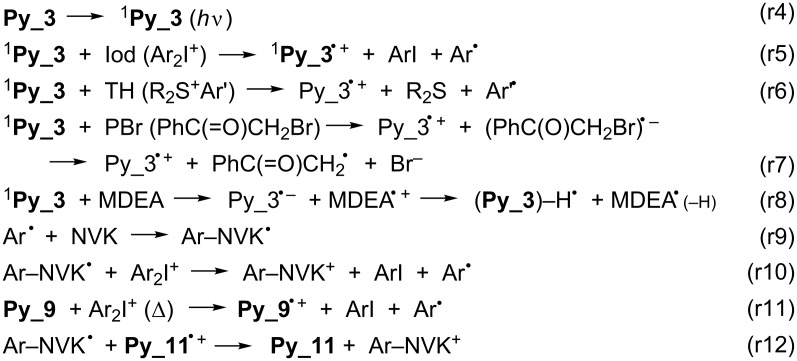Abstract
Eleven di- and trifunctional compounds based on a core-pyrene π structure (Co_Py) were synthesized and investigated for the formation of free radicals. The application of two- and three-component photoinitiating systems (different Co_Pys with the addition of iodonium or sulfonium salts, alkyl halide or amine) was investigated in detail for cationic and radical photopolymerization reactions under near-UV–vis light. The proposed compounds can behave as new photocatalysts. Successful results in terms of rates of polymerization and final conversions were obtained. The strong MO coupling between the six different cores and the pyrene moiety was studied by DFT calculations. The different chemical intermediates are characterized by ESR and laser flash photolysis experiments. The mechanisms involved in the initiation step are discussed, and relationships between the core structure, the Co_Py absorption property, and the polymerization ability are tentatively proposed.
Keywords: cationic photopolymerization, free-radical-promoted cationic photopolymerization, photocatalysts, photoinitiators, radical photopolymerization
Introduction
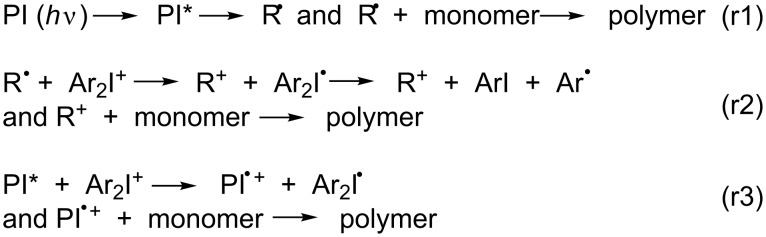
Free radical sources are encountered in various areas such as organic chemistry, biochemistry and polymer chemistry. In the field of polymer photochemistry applied to photopolymerization reactions, they are referred to as photoinitiators (PI) [1]. These PIs are usable in two scenarios, both of which are light induced. Firstly, they are usable in free radical polymerization (FRP), where the PIs work as either cleavable type I PIs or uncleavable type II PIs in dependence of couples formed by the PI and hydrogen or electron donors (r1 in Scheme 1). Secondly, PIs may be used in free-radical-promoted cationic polymerization (FRPCP), in which the produced radical R• is oxidized by an onium salt, e.g., Ar2I+, to form Ar2I• and a cation R+ suitable for the ROP reaction. The Ar2I• species is readily decomposed into ArI and Ar• (r2 in Scheme 1). There is also an usual cationic polymerization (CP; r3 in Scheme 1) [1–2]. When the PIs exhibit a catalytic behavior analogous to a photocatalyst (PC) in organic chemistry, they are designed as photoinitiator catalysts (PIC). PIs as well as PICs are based on pure organic, that is, metal free, or organometallic compounds. In the presence of additives, they constitute a photoinitiating system (PIS).
Scheme 1.
Typical reactions for photoinitiated cationic polymerization.
Among others, two points of interest in the photopolymerization area are (i) the reactivity and efficiency of the PIs and PICs and (ii) the irradiation conditions. Indeed, the absorption properties (wavelengths λ, molar extinction coefficients ε), the excited-state processes, the reactivity of the produced radicals, and the characteristics of the light sources (emission spectra, available luminous power density) govern the polymerization efficiency (rate of polymerization, final monomer conversion).
The photopolymerization reactions in radiation curing technologies are often carried out under high-intensity light sources (>1000 mW cm−2). Applications in this area and in other fields may require to avoid the use of UV rays delivered by Hg lamps or to irradiate with visible light (400–700 nm) and low photon fluxes (~2–10 mW cm−2). Recent works have aimed at working under soft irradiation conditions (near-UV–vis light, low light intensity), which allows the use of Xe lamps, laser diodes, solar radiation, household halogen lamps, fluorescent bulbs and blue, green and white LEDs (see a recent review in [1]).
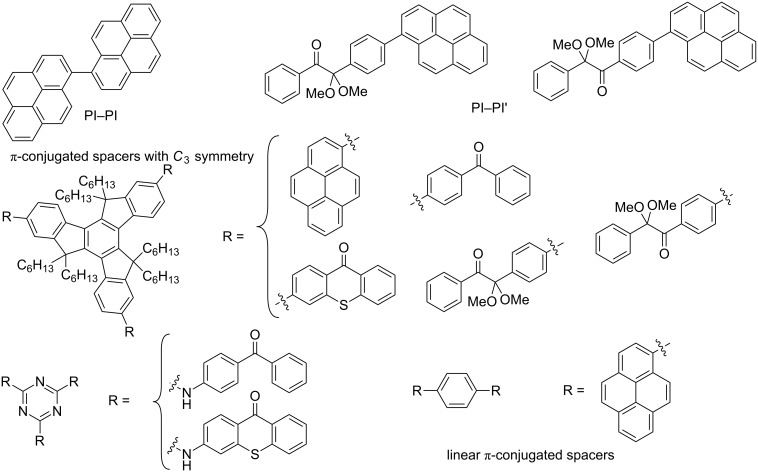
New high-performance PIs and PICs are continuously being developed [1–10] through the design of novel structures (e.g., novel chromophores, novel cleavable bonds) and the modification ofexisting structures by using the classical introduction of electron-donating or -withdrawing substituents, conjugated groups or moieties, and more complex changes on the skeleton (see typical recent examples in [11–41]). To meet the challenge of designing efficient PIs and PICs under soft irradiation, we have recently started the search for suitable multifunctional arrangements exhibiting a strong coupling of the molecular orbitals MOs [42–43]. Improved absorption properties (red-shifted λ, higher ε) while keeping a high reactivity have been already achieved. Examples of these investigated architectures (Scheme 2) [42–44] are linked PI–PI units (e.g., PI = pyrene) or PI-PI’ (e.g., PI = pyrene and PI’ = 2,2’-dimethoxy-2-phenylacetophenone) and PI moieties (e.g., PI = benzophenone, thioxanthone, 2,2’-dimethoxy-2-phenylacetophenone, pyrene) linked to a trifunctional core (truxene, triazine, benzene); the same could be expected by using a difunctional core.
Scheme 2.
Examples of previously investigated architectures.
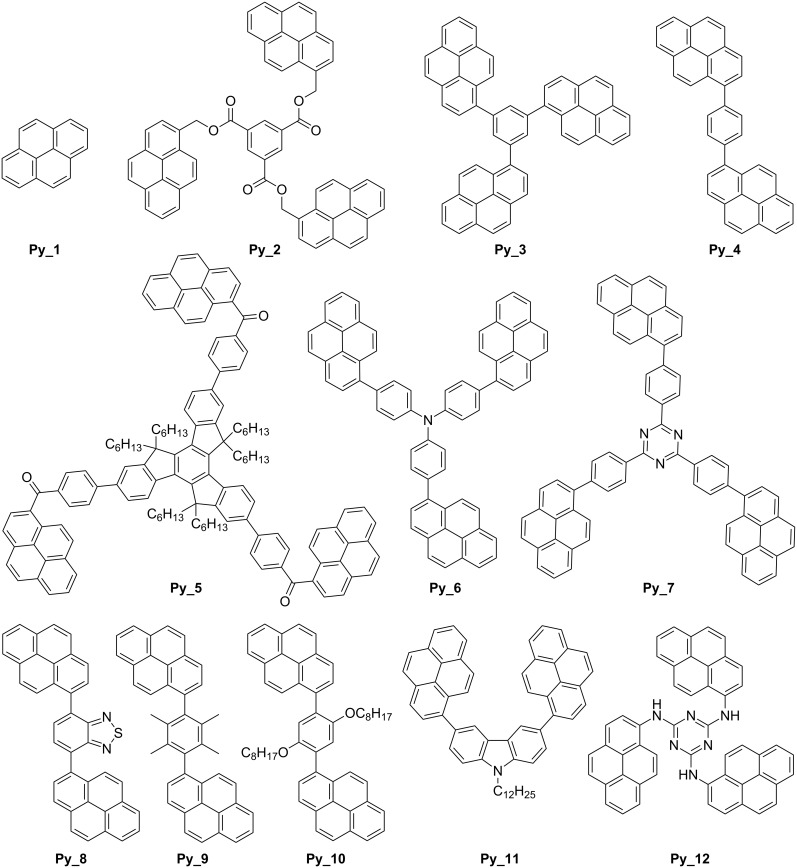
In the present paper, we consider a series of eleven di- and trifunctional core-pyrene π compounds Co_Py, where the pyrene moiety is used as a PI. The cores include a functionalized truxene (Py_5), several substituted phenyl rings (Py_2, Py_3, Py_4, Py_9, Py_10) two functionalized triazines (Py_7, Py_12), a triphenylamine (Py_6), a carbazole (Py_11) and a benzothiadiazole (Py_8) (Scheme 3). The idea is to get a high absorption around 380–410 nm where Xe–Hg lamps, Xe lamps, LED, laser diodes and even household halogen lamps are usable. The large choice of Co_Pys should allow for studying the effect of the core on the MO coupling and the resulting absorption properties. The activity of these Co_Py compounds in the FRP of acrylates and the FRPCP (and eventually the cationic polymerization CP) of epoxides through a ring-opening polymerization (ROP) under exposure to the near-UV–vis light delivered by a Hg–Xe lamp (~30 mW cm−2) and the visible light of a halogen lamp (soft irradiation conditions; ~10 mW cm−2) is investigated. The Co_Pys are used in combination with additives: iodonium or sulfonium salts for ROP and amine or/and alkyl halide for FRP. The monomers investigated are given in Scheme 4. The mechanisms are analyzed by electron spin resonance (ESR), steady-state photolysis, fluorescence and laser flash photolysis (LFP). The ability of Co_Pys to behave as new organophotocatalysts is also discussed.
Scheme 3.
Investigated Co_Pys.
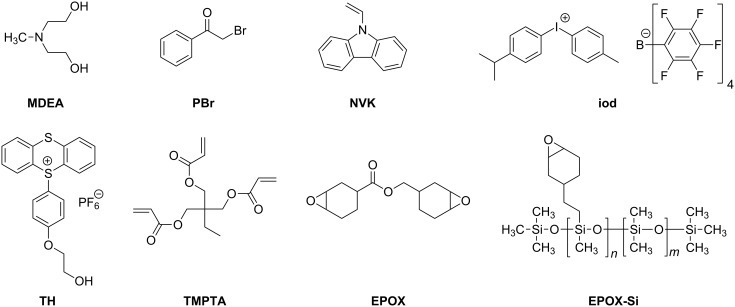
Scheme 4.
Other chemical compounds.
Results and Discussion
Light absorption, redox properties and MO calculations
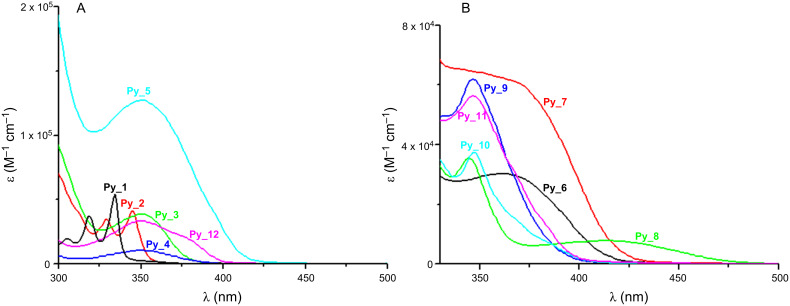
The UV absorption spectra of the different pyrene derivatives are depicted in Figure 1. The solvents were selected for good solubility of the Co_Pys. Absorption maxima are located at ~350 nm for Py_2, Py_3, Py_4, Py_5, Py_12, Py_8, Py_9, Py_10, Py_11, ~370 nm for Py_6, Py_7, and 330 nm for Py_1 (Table 1). This corresponds to a red shift of the absorption for Py_2 to Py_12 compared to the reference compound Py_1. At 365 nm, the maximum emission wavelength of the Hg–Xe lamp, the molar extinction coefficients εmax of the different Co_Pys follow the order Py_5 > Py_7 > Py_11 > Py_9 > Py_6 > Py_12 > Py_3 > Py_10 > Py_8 > Py_4 > Py_1 = Py_2. The highest εmax is obtained for Py_5 (ε353 = 13 × 104 M−1 cm−1, ε346 = 6 × 104 M−1 cm−1 versus ε334 = 6 × 104 M−1 cm−1 for Py_5, Py_9 and Py_1, respectively). Py_4 exhibits low ε values, and thus a low efficiency can be expected for this compound.
Figure 1.
UV–vis absorption spectra of the investigated compounds: (A) In acetonitrile for Py_1 and acetonitrile/toluene (50/50) for the other molecules, (B) in toluene.
Table 1.
Properties of the pyrene derivatives in acetonitrile (*) and toluene. Light absorption and emission properties (experimental λmax,abs,exp and calculated λmax,abs,calc maximum absorption wavelengths, emission wavelengths λmax,em), excited-state energy levels (ES), oxidation potentials (Eox) and free-energy changes ΔG (see text) [1].
| λmax,abs,exp (nm) |
λmax,abs,calca (nm) |
λmax,em (nm) |
Eox (V/SCE) |
ES (eV) |
ΔGPBr (eV) |
ΔGIod (eV) |
|
| Py_1* | 334 | 327 (f = 0.27) | 393 | 1.33 | 3.44 | −1.33 | −1.9 |
| Py_2 | 344 | 334 (f = 0.41) | 380 | 0.8 | 3.45 | −1.87 | −2.45 |
| Py_3 | 351 | 356 (f = 0.50) | 393 | 0.9 | 3.36 | −1.68 | −2.26 |
| Py_4 | 351 | 373 (f = 0.3) | 423 | 0.6 | 3.21 | −1.83 | −2.41 |
| Py_5 | 353 | 387 (f = 1.16) | 404 | 0.7 | 3.24 | −1.76 | −2.34 |
| Py_6 | 365 | 344 (f = 0.52); 403 (f = 0.74) |
441 | 0.9 | 3.04 | −1.36 | −1.94 |
| Py_7 | 367 | 344 (f = 0.68) | 440 | – | 3.02 | – | – |
| Py_8 | 344 | 365 (f = 0.39) | 530 | 0.7 | 2.6 | −1.12 | −1.7 |
| Py_9 | 346 | 336 (f = 0.44) | 428 | 0.8 | 3.2 | −1.62 | −2.2 |
| Py_10 | 347 | 376 (f = 0.51) | 425 | 0.9 | 3.2 | −1.52 | −2.1 |
| Py_11 | 346 | 370 (f = 0.4) | 409 | − | 3.22 | – | – |
| Py_12 | 346 | 377 (f = 1.00) | 397 | 0.6 | 3.22 | −1.84 | −2.42 |
aMolecular orbital MO calculations (using the time-dependent density functional theory at B3LYP/6-31G* level on the relaxed geometries calculated at UB3LYP/6-31G* level); f = oscillator strength.
The oxidation potentials of the Co_Pys (Table 1) range from 1.33 V (Py_1) to 0.6 V (Py_4, Py_12). The singlet-state energy is also affected by the core of the structure, i.e., 2.6 eV for Py_8 versus 3.44 eV for the reference Py_1. The free-energy change ΔG of the Co_Py/phenacyl bromide PBr (or the iodonium salt, Iod) interaction is very favorable (ΔG << 0). Py_2 exhibits the most favorable ΔGs: −2.45 eV (Iod) and −1.87 eV (PBr).
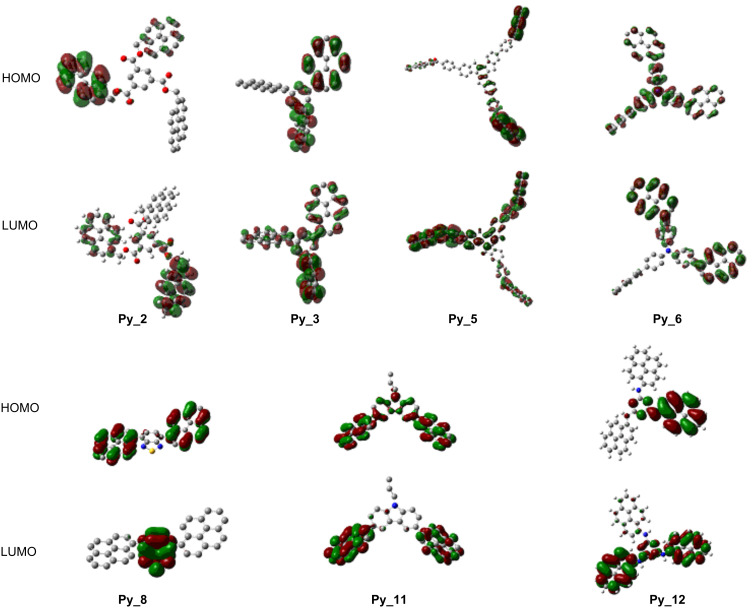
MO calculations show that the calculated and experimental values (Table 1) are approximately the same, e.g., a difference of 5 to 10 nm is observed for Py_2 and Py_3. The oscillator strengths (noted f in Table 1) are higher for the new proposed structures than for Py_1 in agreement with their better light absorption properties (Figure 1). A strong coupling of the π MOs of the core with those of the pyrene substituents is shown supporting a clear ππ* character of the HOMO–LUMO transition, as evidenced in Figure 2 for the investigated derivatives. For the various π-core/pyrene substituent combinations, different coupling strengths can be observed (Figure 2). Strong couplings of the MOs lead to red-shifted transitions compared to Py_1 and enhanced absorption coefficients (Figure 1), e.g., for Py_3, Py_5, Py_6, Py_8, Py_11 and Py_12. A strong delocalization of the HOMOs and LUMOs is observed in line with their better absorption properties. For Py_2, the coupling is weak and the absorption properties are moderately affected compared to Py_1.
Figure 2.
HOMO–LUMO orbitals for Py_2, Py_3, Py_5, Py_6, Py_8, Py_11 and Py_12 involved in the π–π* transition (calculated at UB3LYP/6-31G* level; for Py_5 and Py_11, the alkyl chains have been simplified to reduce the computational cost).
Photochemical reactivity
Fluorescence experiments
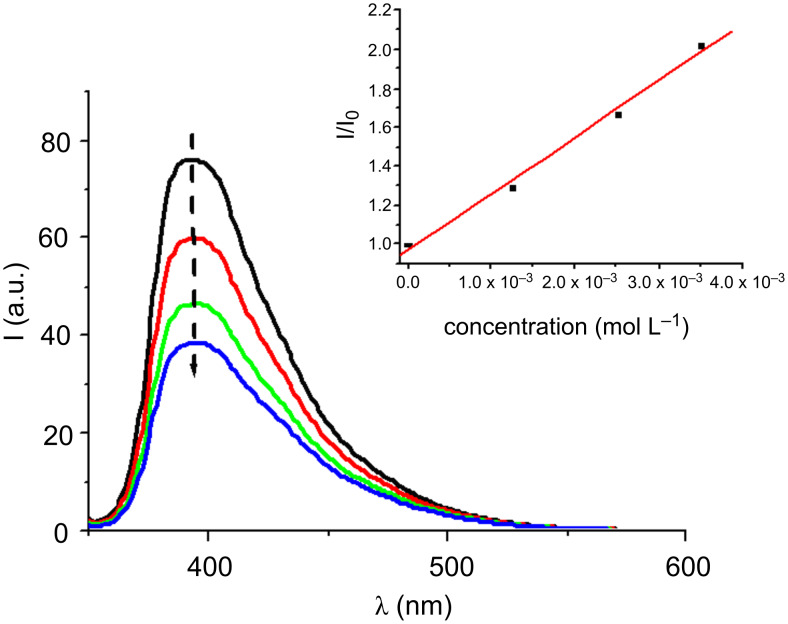
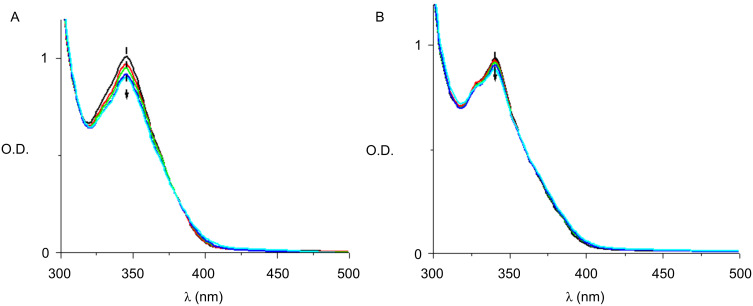
A strong quenching of the Co_Py singlet states by PBr, amine (MDEA), Iod and the sulfonium salt TH is found. Py_3 is selected for this mechanistic study (Figure 3) because of the high reactivity of this compound in photopolymerization experiments (see below). The rate constants in that case are 1.5 × 1010, 9 × 109, 1 × 1010 and 3.7 × 109 M−1 s−1, respectively. The 1Py_3/PBr, 1Py_3/Iod and 1Py_3/TH interactions are diffusion-controlled in full agreement with the highly favorable ΔGs (Table 1; the reduction potential for TH is −1.3 V [1]; the calculated ΔG is −1.16 eV). They correspond to an efficient electron transfer followed by the usual fast generation of radicals from the radical anion species (r5–r7 in Scheme 5) or an electron/proton transfer with the amine (r8 in Scheme 5).
Figure 3.
Fluorescence quenching of 1Py_3 by the phenacylbromide (PBr) in acetonitrile/toluene (50/50). Insert: Stern–Volmer plot (the concentrations used are given in this plot).
Scheme 5.
Photochemical processes for the different Co_Pys.
ESR spin trapping experiments
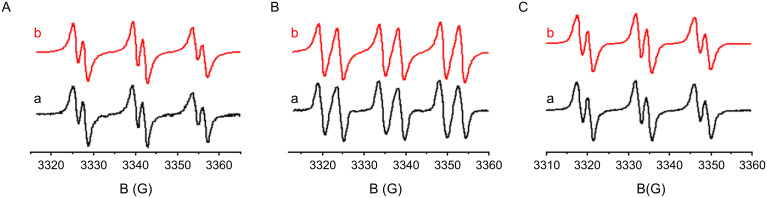
In ESR spin trapping experiments upon UV light exposure, the interaction of the Co_Pys with the iodonium salt Iod (Figure 4A) or the sulfonium salt TH (Figure 1 in Supporting Information File 1) leads to an aryl radical Ar• (e.g., hyperfine coupling constants hfcs of the PBN adduct: aN = 14.2 G and aH = 2.2 G in agreement with known data [22–25,45]) in line with (r5). In the same way, phenacyl radicals and α-aminoalkyl radicals (r7 and r8 in Scheme 5) are observed in 1Py_3/PBr and 1Py_3/EDB (aN = 14.6 G, aH = 4.5 G and aN = 14.3 G, aH = 2.5 G, respectively; Figure 4B and Figure 4C; ethyldimethylaminobenzoate (EDB) instead of MDEA is used to avoid a high polarity of the sample preventing an ESR investigation). The Py_3/Iod or the Py_3/TH couple generates a cation radical (Py_3•+) that can initiate a cationic polymerization whereas the phenacyl and aminoalkyl radicals formed in Py_3/PBr or Py_3/MDEA are susceptible to radical polymerization.
Figure 4.
ESR spectra obtained upon irradiation of (A) Py_3/Iod, (B) Py_3/PBr and (C) Py_3/EDB in tert-butylbenzene. Phenyl-N-tert-butylnitrone (PBN) is used as a spin trap; (a) experimental and (b) simulated ESR spectra.
Laser flash photolysis
Upon laser excitation of Py_3 at 355 nm, a triplet state T1 characterized by a maximum absorption at ~420 nm and a rather long lifetime is formed (t > 4 μs) (Figure S2 in Supporting Information File 1). This T1 state is similar to that of Py_1 [31]. The short S1 lifetime (e.g., ~55 ns under N2 for Py_3) and the diffusion controlled interactions of S1 with Iod, PBr or MDEA (see above) prevent a significant production of T1 in Co_Py/Iod (PBr or MDEA). Therefore, a triplet-state pathway can be neglected in the investigated systems.
Thermal processes
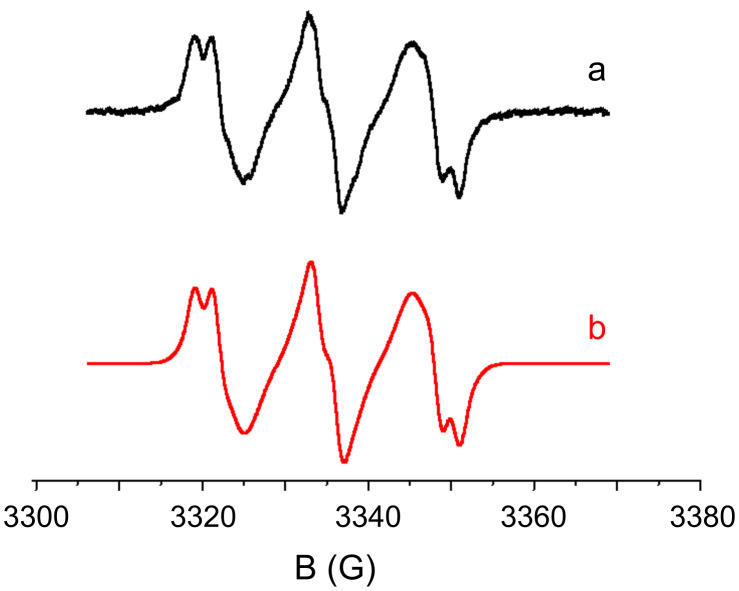
For some Co_Py/Iod couples, a slow thermal redox process can also occur. Indeed, in ESR–ST experiments, aryl radicals are generated without irradiation within 24 h (no free radical was observed in Py_9 or Iod alone). For example, in Py_9/Iod, the same radicals as in r5 are produced (Figure 5; hfccs: aN = 14.2 G and aH = 2.2 G for the PBN radical adduct). Peroxyl radicals are also observed, as aryls easily add to O2 (aN = 13.3 G and aH = 1.6 G for the PBN radical adduct, in agreement with [45]). As in r5 in Scheme 5, a cation radical Py_9•+ is concomitantly formed. This thermal process is slow as Eox(Py_9) > Ered(Iod) (Table 1). The formation of radical cations can be worthwhile to initiate cationic polymerization at room temperature (see below).
Figure 5.
ESR-spin trapping spectra of Py_9/Iod in tert-butylbenzene (storage at rt for 24 h); (a) experimental and (b) simulated spectra.
Ability of the different pyrene structures in photopolymerization reactions
Cationic photopolymerization (CP)
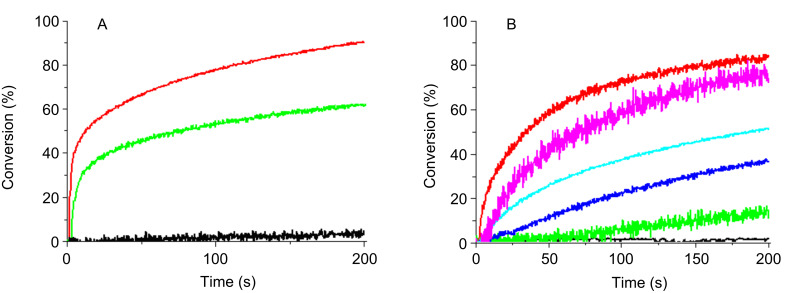
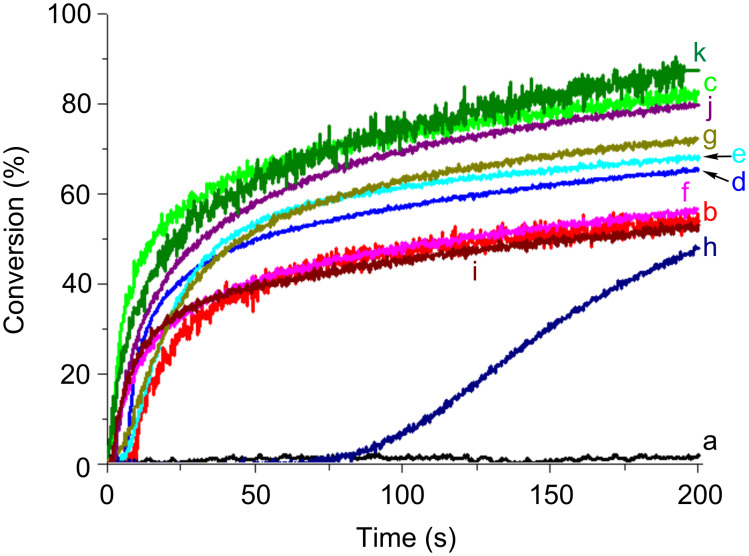
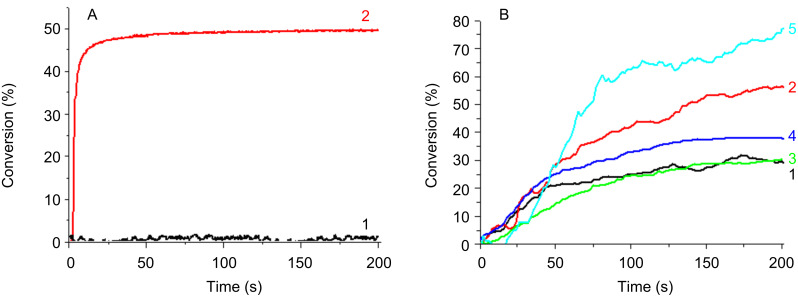
The ring-opening polymerization profiles of EPOX upon exposure to the Xe–Hg lamp under air using two-component PISs are shown in Figure 6, Figure 7 and Figure 8. A comparison of the photopolymerization profiles using the Py_1 (to Py_12)/Iod couples (Figures 6–8) shows that the fastest polymerization rates (Rp) and the highest final conversion (conv) are obtained with Py_11/Iod and Py_3/Iod. No polymerization is observed in the presence of Iod alone Figure 6A and Figure 8A, curves a.) Py_3/Iod leads to a conv ~90% (Figure 6A, curve b) compared to Py_1/Iod (conv ~50%; Figure 6A, curve c). Almost all the new proposed structures are better than the starting structure Py_1 (Figure 7, curve b). This highlights the interest of the present approach associated with the modification of the Py chromophore. CP can also be initiated in the presence of the sulfonium salt TH, e.g., with Py_3, Py_8, Py_9, Py_10 and Py_11 (Figure 6B). The conversions reach 80% and 75% with Py_3/TH and Py_11/TH, respectively (after 200 s light exposure). EPOX-Si can also be easily polymerized (Figure 8). Py_3/Iod (Figure 8A) is still efficient (conv ~50%).
Figure 6.
Photopolymerization profiles of EPOX upon Xe–Hg lamp irradiation (λ > 340 nm) under air for different photoinitiating systems: (A) (a) Iod (2% w/w); (b) Py_3/Iod (1%/2% w/w); (c) Py_1/Iod (1%/2% w/w); (B) (a) TH (2% w/w); (b) Py_3/TH (0.2%/2% w/w); (c) Py_8/TH (0.2%/2% w/w); (d) Py_9/TH (0.2%/2% w/w); (e) Py_10/TH (0.2%/2% w/w); (f) Py_11/TH (0.2%/2% w/w).
Figure 7.
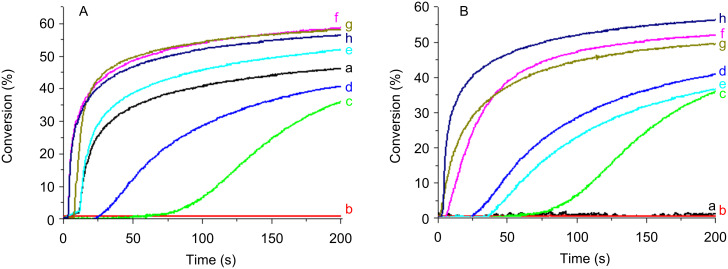
Photopolymerization profiles of EPOX upon a Xe–Hg lamp irradiation (λ > 340 nm) under air for different photoinitiating systems: (a) Iod (2% w/w); (b) Py_1/Iod (0.2%/2% w/w); (c) Py_3/Iod (0.2%/2% w/w); (d) Py_4/Iod (0.2%/2% w/w); (e) Py_5/Iod (0.2%/2% w/w); (f) Py_6/Iod (0.2%/2% w/w); (g) Py_7/Iod (0.2%/2% w/w); (h) Py_8/Iod (0.2%/2% w/w); (i) Py_9/Iod (0.2%/2% w/w); (j) Py_10/Iod (0.2%/2% w/w) ; (k) Py_11/Iod (0.2%/2% w/w).
Figure 8.
(A) Photopolymerization profiles of EPOX-Si upon a Xe–Hg lamp irradiation (λ > 340 nm) under air for different photoinitiating systems: (A) (1) Iod (2% w/w); (2) Py_3/Iod (1%/2% w/w); (B) Photopolymerization profiles of EPOX under air upon halogen-lamp irradiation in the presence of (1) Py_6/Iod (0.2%/2% w/w); (2) Py_7/Iod (0.2%/2% w/w); (3) Py_10/Iod (0.2%/2% w/w); (4) Py_11/Iod (0.2%/2% w/w); (5) Py_11/Iod/NVK (0.2%/2%/2% w/w).
In these systems, the Co_Py•+ generated in r5,r6 (Scheme 5) can initiate the CP process. The relative efficiency of these Co_Py/Iod (or TH) combinations will be affected by different parameters: (i) their relative light absorption properties, (ii) the quantum yields of radical or radical cation formation in the singlet state (the singlet state is predominant, see above), (iii) the rate constants for reactions r5 and r6 and (iv) the ability of Co_Py•+ to initiate a ring-opening polymerization process. Structure–reactivity relationships for the different derivatives can hardly be extracted. This is probably ascribed to a strong interplay between (i) to (iv).
A cationic photopolymerization profile of EPOX under very soft irradiation (halogen lamp exposure) is represented in Figure 8B. In the presence of Iod, several Co_Pys exhibiting an acceptable absorption at λ > 380 nm lead to efficient polymerization reactions. For example, conv = 40 and 55% within 200 s with the Py_7/Iod and Py_11/Iod couples.
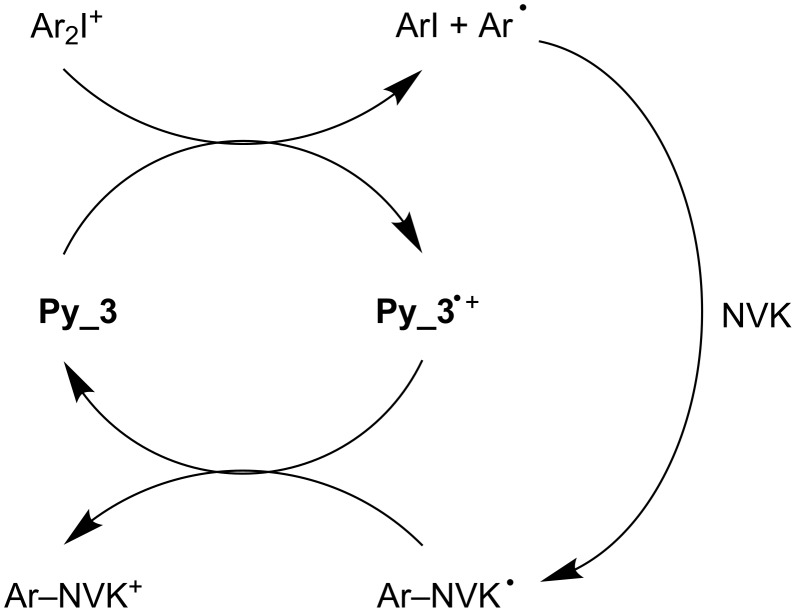
The three-component PIS (Py_11/Iod/NVK) (Figure 8B, curve 5) allows an increase of the EPOX conversion up to 75%. A consumption of the NVK double bond is observed (Figure S3 in Supporting Information File 1). As known in other systems, this is accounted for by the addition of Ar• to the NVK double bond (formation of an Ar–NVK• radical) (r9 in Scheme 5). This electron rich radical is easily oxidized (r10 in Scheme 5) by Iod, the resulting cation Ar–NVK+ being able to initiate the cationic polymerization.
Thermal polymerization at room temperature
Remarkably, the thermal polymerization of EPOX can also be initiated by different Co_Py/Iod (1%/2% w/w) systems. This reaction proceeds smoothly at rt but is completed after 24 h, e.g., with Py_9, Py_6, Py_10 or Py_11/Iod, the final conversion is >55%. As supported by the ESR experiments (see above), the presence of, e.g., Py_9•+ explains the thermal initiation of the cationic polymerization of the epoxide (r11 in Scheme 5). For the other Co_Py, a good thermal stability is found. This dual behavior of some Co_Pys, which are able to initiate both a thermal and a photochemical cationic polymerization, can be very useful to initiate the polymerization in the shadow areas.
Free radical photopolymerization (FRP)
Typical free radical polymerization profiles of TMPTA upon the Xe–Hg lamp exposure in laminate by using the Co_Py/MDEA (or PBr) two-component PISs are represented in Figure 9. In the presence of Co_Py alone, FRP occurs but the inhibition time is high and the final conversion is low (50 s; 35% with Py_3). The addition of PBr (Figure 9A) or MDEA (Figure 9B) shortens the inhibition time and increases the final conversion to ~55%. For the Co_Py/amine or Co_Py/PBr two-component systems, the radicals generated in r7 and r8 in Scheme 5 can initiate the FRP process. Due to its absorption in the UV, PBr alone initiates the acrylate photopolymerization (conv = 45%; Figure 9A, curve a). No striking improvement of the final conversion is observed when using the Py_3/MDEA/PBr three-component PIS instead of Py_3/PBr (~55%; Figure 9A, curve f versus h), but both Rp and conv are better when comparing to Py_3/MDEA.
Figure 9.
Photopolymerization profiles of TMPTA upon Xe–Hg lamp irradiation (λ > 340 nm) in laminate for different photoinitiating systems: (A) (a) PBr (2% w/w); (b) Py_2 (0.2% w/w); (c) Py_3 (0.2% w/w); (d) Py_5 (0.2% w/w); (e) Py_2/PBr (0.2%/2% w/w); (f) Py_3/PBr (0.2%/2% w/w); (g) Py_5/PBr (0.2%/2% w/w); (h) Py_3/MDEA/PBr (0.2%/2%/2% w/w); (B) (a) MDEA (2% w/w); (b) Py_2 (0.2% w/w); (c) Py_3 (0.2% w/w); (d) Py_5 (0.2% w/w); (e) Py_2/MDEA (0.2%/2% w/w); (f) Py_3/MDEA (0.2%/2% w/w); (g) Py_5/MDEA (0.2%/2% w/w); (h) Py_3/MDEA/PBr (0.2%/2%/2% w/w).
Photocatalyst behavior of the Co_Pys
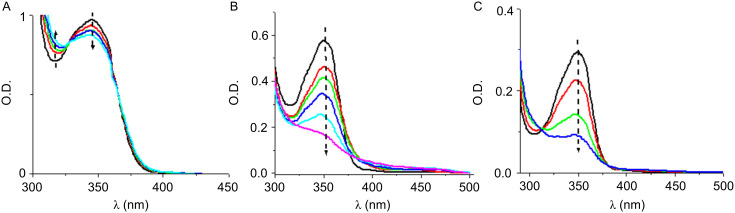
The photocatalytic behavior of the Co_Pys is investigated in the most interesting compounds reported above for the photopolymerization reactions. The steady-state photolysis of Py_3/PBr, Py_3/Iod, Py_3/MDEA couples highlights the consumption of the pyrene moiety under light exposure (Figure 10). In Py_3/PBr and Py_3/MDEA, isosbestic points are present at 330 nm (Figure 10A) and 315 nm (Figure 10B), respectively, suggesting stoichiometric reactions and no other side reactions.
Figure 10.
Photolysis of (A) the Py_3/PBr couple, (B) the Py_3/Iod couple, and (C) the Py_3/MDEA couple; Xe–Hg lamp irradiation. In acetonitrile/toluene (50/50); from t = 0 to 5 min.
The addition of NVK to Py_11/Iod shows that the photolysis is faster with Py_11/Iod than with Py_11/Iod/NVK couples (Figure 11A versus Figure 11B). This difference highlights that in the presence of NVK, Py_11 is regenerated according to an oxidation cycle (Scheme 6). Therefore, Py_11 behaves as a new photocatalyst in agreement with (r12 in Scheme 5).
Figure 11.
Photolysis of (A) the Py_11/Iod and (B) the Py_11/Iod/NVK couple. Halogen lamp irradiation. In acetonitrile/toluene (50/50); from t = 0 to 5 min.
Scheme 6.
The oxidative cycle.
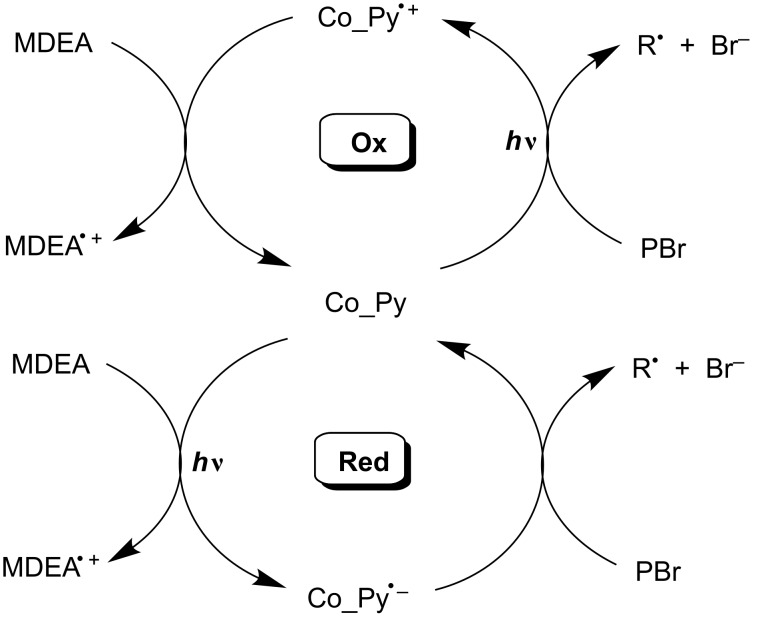
In the Co_Py/PBr/MDEA three-component system, reactions (r7, r8) are probably competitive (e.g., k[MDEA] ~ k[PBr] using Py_3). As a consequence, both a reduction and an oxidation cycle could simultaneously proceed (Scheme 7). However, the ESR and polymerization results (see above) (i) suggest that the contribution of the reduction cycle should be weak and (ii) confirm that the regeneration in the oxidation cycle has a low efficiency. This might be due to a MDEA•+ → MDEA–H• process (in r8 in Scheme 5) less efficient than PBr•− → phenacyl radical (in r7 in Scheme 5). Nevertheless, such an oxidation cycle that can contribute here to some extent appears as one of the rare examples observed in photocatalyst (PC)/PBr/amine systems, because most of them work according to a reduction cycle [30,46] (see other examples in [47]; in a related system, still unpublished, based on another PC, we have demonstrated the true occurrence of a unique oxidation cycle).
Scheme 7.
Oxidation versus reduction cycles.
Conclusion
In this paper, the core-pyrene π structures Co_Pys appear as interesting photoinitiators. Successful cationic and radical photopolymerization reactions were carried out under near-UV–vis irradiation. A photocatalytic behavior is noted, that is, an oxidation cycle for Co_Py/Iod/NVK and a partial oxidation cycle for Co_Py/MDEA/PBr. These experimentally and theoretically investigated di- and trifunctional compounds outline the interest in a suitable strong MO coupling. This could open the way to an a priori choice of absorption spectra driven by the theoretical design of chemical compounds. Other proposals will be provided in the future.
Experimental
Co_Pys
The synthesized compounds are presented in Scheme 3. The procedures are presented in detail in Supporting Information File 1. 1H and 13C NMR spectra and HRMS mass spectral analysis are also given. Py_1 was purchased from Aldrich. 1,4-Di(pyren-1-yl)benzene (Py_4) was prepared by adapting a literature procedure [48]. Py_12 was synthesized as previously reported [49]. The syntheses of Py_2 [50], Py_3 [51], Py_6 [52] and Py_7 [53] were already reported under reaction conditions different to the ones reported in this article and in lower yields. The results obtained in our work agree with the previously reported characterization of these products.
Other chemical compounds
The [methyl-4-phenyl (methyl-1-ethyl)-4-phenyl] iodonium tetrakis(pentafluorophenyl)borate [54–55] (Iod; PI 2074 from Bluestar Silicones - France) was used as the iodonium salt (Scheme 4). Phenacyl bromide (PBr), N-methyldiethanolamine (MDEA) and N-vinylcarbazole (NVK) were obtained from Aldrich and (4-hydroxyethoxyphenyl) thianthrenium hexafluorophosphate (TH; recrystallized form of Esacure 1187) from Lamberti Spa. The (epoxycyclohexylethyl)methylsiloxane-dimethylsiloxane copolymer (EPOX-Si) was obtained from Bluestar Silicones-France (Silcolease UV POLY 200); trimethylolpropane triacrylate TMPTA and (3,4-epoxycyclohexane)methyl 3,4-epoxycyclohexylcarboxylate (EPOX or UVACURE 1500) were provided by Cytec.
Photopolymerization procedures
TMPTA was irradiated at room temperature under lamination conditions: the formulation (25 μm thick) is sandwiched between two polypropylene films. The weight percentages for the photoinitiators are given in the figure captions. The evolution of the acrylate content was continuously followed by real-time FTIR spectroscopy (FTIR NEXUS 870) at ~1620 cm−1. The formulations (25 μm thick) based on the cationic monomers (EPOX, EPOX-Si) deposited on a BaF2 pellet were irradiated under air inside the IR spectrometer cavity at room temperature [56–57]. The evolution of the epoxy content was also continuously followed by real-time FTIR spectroscopy (FTIR NEXUS 870). A Xe–Hg lamp (Hamamatsu, L8252, 150 W, filtered light at λ > 340 nm; intensity ~ 30 mW/cm²) and a halogen lamp (intensity ~10 mW/cm²; the emission spectrum is given in [43]) were used as the irradiation sources.
ESR experiments
ESR spin-trapping (ESR-ST) experiments were carried out by using a X-Band ESR spectrometer (EMX-plus from Bruker Biospin or MS400 from Magnettech). The radicals were produced at RT under Xe–Hg lamp exposure and trapped by phenyl-N-tert-butylnitrone (PBN) according to a procedure described in detail in [58–59].
Redox potentials
The redox potentials were measured in acetonitrile by cyclic voltammetry with tetrabutylammonium hexafluorophosphate (98%) as a supporting electrolyte (0.1 M) (Voltalab 6 Radiometer; the working electrode was a platinum disk and the reference a saturated calomel electrode-SCE). Ferrocene was used as a standard and the potentials were determined from half-peak potentials. The free energy change ΔGet for an electron-transfer reaction is calculated from the classical Rehm–Weller equation (Equation 1) [60] where Eox, Ered, ES and C are the oxidation potential of the donor, the reduction potential of the acceptor, the excited state energy and the coulombic term for the initially formed ion pair, respectively. C is neglected as usually done in polar solvents.
| [1] |
Fluorescence experiments
The fluorescence properties of the different Co_Pys were studied by using a JASCO FP-750 spectrometer.
Computational procedure
Molecular orbital calculations were done with the Gaussian 03 suite of programs [61–62]. The electronic absorption spectra for the different compounds were calculated with the time-dependent density functional theory at B3LYP/6-31G* level on the relaxed geometries calculated at UB3LYP/6-31G* level.
Laser flash photolysis
Nanosecond laser flash photolysis (LFP) experiments were carried out [26–35] by using a Qswitched nanosecond Nd/YAG laser (λexc = 355 nm, 9 ns pulses; energy reduced down to 10 mJ) from Continuum (Minilite) and an analyzing system consisting of a ceramic Xenon lamp, a monochromator, a fast photomultiplier, and a transient digitizer (Luzchem LFP 212).
Supporting Information
Experimental procedures, characterization data, and additional spectra.
Acknowledgments
This work was partly supported by the “Agence Nationale de la Recherche” grant ANR 2010-BLAN-0802. JL thanks the Institut Universitaire de France for the financial support.
This article is part of the Thematic Series "Organic free radical chemistry".
Contributor Information
Didier Gigmes, Email: didier.gigmes@univ-amu.fr.
Jacques Lalevée, Email: jacques.lalevee@uha.fr.
References
- 1.Fouassier J-P, Lalevée J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency. Weinheim, Germany: Wiley-VCH; 2012. [DOI] [Google Scholar]
- 2.Fouassier J-P. Photoinitiation, Photopolymerization, Photocuring. Münen, Germany: Hanser; 1995. [Google Scholar]
- 3.Fouassier J-P, Rabek F J, editors. Radiation Curing in Polymer Science and Technology. London, U.K.: Chapman & Hall; 1993. [Google Scholar]
- 4.Crivello J V, Dietliker K. In: Photoinitiators for Free Radical, Cationic and Anionic Photopolymerization. Bradley G, editor. III. Wiley/SITA; 1999. ((Surface Coatings Technology)). [Google Scholar]
- 5.Dietliker K A. Compilation of Photoinitiators Commercially Available for UV Today. London, U.K.: Sita Technology Ltd; 2002. [Google Scholar]
- 6.Belfied K D, Crivello J V, editors. Photoinitiated Polymerization. Vol. 847. Washington DC, U.S.A.: American Chemical Society; 2003. ((ACS Symposium Series)). [DOI] [Google Scholar]
- 7.Allen N S, editor. Photochemistry and Photophysics of Polymer Materials. Hoboken: Wiley; 2010. [Google Scholar]
- 8.Scranton A B, Bowman A, Peiffer R W, editors. Photopolymerization: Fundamentals and Applications. Vol. 673. Washington DC, U.S.A.: American Chemical Society; 1997. ((ACS Symposium Series)). [DOI] [Google Scholar]
- 9.Green W A. Industrial Photoinitiators. Boca Raton: CRC Press; 2010. [DOI] [Google Scholar]
- 10.Mishra M K, Yagci Y, editors. Handbook of Vinyl Polymers. CRC Press; 2008. [Google Scholar]
- 11.Wang K, Jiang S, Liu J, Nie J, Yu Q. Prog Org Coat. 2011;72:517–521. doi: 10.1016/j.porgcoat.2011.06.011. [DOI] [Google Scholar]
- 12.Temel G, Enginol B, Aydin M, Karaca Balta D, Arsu N. J Photochem Photobiol, A: Chem. 2011;219:26–31. doi: 10.1016/j.jphotochem.2011.01.012. [DOI] [Google Scholar]
- 13.Wei J, Wang B. Macromol Chem Phys. 2011;212:88–95. doi: 10.1002/macp.201000535. [DOI] [Google Scholar]
- 14.Asvos X, Siskos M G, Zarkadis A K, Hermann R, Brede O. J Photochem Photobiol, A: Chem. 2011;219:255–264. doi: 10.1016/j.jphotochem.2011.02.028. [DOI] [Google Scholar]
- 15.Kabatc J, Jurek K. Polymer. 2012;53:1973–1980. doi: 10.1016/j.polymer.2012.03.027. [DOI] [Google Scholar]
- 16.Rosspeintner A, Griesser M, Pucher N, Iskra K, Liska R, Gescheidt G. Macromolecules. 2009;42:8034–8038. doi: 10.1021/ma901570h. [DOI] [Google Scholar]
- 17.Karaka-Balta D, Temel G, Goksu G, Okal N, Arsu N. Macromolecules. 2012;45:119–125. doi: 10.1021/ma202168m. [DOI] [Google Scholar]
- 18.Yilmaz G, Acik G, Yagci Y. Macromolecules. 2012;45:2219–2224. doi: 10.1021/ma3000169. [DOI] [Google Scholar]
- 19.Kumbaraci V, Aydogan B, Talinli N, Yagci Y. J Polym Sci, Part A: Polym Chem. 2012;50:2612–2618. doi: 10.1002/pola.26034. [DOI] [Google Scholar]
- 20.Tunc D, Yagci Y. Polym Chem. 2011;2:2557–2563. doi: 10.1039/c1py00269d. [DOI] [Google Scholar]
- 21.Kreutzer J, Dogan Demir K, Yagci Y. Polym J. 2011;47:792–799. [Google Scholar]
- 22.Lalevée J, Blanchard N, Tehfe M A, Morlet-Savary F, Fouassier J P. Macromolecules. 2010;43:10191–10195. doi: 10.1021/ma1023318. [DOI] [Google Scholar]
- 23.Zhang G, Song I Y, Ahn K H, Park T, Choi W. Macromolecules. 2011;44:7594–7599. doi: 10.1021/ma201546c. [DOI] [Google Scholar]
- 24.Lalevée J, Peter M, Dumur F, Gigmes D, Blanchard N, Tehfe M-A, Morlet-Savary F, Fouassier J P. Chem–Eur J. 2011;17:15027–15031. doi: 10.1002/chem.201101445. [DOI] [PubMed] [Google Scholar]
- 25.Tehfe M-A, Ma L, Graff B, Morlet-Savary F, Fouassier J-P, Zhao J, Lalevée J. Macromol Chem Phys. 2012;213:2282–2286. doi: 10.1002/macp.201200489. [DOI] [Google Scholar]
- 26.Lalevée J, Blanchard N, Fries C, Tehfe M A, Morlet-Savary F, Fouassier J P. Polym Chem. 2011;2:1077–1084. doi: 10.1039/c0py00392a. [DOI] [Google Scholar]
- 27.Tehfe M-A, Zein-Fakih A, Lalevée J, Dumur F, Gigmes D, Graff B, Morlet-Savary F, Hamieh T, Fouassier J P. Eur Polym J. 2013;49:567–574. doi: 10.1016/j.eurpolymj.2012.10.010. [DOI] [Google Scholar]
- 28.Telitel S, Lalevée J, Blanchard N, Kavalli T, Tehfe M-A, Schweizer S, Morlet-Savary F, Graff B, Fouassier J P. Macromolecules. 2012;45:6864–6868. doi: 10.1021/ma301293m. [DOI] [Google Scholar]
- 29.Tehfe M-A, Lalevée J, Morlet-Savary F, Graff B, Blanchard N, Fouassier J P. ACS Macro Lett. 2012;1:198–203. doi: 10.1021/mz200140y. [DOI] [PubMed] [Google Scholar]
- 30.Tehfe M-A, Lalevée J, Morlet-Savary F, Graff B, Blanchard N, Fouassier J P. Macromolecules. 2012;45:1746–1752. doi: 10.1021/ma300050n. [DOI] [Google Scholar]
- 31.Tehfe M-A, Dumur F, Contal E, Graff B, Morlet-Savary F, Gigmes D, Fouassier J P, Lalevée J. Polym Chem. 2013;4:1625–1634. doi: 10.1039/c2py20950k. [DOI] [Google Scholar]
- 32.Tehfe M-A, Lalevée J, Morlet-Savary F, Graff B, Fouassier J-P. Macromolecules. 2012;45:356–361. doi: 10.1021/ma202307x. [DOI] [Google Scholar]
- 33.Lalevée J, Dumur F, Tehfe M-A, Zein-Fakih A, Gigmes D, Morlet-Savary F, Fouassier J P. Polymer. 2012;53:4947–4954. doi: 10.1016/j.polymer.2012.08.067. [DOI] [Google Scholar]
- 34.Lalevée J, Tehfe M-A, Dumur F, Gigmes D, Blanchard N, Morlet-Savary F, Graff B, Fouassier J P. ACS Macro Lett. 2012;1:286–290. doi: 10.1021/mz2001753. [DOI] [PubMed] [Google Scholar]
- 35.Tehfe M-A, Lalevée J, Telitel S, Sun J, Zhao J, Graff B, Morlet-Savary F, Fouassier J-P. Polymer. 2012;53:2803–2808. doi: 10.1016/j.polymer.2012.05.009. [DOI] [Google Scholar]
- 36.Aydogan B, Yagci Y, Toppare L, Jockusch S, Turro N J. Macromolecules. 2012;45:7829–7834. doi: 10.1021/ma301546d. [DOI] [Google Scholar]
- 37.Aydogan B, Durmaz Y Y, Kahveci M U, Uygun M, Tasdelen M A, Yagci Y. Macromol Symp. 2011;308:25–34. doi: 10.1002/masy.201151005. [DOI] [Google Scholar]
- 38.Aydogan B, Gunbas G E, Durmus A, Toppare L, Yagci Y. Macromolecules. 2010;43:101–106. doi: 10.1021/ma901858p. [DOI] [Google Scholar]
- 39.Bulut U, Gunbas G E, Toppare L. J Polym Sci, Part A: Polym Chem. 2010;48:209–213. doi: 10.1002/pola.23779. [DOI] [Google Scholar]
- 40.Bulut U, Balan A, Caliskan C. J Polym Sci, Part A: Polym Chem. 2011;49:729–733. doi: 10.1002/pola.24485. [DOI] [Google Scholar]
- 41.Yagci Y, Jockusch S, Turro N J. Macromolecules. 2007;40:4481–4485. doi: 10.1021/ma070586a. [DOI] [Google Scholar]
- 42.Lalevée J, Tehfe M A, Dumur F, Gigmes D, Graff B, Morlet-Savary F, Fouassier J P. Macromol Rapid Commun. 2013;34:239–245. doi: 10.1002/marc.201200578. [DOI] [PubMed] [Google Scholar]
- 43.Tehfe M-A, Dumur F, Graff B, Clément J-L, Gigmes D, Morlet-Savary F, Fouassier J P, Lalevée J. Macromolecules. 2013;46:736–746. doi: 10.1021/ma3024359. [DOI] [Google Scholar]
- 44.Tehfe M-A, Lalevée J, Telitel S, Contal E, Dumur F, Gigmes D, Bertin D, Nechab M, Graff B, Morlet-Savary F, et al. Macromolecules. 2012;45:4454–4460. doi: 10.1021/ma300760c. [DOI] [Google Scholar]
- 45.Fisher H, editor. Landolt-Börnstein - Group II, Molecules and Radicals. 26d. Berlin, Germany: Springer Verlag; 2005. Magnetic Properties of Free Radicals, Nitroxide Radicals and Nitroxide Based High-Spin Systems. [Google Scholar]
- 46.Narayanam J M R, Stephenson C R J. Chem Soc Rev. 2011;40:102–113. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen J D, Tucker J W, Konieczynska M D, Stephenson C R J. J Am Chem Soc. 2011;133:4160–4163. doi: 10.1021/ja108560e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang C-H, Guo T-F, Sun I-W. J Lumin. 2007;124:93–98. doi: 10.1016/j.jlumin.2006.02.003. [DOI] [Google Scholar]
- 49.Tehfe M-A, Dumur F, Graff B, Morlet-Savary F, Fouassier J-P, Gigmes D, Lalevée J. Macromolecules. 2012;45:8639–8647. doi: 10.1021/ma301931p. [DOI] [Google Scholar]
- 50.Rajakumar P, Visalakshi K, Ganesan S, Maruthamuthu P, Suthanthiraraj S A. Bull Chem Soc Jpn. 2012;85:902–911. doi: 10.1246/bcsj.20110280. [DOI] [Google Scholar]
- 51.Lee D-H, Jin M-J. Org Lett. 2011;13:252–255. doi: 10.1021/ol102677r. [DOI] [PubMed] [Google Scholar]
- 52.Kim M K, Kwon J, Hong J P, Lee S, Hong J I. Bull Korean Chem Soc. 2011;32:2899–2905. doi: 10.5012/bkcs.2011.32.8.2899. [DOI] [Google Scholar]
- 53.Ishi-i T, Yaguma K, Thiemann T, Yashima M, Ueno K, Mataka S. Chem Lett. 2004;33:1244–1245. doi: 10.1246/cl.2004.1244. [DOI] [Google Scholar]
- 54.Castellanos F, Fouassier J P, Priou D, Cavezzan A, inventors. Onium borates/borates of organometallic complexes and cationic initiation of polymerization therewith. 5,668,192. U.S. Patent. 1997 Sep 16;
- 55.Castellanos F, Fouassier J P, Priou C, Cavezzan J. J Appl Polym Sci. 1996;60:705–713. doi: 10.1002/(SICI)1097-4628(19960502)60:5<705::AID-APP7>3.0.CO;2-U. [DOI] [Google Scholar]
- 56.Tehfe M-A, Lalevée J, Gigmes D, Fouassier J P. Macromolecules. 2010;43:1364–1370. doi: 10.1021/ma9025702. [DOI] [Google Scholar]
- 57.Tehfe M-A, Lalevée J, Gigmes D, Fouassier J P. J Polym Sci, Part A: Polym Chem. 2010;48:1830–1837. doi: 10.1002/pola.23956. [DOI] [Google Scholar]
- 58.Tordo P. Spin-trapping: recent developments and applications. In: Gilbert B C, Atherton N M, Davies M J, editors. Electron Pragmagnetic Resonance. Vol. 16. Cambridge, U. K.: The Royal Society of Chemistry; 1998. [Google Scholar]
- 59.Lalevée J, Dumur F, Mayer C R, Gigmes D, Nasr G, Tehfe M-A, Telitel S, Morlet-Savary F, Graff B, Fouassier J P. Macromolecules. 2012;45:4134–4141. doi: 10.1021/ma3005229. [DOI] [Google Scholar]
- 60.Rehm D, Weller A. Isr J Chem. 1970;8:259–271. [Google Scholar]
- 61.Gaussian 03. Wallingford, CT: Gaussian, Inc.; 2003. [Google Scholar]
- 62.Foresman J B, Frisch A. Exploring Chemistry with Electronic Structure Methods. 2nd ed. Wallingford, CT: Gaussian, Inc.; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data, and additional spectra.