Ctrl, DW, dry weight; TSW, thousand seed weight.
In contrast to the response pattern seen under elevated [CO2], higher temperatures led to reductions in final AG DW in B. napus (P < 0.001, Fig. 2A). The decline in total AG DW under elevated temperatures was aligned with decreasing weights of the seed fraction (P < 0.001), while leaf and stem biomass did not reveal a response to the applied temperature regime. Under both concentrations of CO2, the DW of seeds in B. napus was significantly reduced in response to higher temperatures (Fig. 2D). The temperature-driven depression in seed number per plant was ∼50% and showing similar reductions under both [CO2] regimes (Fig. 2E).
As the effect of temperature on the sum of all AG biomass fractions had a larger magnitude than the promoting effect of high [CO2], AG DW tended to be slightly reduced when [CO2] and temperature were elevated concurrently compared with the corresponding control conditions (Fig. 2A).
The cultivation of B. napus in the experimental environments altered its responsiveness over the range of generations investigated here. As a specific response to the CO2 concentration, AG DW (P = 0.0118), DW of seeds (P = 0.0017), and their total number (P = 0.0624) were different between the ancestral stock population (F0) and the populations selected in the manipulated environments over four generations (F4, Fig. 2A, D and E). Throughout the multi-generational cultivation, the sum of accumulated AG biomass deviated to higher values from F0 to F4 in high [CO2] environments compared to the pattern found under ambient [CO2] (Fig. 2A). Under high [CO2] growing conditions, AG DW increased ∼4.8% and 4.1% at concurrently elevated temperatures, respectively, from F0 to F4. This CO2-specific response between the start (F0) and final offspring generation (F4) of the experiment significantly contrasts the decreasing of 0.7% and 3.4%, respectively, under low [CO2] conditions. Hence, throughout the trans-generational cultivation process, plants achieving higher final AG DW were selectively favored in environments applying the elevated [CO2] regime. This trans-generational alteration of the final aboveground biomass, which was found specifically in high [CO2] environments, was associated with a similar pattern of response in the seed fraction, whereas vegetative fractions, stems, and leaves, did not reveal any environment specific responses to the selection process. Concurrent to the pattern found for AG DW, the trans-generational alteration in the quantity of seeds produced per plant was revealed to be different according to the two CO2 concentrations investigated here. The absolute differentials of increase in seed yields from the F0 to the F4 generation appeared larger under lower temperature conditions (Fig. 2D and E). Hence, under elevated [CO2], reproductive output was increased in numbers and total weights in response to the selection regime established by elevated [CO2] compared to the pattern found at ambient CO2 concentrations (Fig. 2D and E). However, although individual seed weight was reduced from F0 to F4 in the elevated CO2 environment, applying the lower temperature regime, the pattern under increased temperatures was significantly different (P = 0.0196) and revealed an increase in the weight of individual seeds (Fig. 2F).
Within the present experiment, no differences were detected for any biomass fraction specifically to the applied temperature regimes within the generational range of this study.
Discussion
The causes and implications of the promoting effect of [CO2] on plant productivity are well understood and described for numerous plant species and ecosystems (Ainsworth and Rogers 2007). In consistency with earlier greenhouse and free air CO2 enrichment studies (FACE, Qaderi et al. 2006; Franzaring et al. 2008), vegetative biomass in B. napus was increased under elevated [CO2]. The lack of [CO2]-response for seeds was earlier explained by restrictions in the sink capacity of the reproductive apparatus in B. napus (Reekie et al. 1998). An additional reduction in the sink potential of reproductive organs might be caused by the adverse effects of increased canopy temperatures at elevated [CO2] (Fuhrer 2003) as explained below. This evidence already suggests that an increase in sink strength of reproductive organs has the potential to enhance the gain in biomass productivity caused by high [CO2] in B. napus.
The decline of AG DW under elevated temperatures was associated with a decrease in reproductive productivity while other biomass fractions remained unaffected. Supra-optimal temperatures adversely affect functions of reproductive development on multiple levels (cf. Barnabás et al. 2008 for review). Consequently, increased temperatures reduce the sink strength of the reproductive apparatus in particular, while reducing the source potential of plants at the same time (Stone 2001).
The direct and negative effect of temperature on reproductive fitness demonstrates the strong selection pressure imposed by non-optimal thermal conditions, which resulted in frequent differentiations of natural populations with respect to temperature (Jump and Peñuelas 2005). In turn, rapidly changing climatic conditions have the potential to overwhelm the plastic response potential, and cause fast declines in the fitness of natural populations. If fitness cannot be maintained or restored by an adaptation to the altered conditions, the fast changes in environmental characteristics may increase the risk of local extinctions as migration rates are often likely to be insufficient for tracking the climatic conditions in which plants are currently adapted to (cf. Jump and Peñuelas 2005 for review). A study performed by Franks et al. (2007) recently provided an example of Brassica rapa responding adaptively to a natural multi-year drought in just a small number of generations. The evolved descendants flowered earlier to adapt their development to the drought abbreviated growing season.
In annual plants, seed production has to be maximized within a single growing season, as the recruitment of new individuals is only possible from seeds. Hence, reproductive output and fecundity become central and major components of fitness in annuals (Cheplick 2005; Hirose et al. 2005). Consequently, for an intra-specific comparison, it can be assumed that the genotype with higher reproductive quantities is likely to be the one with the higher fitness, and the one better adapted to the environment of comparison. This, however, only holds true, if no trade-offs with other fitness-related traits are implied and the competition and resource regime can be considered constant, like in the experimental setup presented here.
In our study, increased numbers and total weights of seeds produced per plant were found as a trans-generational response specific to high [CO2] growing conditions, when compared to the response pattern revealed under ambient CO2 concentration. The manipulation of the CO2 regime in the experimental environments significantly altered the trans-generational response pattern in reproductive output from the F0 to the F4 generation. As this was a specific response to the selective environment generated by elevated CO2 concentrations, seed quantities and likely associated reproductive fitness were significantly increased in the F4 generation compared with the response in environments applying lower concentrations of CO2. However, the pattern of the trans-generational response to elevated [CO2] for individual seed weights suggests different mechanisms for the increase in total seeds weights and numbers in the two different temperature regimes (Fig. 2F).
A positive selection response of seed production under manipulated [CO2] shown for Arabidopsis thaliana in an artificial selection regime (Ward et al. 2000) suggested that [CO2] provides a sufficient pressure to act on phenotypic divergence of populations. However, artificial selection regimes, where the investigator selects for a single trait which is believed to provide a fitness advantage in the wild, impose specific artifacts on the response to selection. Selection for a single, deliberately chosen trait will interfere with the genetic architecture of the trait selected for in terms of heritability, correlations, and trade-offs with other traits (Reusch and Wood 2007). Therefore, the magnitude, direction, and phenotypic integration of trait differentials revealed in artificial selection experiments might be biased and/or misleading, when they are discussed in a natural context.
A positive selection response to elevated [CO2], as evidenced in our study, might provide a potential to improve reproductive fitness in some plants species within a foreseeable number of generations. Thereby, fitness depressions imposed by co-emerging stressors as, in the present example, increased temperatures can partially be ameliorated by the positive effect of selection imposed by high [CO2], if the pattern seen in this study is representative for natural conditions.
Our results show no phenotypic divergence of the B. napus linages in response to the temperature manipulations. We hypothesize that the selection regime imposed by the thermal conditions in the experiment was stabilizing. This stabilizing selection might have been result of the limited temperature variability in the experimental environments. Spatial and temporal environmental variation is an important driver to maintain genotypic and consequently phenotypic diversity of populations by creating alternating selection regimes (Gutschick and Bassirirad 2003; Jump et al. 2009). Hence, it appears plausible that in the absence of environmental heterogeneity and variability, like in the experimental system presented here, populations will become more “streamlined”.
The overall increase in AG DW from F0 to F4 suggests a trend to higher biomass productivity of the plants selected in high [CO2] environments over the generational range of this study. Our results suggest a likely qualitative alteration of plant-mediated carbon accumulation as a trans-generational response to the manipulated CO2 levels in the experiment. Today, the abundance of projections considering the long-term trajectory of plant-atmosphere feedbacks assume plant responses to be stable over the predicted timescales. However, our study shows that important functions related to carbon assimilation and plant fitness might change in response to selection pressure imposed by increasing [CO2]. Therefore, the presented experimental evidence reinforces earlier propositions that long-term projections regarding future biogeochemical dynamics may lead to incomplete or incorrect conclusions, if they neglect likely responses to changed environments, which occur over progressing generations or fail to incorporate these effects (Bazzaz et al. 1995; Ward and Kelly 2004). Wieneke et al. (2004) detected a weak adaptive response toward increased biomass production in Sanguisorba minor under increased concentrations of [CO2]. However, for Sanguisorba minor, embryological data document gametophytic apomixes (Dickinson et al. 2007), implying that the establishment of any treatment-specific offspring during their experiment is speculative and requires further clarification, for example, by genetic data.
A potential explanation of the rapid phenotypic divergence seen in the experimental populations of the present experiment as a trans-generational response to the environmental manipulations may be provided by the selection of beneficial genotypic configurations. Fast genetic adaptation responses can occur within a small number of subsequent generations (Barrett and Schluter 2008). However, non-genetic inheritance mechanisms may offer an alternative explanation for the fast and specific responses to the different selection scenarios in this study (Bonduriansky 2012). Even though this study cannot fully resolve if the underlying molecular mechanism of the trans-generational response is DNA-sequence based or epigenetic (including parental/maternal), it clearly suggests a selection response as fitness differentials of the experimental populations were revealed over the range of generations investigated. We further argue that the separation of genetic and non-genetic causes for the observed divergence is unsubstantial for the discussion of functional differentiation of plant phenotypes in a [CO2]-context. Both, DNA-sequence based and non-genetic pathways provide heritable phenotypic adaptation to environmental change, which is persistent over multiple generations and only differ in the speed of the response. However, fitness enhancing, maternal environmental effects are unlikely to produce phenotypic divergence in response to environmental conditions experienced by all individuals of a given population, like, for example, elevated CO2 concentrations. In such scenarios, natural selection is expected to be the primary force to define adaptive responses of a given population (Galloway 2005).
The relatively small number of individuals in the present experiment might have imposed a genetic bottleneck to the experimental populations. Such bottlenecks have been reported to increase additive genetic variance, leading to faster selection responses as in more diverse populations as high levels of genetic diversity may dampen the immediate response to selection (Edwards and Lamkey 2003). Therefore, our experiment is likely to have detected an accelerated response to selection imposed by the environmental manipulations. Rapid adaptation responses strongly depend on the pool of immediately available alleles, rather than beneficial mutation events (Barrett and Schluter 2008). Consequently, the rate at which recombination generates new allelic combinations and all parameters which affect the amount of recombination events per unit time define the potential speed of adaptation. Hence, the rates of micro-evolution will, for every natural population, depend on a unique and specific set of multiple abiotic and biotic parameters (e.g., habitat structure, gene flow, generation time, breeding strategy, self-compatibility, etc.), making general predictions virtually impossible (Bone and Farres 2001).
In this study, the potential of plants to adjust phenotypes to rapid environmental change over a range of progressing generations beyond the initial plastic response was clearly shown by the significant and specific trans-generational response. Also, the direction of this response under elevated [CO2] conditions was clearly indicated for a higher plant species model. The chances of a seed of any given mother plant to be selected for the next generation in the experiment was proportional to the mother plants relative contribution to the seed output of the entire population. Hence, selection by competitive exclusion, similar to natural conditions, was warranted on the basis of quantity of reproductive output. Irrespective to the underlying molecular basis of the phenotypic selection response, we were able to reveal a dimension of plant-environment feedbacks, which is currently insufficiently investigated and described for the response of plants to future CO2 concentrations. The results of this study reinforce that fast phenotypic divergence in response to selection imposed by anthropogenic environmental change can alter fitness distributions in plant populations over a short range of generations and imply that future ecological dynamics might differ from projections that do not incorporate adaptive change in plants. Therefore, this experiment demonstrates the importance of responses across several generations in relation to our understanding of future plant-environment feedbacks. The results of this study ask for a broader scientific approach and further investigations in order to define the magnitude of plant responses to rapid environmental change in a multi-generational and evolutionary context.
Acknowledgments
The authors thank Bente A. Andersen, Lis Brandt, Nina Wiese Thomsen, Poul T. Sørensen (all working at Risø-DTU, DK) for their practical support and assistance on the cultivation procedures. Technical support regarding the phytotron was kindly provided by Esben Højrup and Allan A. Murphy (Risø-DTU, DK). We thank Alastair Jump (University of Stirling, UK) and Markus Fischer (University of Bern, CH) for their valuable comments and advices on the manuscript and Ansgar Prüwer for his help compiling Fig. 2. The study presented here benefited from support by the NordForsk network “Sustainable primary production in a changing climate”. G. F. was partly supported by the International School of Biodiversity Science, Denmark – ISOBIS – throughout the period of this study. The CO2 applied in the RERAF phytotron environment was generously donated by Air Liquide Denmark A/S.
Appendix
Table A1.
Analysis of variance (ANOVA) results for linear mixed-effects models integrating the independent and all interactive effects (×) of [CO2] (C), temperature (T), and generation (G) on finally achieved aboveground biomass fractions for four replicate selection lines of Brassica napus grown under two temperature regimes and two CO2 concentrations (Table 1) in a phytotron environment. Only effects, which explain a significant amount of variation in the data and/or are included in the most parsimonious model, are shown here: df, degrees of freedom; den. df, degrees of freedom for the denominator; upper and lower bound den. df and P-values shown as extracted with the method provided by Tremblay (2012); P-values below 0.05 are presented in bold with standard significance indices; DW, dry weight
| Response | Source | df | F-value | Upper den. df | Upper P-value | Lower den. df | Lower P-value |
|---|---|---|---|---|---|---|---|
| Aboveground DW | [CO2] (C) | 1 | 31.3071 | 283 | 0*** | 257 | 0*** |
| Temperature (T) | 1 | 40.2058 | 283 | 0*** | 257 | 0*** | |
| Generation (G) | 1 | 0.7873 | 283 | 0.3757 | 257 | 0.3758 | |
| C × G | 1 | 6.431 | 283 | 0.0118* | 257 | 0.0118* | |
| Leaves DW | [CO2] | 1 | 7.5758 | 286 | 0.0063** | 260 | 0.0063** |
| Stems DW | [CO2] | 1 | 8.9545 | 286 | 0.003** | 260 | 0.003** |
| Seeds DW | [CO2] | 1 | 1.0453 | 283 | 0.3075 | 257 | 0.3075 |
| Temperature | 1 | 32.7189 | 283 | 0*** | 257 | 0*** | |
| Generation | 1 | 0.0578 | 283 | 0.8102 | 257 | 0.8102 | |
| C × G | 1 | 10.024 | 283 | 0.0017** | 257 | 0.0017** | |
| Seed number | [CO2] | 1 | 3.4348 | 283 | 0.0649 | 231 | 0.0651 |
| Temperature | 1 | 38.5349 | 283 | 0*** | 231 | 0*** | |
| Generation | 1 | 0.0013 | 283 | 0.9715 | 231 | 0.9715 | |
| C × G | 1 | 3.5074 | 283 | 0.0621. | 231 | 0.0624. | |
| 1000 Seed weight | [CO2] | 1 | 0.3253 | 280 | 0.5689 | 228 | 0.569 |
| Temperature | 1 | 5.4091 | 280 | 0.0207* | 228 | 0.0209* | |
| Generation | 1 | 0.0197 | 280 | 0.8884 | 228 | 0.8884 | |
| C × T | 1 | 0.4343 | 280 | 0.5104 | 228 | 0.5105 | |
| C × G | 1 | 0.4083 | 280 | 0.5234 | 228 | 0.5235 | |
| T × G | 1 | 0.1296 | 280 | 0.7191 | 228 | 0.7192 | |
| C × T × G | 1 | 5.5216 | 280 | 0.0195* | 228 | 0.0196* |
Conflict of Interest
None declared.
References
- Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell Environ. 2007;30:258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Barnabás B, Jäger K, Fehér A. The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell Environ. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Barrett RDH, Schluter D. Adaptation from standing genetic variation. Trends Ecol. Evol. 2008;23:38–44. doi: 10.1016/j.tree.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Bazzaz FA, Jasieński M, Thomas SC, Wayne P. Microevolutionary responses in experimental populations of plants to CO2-enriched environments: parallel results from two model systems. Proc. Natl. Acad. Sci. USA. 1995;92:8161–8165. doi: 10.1073/pnas.92.18.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Collins S. Adaptation, extinction and global change. Evol. Appl. 2008;1:3–16. doi: 10.1111/j.1752-4571.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Gonzalez A. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 2009;12:942–948. doi: 10.1111/j.1461-0248.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R. Rethinking heredity, again. Trends Ecol. Evol. 2012;27:330–336. doi: 10.1016/j.tree.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Bone E, Farres A. Trends and rates of microevolution in plants. Genetica. 2001;112–113:165–182. [PubMed] [Google Scholar]
- Carter TR, Jones RN, Lu X, Bhadwal S, Conde C, Mearns LO. New assessment methods and the characterisation of future conditions. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, et al., editors. Climate change 2007: impacts, adaptation and vulnerability. Cambridge, U.K: Cambridge University Press; 2007. pp. 133–171. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Cheplick GP. The allometry of reproductive allocation. In: Reekie EG, Bazzaz FA, editors. Reproductive allocation in plants. Oxford, U.K: Elsevier Academic Press; 2005. pp. 97–128. [Google Scholar]
- Dickinson TA, Lo E, Talent N. Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. Plant Syst. Evol. 2007;266:59–78. [Google Scholar]
- Edwards JW, Lamkey KR. Dominance and genetic drift: predicted effects of population subdivision in a maize population. Crop Sci. 2003;43:2006–2017. [Google Scholar]
- Fischlin A, Midgley GF, Price JT, Leemans R, Gopal B, Turley C. Ecosystems, their properties, goods, and services. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, et al., editors. Climate change 2007: impacts, adaptation and vulnerability. Cambridge, U.K: Cambridge University Press; 2007. pp. 211–272. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW. Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, et al., editors. Climate change 2007: the physical science basis. Cambridge, U.K. and New York, NY, USA: Cambridge University Press; 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Franks SJ, Sim S, Weis AE. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl. Acad. Sci. USA. 2007;104:1278–1282. doi: 10.1073/pnas.0608379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzaring J, Högy P, Fangmeier A. Effects of free-air CO2 enrichment on the growth of summer oilseed rape (Brassica napus cv. Campino) Agric. Ecosyst. Environ. 2008;128:127–134. [Google Scholar]
- Fuhrer J. Agroecosystem responses to combinations of elevated CO2, ozone, and global climate change. Agric. Ecosyst. Environ. 2003;97:1–20. [Google Scholar]
- Fuller RC, Baer CF, Travis J. How and when selection experiments might actually be useful. Integr. Comp. Biol. 2009;45:391–404. doi: 10.1093/icb/45.3.391. [DOI] [PubMed] [Google Scholar]
- Galloway LF. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 2005;166:93–100. doi: 10.1111/j.1469-8137.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- Gutschick VP, Bassirirad H. Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 2003;160:21–42. doi: 10.1046/j.1469-8137.2003.00866.x. [DOI] [PubMed] [Google Scholar]
- Heimann M, Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature. 2008;451:289–292. doi: 10.1038/nature06591. [DOI] [PubMed] [Google Scholar]
- Hirose T, Kinugasa T, Shitaka Y. Time of flowering, costs of reproduction, and reproductive output in annuals. In: Reekie EG, Bazzaz FA, editors. Reproductive allocation in plants. Oxford, U.K: Elsevier Academic Press; 2005. pp. 159–188. [Google Scholar]
- Jump AS, Peñuelas J. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005;8:1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- Jump AS, Marchant R, Peñuelas J. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009;14:51–58. doi: 10.1016/j.tplants.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nosberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed effects models in S and S-plus New York. New York, NY: Springer; 2000. [Google Scholar]
- Qaderi MM, Kurepin LV, Reid DM. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: temperature, carbon dioxide and drought. Physiol. Plant. 2006;128:710–721. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. ISBN 3-900051-07-0. Available at: http://www.R-project.org/ [Google Scholar]
- Reekie EG, Macdougall C, Wong I, Hicklenton PR. Effect of sink size on growth response to elevated atmospheric CO2 within the genus Brassica. Can. J. Bot. 1998;76:829–835. [Google Scholar]
- Reusch TBH, Wood TE. Molecular ecology of global change. Mol. Ecol. 2007;16:3973–3992. doi: 10.1111/j.1365-294X.2007.03454.x. [DOI] [PubMed] [Google Scholar]
- Stone P. The effects of heat stress on cereal yield and quality. In: Basra AS, editor. Crop responses and adaptations to temperature stress. Binghamton, NY, USA: Food Products Press; 2001. pp. 243–291. [Google Scholar]
- Swart R, Mitchell J, Morita T, Raper S. Stabilisation scenarios for climate impact assessment. Glob. Environ. Change. 2002;12:155–165. [Google Scholar]
- Thomas SC, Jasienski M. Genetic variability and the nature of microevolutionary responses to elevated CO2. In: Körner C, Bazzaz FA, editors. Carbon dioxide, populations, and communities. San Diego: Academic Press; 1996. pp. 51–81. [Google Scholar]
- Tremblay A. 2012. LMERConvenienceFunctions: a suite of functions to back-fit fixed effects and forward-fit random effects, as well as other miscellaneous functions [Online]. Available at http://CRAN.R-project.org/package=LMERConvenienceFunctions (accessed March 6, 2013)
- Ward JK, Kelly JK. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol. Lett. 2004;7:427–440. [Google Scholar]
- Ward JK, Antonovics J, Thomas RB, Strain BR. Is atmospheric CO2 a selective agent on model C3 annuals? Oecologia. 2000;123:330–341. doi: 10.1007/s004420051019. [DOI] [PubMed] [Google Scholar]
- Wieneke S, Prati D, Brandl R, Stöcklin J, Auge H. Genetic variation in Sanguisorba minor after 6 years in situ selection under elevated CO2. Glob. Change Biol. 2004;10:1389–1401. [Google Scholar]


