Abstract
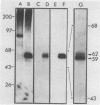
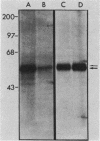
We raised antibodies in rabbits against the amino-terminal portion of the viral yes protein produced in bacteria with the use of an expression vector based on the lac operon. The anti-yes serum thus obtained precipitated P90gag-yes from Yamaguchi 73 virus-transformed chicken embryo fibroblasts, and this immunoprecipitation was blocked by the purified antigen. The anti-yes serum did not recognize viral src, fps, or fgr proteins. Affinity-purified anti-yes immunoglobulin G (IgG) precipitated two proteins of 59 and 62 kilodaltons from lysates of normal chicken embryo fibroblasts. Two-dimensional tryptic peptide mapping showed that these proteins are closely related to P90gag-yes and that they are different from pp60c-src. Similar to P90gag-yes, the 59- and 62-kilodalton proteins were phosphorylated exclusively on tyrosine in an in vitro kinase reaction, whereas in vivo they were phosphorylated on serine and, to a lesser extent, on tyrosine as well. Expression of the 59- and 62-kilodalton proteins, determined by the immune complex kinase assay, was relatively high in brain, retina, kidney, and liver. The presence in normal chicken embryo fibroblasts and in chicken kidney of two transcripts, 3.7 and 3.9 kilobases in length, that hybridize with a yes-specific DNA probe, as well as the two proteins recognized by anti-yes IgG, suggests either differential splicing of cellular yes gene transcripts or the existence of another yes-related gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Foster D. A., Levy J. B., Daley G. Q., Simon M. C., Hanafusa H. Isolation of chicken cellular DNA sequences with homology to the region of viral oncogenes that encodes the tyrosine kinase domain. Mol Cell Biol. 1986 Jan;6(1):325–331. doi: 10.1128/mcb.6.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Neil J. C., Vogt P. K. A third class of avian sarcoma viruses, defined by related transformation-specific proteins of Yamaguchi 73 and Esh sarcoma viruses. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2611–2615. doi: 10.1073/pnas.78.4.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Neil J. C., Wallbank A. M., Vogt P. K. Esh avian sarcoma virus codes for a gag-linked transformation-specific protein with an associated protein kinase activity. Virology. 1981 Jun;111(2):386–400. doi: 10.1016/0042-6822(81)90342-1. [DOI] [PubMed] [Google Scholar]
- Gilmer T. M., Parsons J. T., Erikson R. L. Construction of plasmids for expression of Rous sarcoma virus transforming protein, p60src, in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2152–2156. doi: 10.1073/pnas.79.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. R., Colot H. V., Guarente L., Rosbash M. Open reading frame cloning: identification, cloning, and expression of open reading frame DNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6598–6602. doi: 10.1073/pnas.79.21.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Hirata K., Inoue M., Hatsuoka M., Sato A. Isolation of a sarcoma virus from a spontaneous chicken tumor. Gan. 1978 Dec;69(6):825–830. [PubMed] [Google Scholar]
- Karess R. E., Hanafusa H. Viral and cellular src genes contribute to the structure of recovered avian sarcoma virus transforming protein. Cell. 1981 Apr;24(1):155–164. doi: 10.1016/0092-8674(81)90511-0. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Westbrook C. A., Diaz M. O., Rowley J. D. Evidence for two distinct c-src loci on human chromosomes 1 and 20. Nature. 1984 Nov 1;312(5989):70–71. doi: 10.1038/312070a0. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Naharro G., Tronick S. R., Rasheed S., Gardner M. B., Aaronson S. A., Robbins K. C. Molecular cloning of integrated Gardner-Rasheed feline sarcoma virus: genetic structure of its cell-derived sequence differs from that of other tyrosine kinase-coding onc genes. J Virol. 1983 Sep;47(3):611–619. doi: 10.1128/jvi.47.3.611-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Nucleotide sequence of avian sarcoma virus UR2 and comparison of its transforming gene with other members of the tyrosine protein kinase oncogene family. J Virol. 1985 Mar;53(3):879–884. doi: 10.1128/jvi.53.3.879-884.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Semba K., Yamamoto T., Toyoshima K. Human c-f gr gene does not contain coding sequence for actin-like protein. Jpn J Cancer Res. 1985 Mar;76(3):155–159. [PubMed] [Google Scholar]
- Nishizawa M., Semba K., Yoshida M. C., Yamamoto T., Sasaki M., Toyoshima K. Structure, expression, and chromosomal location of the human c-fgr gene. Mol Cell Biol. 1986 Feb;6(2):511–517. doi: 10.1128/mcb.6.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleuning W. D., Sudol M., Reich E. A proenzyme from chicken plasma similar to human plasma prekallikrein. J Biol Chem. 1983 Dec 10;258(23):14106–14115. [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Semba K., Yamanashi Y., Nishizawa M., Sukegawa J., Yoshida M., Sasaki M., Yamamoto T., Toyoshima K. Location of the c-yes gene on the human chromosome and its expression in various tissues. Science. 1985 Mar 1;227(4690):1038–1040. doi: 10.1126/science.2983418. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sudol M., Lerner T. L., Hanafusa H. Polymerase-defective mutant of the Bryan high-titer strain of Rous sarcoma virus. Nucleic Acids Res. 1986 Mar 11;14(5):2391–2405. doi: 10.1093/nar/14.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M., Reich E. Purification and characterization of a plasminogen activator secreted by a pig kidney cell line. Biochem J. 1984 May 1;219(3):971–978. doi: 10.1042/bj2190971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallbank A. M., Sperling F. G., Hubben K., Stubbs E. L. Isolation of a tumour virus from a chicken submitted to a poultry diagnostic laboratory--Esh sarcoma virus. Nature. 1966 Mar 19;209(5029):1265–1265. doi: 10.1038/2091265a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M. C., Sasaki M., Mise K., Semba K., Nishizawa M., Yamamoto T., Toyoshima K. Regional mapping of the human proto-oncogene c-yes-1 to chromosome 18 at band q21.3. Jpn J Cancer Res. 1985 Jul;76(7):559–562. [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Unifected avian cells contain structurally unrelated progenitors of viral sarcoma genes. Nature. 1980 Oct 16;287(5783):653–654. doi: 10.1038/287653a0. [DOI] [PubMed] [Google Scholar]