Abstract
Mouse embryonic chimeras are a well-established tool for studying cell lineage commitment and pluripotency. Experimental chimeras were successfully produced by combining of two or more preimplantation embryos or, by introduction into a host embryo cultured pluripotent embryonic stem cells (ESCs). Chimera production using genetically modified ESCs became the method of choice for generation of knockout or knockin mice. Although the derivation of ESCs or ESC-like cells has been reported for other species, only mouse and rat pluripotent stem cells have been shown to contribute to germline competent chimeras, which is the defining feature of ESCs. Herein we describe different approaches employed for the generation of embryonic chimeras, define chimera competent cell types and describe cases of spontaneous chimerism in humans. We also review the current state of derivation of pluripotent stem cells in several species and discuss outcomes of various chimera studies when such cells are used.
Developmental potential and embryonic chimerism
The term chimerism is derived from the Greek Χíμαιρα ‘she-goat or monster’, a monstrous fire-breathing creature composed of the parts of three animals: a lion, a serpent and a goat (http://en.wikipedia.org/wiki). In biology, the term chimera usually refers to a single organism composed of two or more different populations of genetically distinct cells originated from different zygotes. Typically, experimental embryonic chimeras are formed by aggregation of two or more whole early cleaving embryos or by combining isolated blastomeres from two or more embryos. The contribution level of the each parental cell types in tissues and organs of chimeric offspring can vary. For example, a chimeric organism could potentially consist of an equal mixture of parental embryonic cells in all cell and tissue types or contain only limited contribution of one of the genotypes in some tissues (microchimerism or Mc).
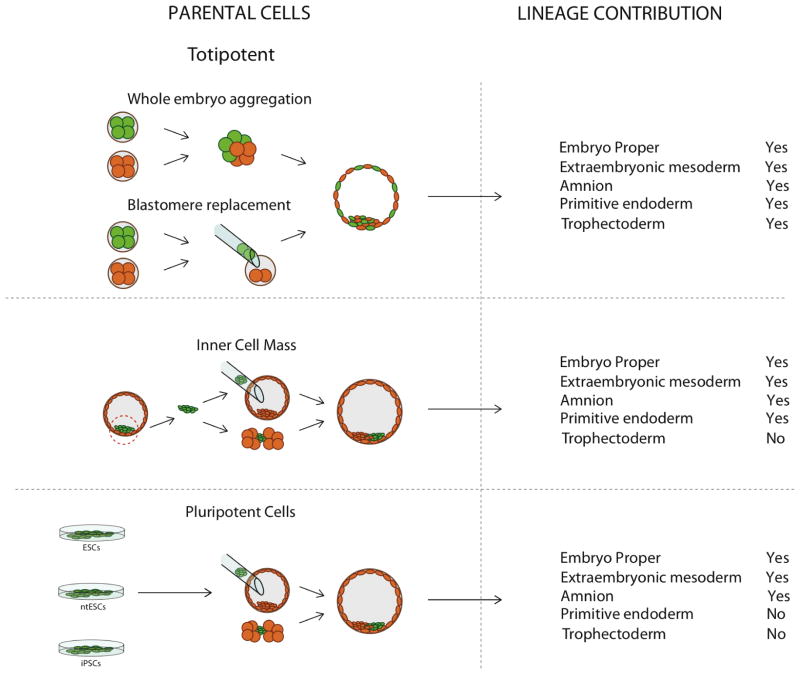
The level of contribution in embryonic chimeras is largely depends on the developmental potency of parental cells. Mammalian development originates from a state of totipotency, an attribute of zygotes and early cleaving blastomeres. Totipotency is defined as the ability of a single cell to divide and produce all the differentiated cells of an organism including extra-embryonic (placental) and embryonic tissues (embryo proper) (Mitalipov and Wolf 2009). As development advances, the totipotent cells undergo differentiation and segregation into developmentally more restricted cell lineages. The first visual differentiation of an embryo takes place during formation of a blastocyst, a stage consisting of two cell lineages: the inner cell mass (ICM) and the trophectoderm (TE). The ICM further segregates into epiblast and primitive endoderm, which subsequently form the embryo proper and parts of the yolk sac, respectively. The trophectoderm contributes to the extraembryonic tissues including primary and secondary giant cells, spongio trophoblast and chorionic ectoderm. The epiblast and its in vitro counterparts, embryonic stem cells are termed pluripotent based on their ability to give rise to all three germ layers (ectoderm, mesoderm and endoderm) of the embryo proper. However, epiblast and ESCs also contribute to some extraembryonic tissues (Beddington and Robertson 1989). Early studies have demonstrated that in addition to the embryo proper, mouse ESCs contribute to the amnion and the extraembryonic mesoderm of allantois, chorion and yolk sac. Since this phenomenon has not been thoroughly investigated, it could be one of the defining factors responsible for chimera competency of ESCs. It is also important to note that developmental potential of ESCs is more restricted than that of whole ICMs (Rossant and Lis 1979). As previously stated, it is well documented that in addition to the epiblast lineage, early ICM cells also contribute to the primitive endoderm (PE) that subsequently forms part of the yolk sac. In contrast, ESCs and other pluripotent cell types are not capable of forming the PE and rely on the host embryo complementation for this extraembryonic compartment in chimeras.
The early experimental mouse chimeras were produced in the sixties by aggregating of two or more whole 8-cell embryos that resulted in normal-sized mice whose tissues consist of a mixture of parental embryos (Mintz 1962, Tarkowski 1961). Chimerism in such embryos extends to the epiblast, the trophectoderm and primitive endoderm. Mouse aggregation chimeras have provided an invaluable tool to study important questions in developmental biology, cell lineage commitment, genetics and immunology (Alexandre 2001, McLaren 1976a, b, Tarkowski 1998). Gardner was able to produce mouse chimeras by an injection of isolated ICM cells into a host blastocyst cavity (Gardner 1968). Later on, as more mouse chimeric studies were conducted, it became apparent that despite introduction into the 8-cell, morula or blastocyst host embryos, the ICM contribution was always limited to the epiblast and the PE (Tam and Rossant 2003). Since the earlier studies were conducted, a range of other pluripotent cell types has been shown to contribute to mouse embryo proper chimeras. Specifically, pluripotent teratocarcinoma cells (Mintz and Illmensee 1975), ESCs (Bradley, et al. 1984), primordial germ cells (Matsui, et al. 1992), reprogrammed to the pluripotency by somatic cell nuclear transfer ESCs (ntESCs) (Wakayama, et al. 2001) and induced pluripotent stem cells (iPSCs) (Okita, et al. 2007) can also contribute to chimeras. The combination of host and donor cell types capable of forming mouse chimeras is illustrated in Fig. 1.
Figure 1. Developmental potential and contribution to chimeras.
Aggregation of whole embryos or totipotent blastomeres from early embryos results in chimeras with contribution to all embryonic (embryo proper) and extraembryonic lineages. Whole mouse ICMs injected into blastocysts or cleaving embryos participate in the formation of the embryo proper and most extraembryonic tissues in chimeras except the trophectoderm lineage. Contribution of pluripotent ESCs, ntESCs or iPSCs in chimeras is restricted to the embryo proper, amnion and the extraembryonic mesoderm of the allantois, chorion and visceral yolk sac.
Mouse chimeras can be also successfully produced with tetraploid host embryos. Experimentally produced tetraploid embryos can be derived by the fusion of mouse 2-cell embryos. Such embryos are not capable of forming viable offspring; however, they have an ability to contribute to functional extraembryonic tissues. When ESCs are introduced to mouse tetraploid embryos, they colonize the embryo proper, the amnion, the allantois and the mesoderm layer of the yolk sac; while tetraploid cells are restricted to the other remaining extraembryonic tissues resulting in almost completely ESC-derived postnatal offspring (Nagy, et al. 1993). Mutant and wild type embryos have been also used for aggregation chimeras to pinpoint timing and nature of developmental defects in mutant embryos. The failure or abnormal contribution of defective embryonic cells to a particular lineage, tissue, or organ in chimeras can be complemented and rescued by the wild type embryo cells thus allowing development to continue (Tam and Rossant 2003). ESC-tetraploid chimeras can be particularly useful to study defects in development of the extraembryonic lineages. A classical example is the targeted mutation of Hnf4, a gene encoding for transcription factor known as Hepatocyte nuclear factor 4. Homozygous mutant (Hnf4−/−) embryos fail to complete gastrulation resulting in early embryonic arrest (Chen, et al. 1994). Rescue of the extraembryonic compartment in chimeras by tetraploid host embryos allowed Hnf4−/− ESCs to undergo normal gastrulation suggesting that Hnf4−/− defects at this stage of development are affecting primarily the PE lineage (Duncan, et al. 1997).
While conventional chimeras are generated by aggregation of embryos or embryonic cells derived from the same developmental stage, chimeric offspring can be produced by mixing embryonic cells from different developmental stages (Gearhart and Oster-Granite 1981, Nagashima, et al. 2004). As pointed above, isolated ICMs can contribute to chimeras not only when injected into the blastocoelic cavity of host blastocysts, but also when introduced into 8-cell or morula stage embryos (Butler, et al. 1987, Nagy, et al. 1990, Picard, et al. 1990, Polzin, et al. 1987, Roth, et al. 1989).
It is critical to utilize genetic, biochemical or phenotypic markers that would allow distinguishing contribution of the parental cells in a chimeric organism. Aggregation of embryos with a distinct coat color pattern (different strain of mice) has been routinely used as one of the key marker of chimerism during early research studies on chimeras. Other common markers used initially were the electrophoretic variants of the housekeeping enzyme glucose-6-phosphase isomerase (GPI). The major limitation of the GPI is the inability to detect variants at the spatial histological level (Buehr and McLaren 1981). An ideal marker would detect a putative chimera at the single-cell level in situ. The first generation of genetic markers that were used to distinguished cells of different origins in chimeras were strain-specific DNA satellite markers (Rossant, et al. 1983). These markers allowed cell lineage tracking based on in situ hybridization of histological samples. At the present time, the most broadly utilized markers are the β-galactosidase enzyme encoded by the E. coli lacZ gene and the green fluorescent protein (GFP). Expression of lacZ can be detected by histochemical staining, while GFP expression detection requires epifluorescent microscopy. Since such transgenes are not readily available for other mammal, additional genetic markers such as microsatellites, also known as short tandem repeats (STRs), can be employed (Tachibana, et al. 2012).
Chimera competent pluripotent stem cells in the mouse
As discussed, the chimera assay is used as an ultimate pluripotency test for experimental cultured mouse pluripotent stem cells, such as ESCs and more recently, iPSCs. While natural pluripotent cells within developing embryos exist transiently, when isolated and explanted using adaptive culture conditions, they can grow in vitro but remain pluripotent. Embryo derived stem cells termed ESCs can be propagated indefinitely, providing an unlimited source of pluripotent cells. When a few ESCs are reintroduced into a host embryo, they can resume normal developmental program and contribute to all tissues and organs of chimeric offspring. ESCs can be readily isolated using standard techniques, but only from a very few so-called “permissive” mouse strains (129, C57Bl/6 and BALB/C).
Pluripotent cell lines also have been derived from the postimplantation mouse epiblasts (day E5.5–7.5) and were termed EpiSCs (Tesar, et al. 2007). Such cells are morphologically distinct from the mouse ESCs and also differ based on their growth and culture requirements (Tesar, et al. 2007). The fundamental distinction from mouse ESCs is that EpiSCs do not contribute to chimeras (Guo, et al. 2009, Rossant 2008). Speculation is that EpiSCs represent more restricted (“primed”) pluripotent cells while ESCs symbolize developmentally more potent (“naïve”) state of pluripotency (Nichols and Smith 2009). The inability of EpiSCs to form chimeras could simply be due to their failure to contribute to the vital extraembryonic tissues. As we have discussed, the epiblast and ESCs colonize the amnion, the allantois as well as the mesoderm layer of the yolk sac. It is conceivable that their initial contribution to the extraembryonic niche is required for subsequent development into the lineages of the embryo proper.
Experimental pluripotent stem cells can be also produced by reprogramming somatic cells. Reprogrammed somatic cells may have a particularly important role in future clinical applications of autologous cell replacement therapies since the patient’s own cells would evade host immune based rejection. Reprogramming of somatic cells to pluripotency can be achieved using two alternative approaches: 1) by direct reprogramming into iPSCs or 2) by somatic cell nuclear transfer into ntESCs. Direct reprogramming by current techniques operates by delivering and expressing exogenous genes known to be essential for pluripotency (Takahashi and Yamanaka 2006). Somatic cell nuclear transfer, often referred as cloning, is based on oocyte-assisted reprogramming, where the cytoplasm of an enucleated oocyte delivers the essential epigenetic and cytoplasmic factors that support natural reprogramming processes and thus recapitulates the normal development of totipotent and pluripotent cells.
Both mouse ntESCs and iPSCs share the defining feature of embryo derived ESCs; that is, their ability to contribute to chimeras (Figure 2). In addition, ntESCs and iPSCs have met the current most stringent pluripotency assays and have generated all-stem cell derived mice following tetraploid complementation (Boland, et al. 2009, Lin, et al. 2010). However, when ntESCs and iPSCs were derived from genetically identical donor cells and then compared for their ability to generate mice through tetraploid embryo complementation, only ntESCs resulted in a successful outcome; iPSCs failed to produce whole iPSC mice. This demonstrates that nuclear transfer can generate ESC-equivalent pluripotent stem cells more effectively than factor-based reprogramming (Jiang, et al. 2011).
Figure 2. Mouse and rhesus monkey chimeras.
Upper Panel: mouse chimeric pups produced with iPSCs in the Mitalipov laboratory (unpublished results). iPSCs were derived by transduction of skin fibroblasts isolated from C57Bl/6 (black) strain with lentiviral vectors carrying Yamanaka’s reprogramming factors (Oct4, Sox2, Klf4 and c-Myc). To evaluate developmental competence, approximately 8–10 isolated iPSCs were injected into cleaving 4–8-cell embryos from ICR (albino) mice and transplanted into recipients. Note black coat color spots in offspring demonstrating contribution of iPSCs to chimeras.
Lower panel: Roku and Hex, chimeric rhesus infants each produced by aggregating of six individual 4-cell embryos (Tachibana et al., 2012). Extensive contribution of parental embryos in the blood and extraembryonic tissues was confirmed using microsatellite and mtDNA genotyping. In addition, gender specific PCR, G-banding and fluorescent in situ hybridization (FISH) based cytogenetic assays detected presence of both male (XY) and female (XX) cells in Roku suggesting sex chimerism.
Experimental Embryonic Chimeras in Other Species
Aggregation of early cleaving embryos (or isolated blastomeres) have resulted in the birth of chimeric animals in number of mammals including sheep (Tucker, et al. 1974), rats (Mayer and Fritz 1974), rabbits (Gardner and Munro 1974), cattle (Brem, et al. 1984) and more recently in nonhuman primates (Tachibana, et al. 2012). Moreover, aggregation of goat and sheep early embryos or injection of goat ICMs into sheep blastocysts can also result in a birth of live interspecies chimeras (Fehilly, et al. 1985, Fehilly, et al. 1984, Polzin, et al. 1987, Roth, et al. 1989). Chimeric cattle fetuses were produced by aggregation of embryos generated through somatic cell nuclear transfer with IVF embryos (Stice, et al. 1996).
In the rhesus monkey, transplantation of ICMs into blastocysts will not result in efficient integration into the host ICM (Tachibana et al 2012). Unlike mouse ICM chimeras, monkey donor and host ICMs develop into separate offspring while sharing the TE compartment of host blastocysts. Thus, whole ICM injection into a host blastocyst will often produce twin fetuses. Analysis of such rhesus fetuses demonstrated limited chimerism in their bodies (embryo proper) (Tachibana 2012). Specifically, chimerism was only detected in livers and spleens that possibly could result from the exchange of blood and blood stem cells through placental perfusions. However, chimerism in the extraembryonic compartment consisting of chorionic and amniotic tissues was extensive indicating that both the host embryo and injected ICMs contributed to these lineages.
To investigate possible mechanisms responsible for inability of injected ICMs to incorporate with host ICMs and form embryo proper chimeras, monkey blastocysts and ICMs were analyzed for lineage segregation. Cells within an ICM in mouse preimplantation stage (E3.5) blastocysts are relatively homogeneous and not visibly differentiated into epiblast and PE fates. Such segregation is apparent in peri-implantation blastocysts (E4.5), where the layer of PE is spatially separated and covers underlying epiblast cells (Cockburn and Rossant 2010). This segregation coincides with significant decline in the ability of host ICM to incorporate injected pluripotent cells and form mouse chimeras (Ohta, et al. 2008).
Analysis of preimplantation blastocysts has demonstrated that monkey ICMs have already segregated into the epiblast and PE lineages. Even in early blastocysts, ICMs consisted of a cluster of NANOG positive epiblast that was covered by GATA-6 positive PE cells. This event likely prevents efficient aggregation of injected ICMs or ESCs with donor ICMs and the formation of embryo proper chimeras.
In contrast to ICMs, monkey chimeras were efficiently produced by aggregation of cleaving 4-cell embryos (Figure 2). It was demonstrated that blastomeres of the 4-cell monkey embryos are totipotent since a single blastomere can support full-term development (Chan, et al. 2000). Between 3 to 6 individual 4-cell monkey embryos were aggregated together and 14 resulting blastocysts were transplanted into 5 recipients (Tachibana, et al. 2012). Remarkably, all 5 recipients become pregnant − 2 females carried singletons, another 2 carried twins and one recipient had quadruplets. Such high pregnancy (100%) and implantation rates (10/14 or 71%) were remarkable considering that average rhesus embryo transfer outcomes with non-chimeric embryos do not exceed 36% pregnancy and 17% implantation rates (Wolf, et al. 2004). Since chimeric blastocysts consisted of much higher cell counts (double or triple of regular numbers), it is possible that sufficient cell numbers in preimplantation embryos is critical for pregnancy initiation.
When several midgestation rhesus fetuses were recovered and analyzed for genetic contribution of parental embryos, all offspring were confirmed to be chimeras. Remarkably, chimerism was detected in all tested tissues and organs including placenta, spleen, reproductive tract, bladder, pancreas, stomach, small-intestine, adrenal gland, kidney and muscle. Since, chimeric embryos were generated by aggregating together of 3 or more cleaving embryos, some fetuses displayed contribution of multiple parental genotypes. Chimerism was also confirmed in 3 live born infants that were phenotypically normal males (Figure 2). However, detailed cytogenetic analysis of blood showed that one infant contained both male (XY) and female (XX) cells confirming gender chimerism.
ESCs in Other Species
Although derivation of ESCs or ESC-like cells expressing pluripotency markers has been reported for several species, only mouse and rat ESCs have been shown so far to contribute to germline chimeras. The first germline-competent rat ESCs were recently derived using modified culture conditions (Li, et al. 2008). These cells express typical pluripotency markers and retain the capacity to differentiate into derivatives of all three germ layers. Most importantly, they efficiently induce chimeras when reintroduced into early cleaving embryos (Li, et al. 2008). Rat ESCs were also capable of contributing to the germline and some extraembryonic lineages (Demers, et al. 2011).
It is currently assumed that species-specific differences in ESC maintenance and culture between mouse and farm animals are the posing factors challenging the identification and derivation of putative ESCs. As an example, enzymatic dissociation of cattle ESCs results in cell death or differentiation (Cibelli, et al. 1998, Stice, et al. 1996). In addition, there is inconsistency among proposed markers for defining genuine bovine ESCs (Malaver-Ortega, et al. 2012). It has been reported that livestock ESCs share some morphologic features with mouse EpiSCs rather than with mouse ESCs. These unique characteristics include flat epithelial-like cells that have low tolerance to enzymatic single cell dissociation (Tesar, et al. 2007). Several studies have reported the isolation of bovine ESC-like cells that are capable of contributing to the somatic tissues in chimeric offspring (Cibelli, et al. 1998, Saito 1992). Putative ESCs were also described from porcine blastocysts and have been shown to contribute to some somatic tissues in chimeras (Brevini, et al. 2010, Chen, et al. 1999, Vassiliev, et al. 2010, Wheeler 1994). There was no germ line contribution of ESCs documented in these chimeras. Caprine and ovine embryo-derived cell lines have been also isolated, however pluripotency was not tested in chimeras (Behboodi, et al. 2011, Dattena, et al. 2006, Kumar De, et al. 2011, Notarianni, et al. 1991, Pawar, et al. 2009, Wells, et al. 1997).
Recent advances in iPSC technology have opened new opportunities for generation of pluripotent stem cells from many species including rat (Liao, et al. 2009), rhesus monkey (Liu, et al. 2008), cattle (Han, et al. 2011), sheep (Liu, et al. 2012) and pig (Ezashi, et al. 2009, Wu, et al. 2009). Porcine iPSCs were recently reported to induce chimeras contributing to multiple tissues that represented all 3 germ layers (West, et al. 2010). Fujishiro and co-workers demonstrated generation of naïve-like porcine iPSCs also capable of contributing to a variety of organs in chimeric fetuses (head, brachial arch, atrium, ventricle, liver, and limb bud) (Fujishiro, et al. 2012). However, no germline contribution has been observed.
Chimerism in Humans
Embryonic chimerism in humans generally occurs by spontaneous aggregation of two different zygotes or embryos. Lacking the visible features of chimerism, the condition goes underdiagnosed in most cases. Therefore, reported cases of chimerism are generally those associated with either developmental anomalies or genotype/sex discordance (Boklage 2006). One classic example of tetragametic chimerism was a woman waiting for organ donation from her biological children. However, the histocompatibility testing indicated that the children were of different genotypes. She was later confirmed to be a germ-line chimera producing two different germ cell populations (Yu, et al. 2002). Interestingly, she was phenotypically normal XX/XX chimera with no detectable chimerism in her peripheral blood.
Infertility treatments by in vitro fertilization (IVF) could potentially increase the risk of spontaneous tetragametic chimerism. Typically, improved pregnancy rates are achieved by placing more than one embryo into a patient. This can result in up to 30-to-35-fold increase in dizygotic-twin deliveries. Multiple embryo transfers could also lead to the increased risk of chimerism (Strain, et al. 1998).
Human chimerism can be also caused by aggregation of fertilized embryos with unfertilized parthenogenetic or androgenetic embryos or by aggregation with the fertilized second polar body (Malan, et al. 2006, Strain, et al. 1995).
The term microchimerism refers to a small population of donor cells in the body (fewer than 1 in 100 cells) (Gammill and Nelson 2010). Naturally acquired Mc originates primarily from fetal cells in the mother during pregnancy (fetal microchimerism) or maternal cells in her children (maternal microchimerism). Exchange of hematopoietic and other cells between twin fetuses can also result in microchimerism (De Moor, et al. 1988). Feto-maternal cell exchange starts as early as six weeks of gestation (Ariga, et al. 2001) and increases as pregnancy progresses. At 36 weeks of gestation, pregnant women have detectable fetal cells in their circulation. After delivery, the presence of fetal cells in a mother rapidly declines. However, sensitive PCR assays have shown that between 30–50% of women carry detectable fetal cells in the blood and hematopoietic tissues (e.g. spleen, lymph nodes) for several months and in some cases for decades post-partum (Evans, et al. 1999). Most of the fetal origin cells in women express CD45, the common leukocyte antigen, indicating a likely hematopoietic origin. Y-chromosome in situ hybridization techniques identified male fetal origin cells in lung, lymph note, skin, thyroid, kidney, liver and heart of postpartum women (Koopmans, et al. 2008). The persistence of fetal cells long-term in the maternal environment strongly indicates that this population contains stem cells, termed pregnancy associated progenitor cells (PAPCs) (Khosrotehrani and Bianchi 2005). One possible origin of PAPCs is fetal hematopoietic stem cells (HSCs) as placenta has 2–4 times more HSCs than other hematopoietic tissues (e.g., liver or yolk sac) (Alvarez-Silva, et al. 2003). However, Mc is not limited to the hematopoietic lineage. For example, differentiated maternal origin cells in were found among hepatocytes in liver, renal tubular cells in kidney and β-islet cells in pancreas (Oliver-Krasinski and Stoffers 2008). Male mesenchymal stem cells (presumably fetal origin) were found in a bone marrow samples obtained from women who had sons from 13 to 51 years of age (O’Donoghue, et al. 2004).
The widespread presence of maternal and fetal Mc in humans in a variety of tissues and organs raises some questions about the biological significance of this phenomenon (Oliver-Krasinski and Stoffers 2008). Mouse studies suggested that fetal cells in postpartum females may be involved in tissue repair. For example, fetal cells migrated to injured liver, heart and brain tissues (Zeng, et al. 2010) (Kara, et al. 2012).
Conclusions
Naturally occurring embryonic chimeras in mammals are rare but experimental induction of chimeric animals by mixing early embryonic cells has gained a lot of momentum and is currently being used in biomedical research for potency determination of various cell types. Mouse chimeras in particular with ESCs have become a revolutionary assay to study the gene function in knockout models. The chimera assay is the most rigorous and ultimate measure of pluripotency in experimental pluripotent cells derived by the reprogramming of somatic cells. Specifically, tetraploid chimeras are considered as the most comprehensive test as it enables production of whole stem cell-derived mouse offspring. Chimerism in humans is a much more common phenomenon than originally thought. Particularly, microchimerism resulting from mutual penetration of fetal and maternal cells is common in children and their mothers.
Acknowledgments
The authors would like to acknowledge Drs. Masahito Tachibana and Eunju Kang for assistance in writing this review and for proving with illustrative materials. We are grateful to Dr. Thomas Bunch for helpful discussions and critical reading of the manuscript. This work was supported by grants from the National Institutes of Health HD063276, HD057121, HD059946, EY021214, 8P51OD011092 and funds from the Leducq Fondation to S.M. I.P. was supported by the Utah Science Technology and Research Initiative and Utah Multidisciplinary Arrhythmia Consortium.
Footnotes
The authors have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Alexandre H. A history of mammalian embryological research. The International journal of developmental biology. 2001;45:457–467. [PubMed] [Google Scholar]
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. doi: 10.1242/dev.00755. [DOI] [PubMed] [Google Scholar]
- Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, Lee TH. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41:1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- Behboodi E, Bondareva A, Begin I, Rao K, Neveu N, Pierson JT, Wylie C, Piero FD, Huang YJ, Zeng W, Tanco V, Baldassarre H, Karatzas CN, Dobrinski I. Establishment of goat embryonic stem cells from in vivo produced blastocyst-stage embryos. Molecular reproduction and development. 2011;78:202–211. doi: 10.1002/mrd.21290. [DOI] [PubMed] [Google Scholar]
- Boklage CE. Embryogenesis of chimeras, twins and anterior midline asymmetries. Human reproduction. 2006;21:579–591. doi: 10.1093/humrep/dei370. [DOI] [PubMed] [Google Scholar]
- Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, Baldwin KK. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brem G, Tenhumberg H, Krausslich H. Chimerism in cattle through microsurgical aggregation of morulae. Theriogenology. 1984;22:609–613. doi: 10.1016/0093-691x(84)90061-x. [DOI] [PubMed] [Google Scholar]
- Brevini TA, Pennarossa G, Attanasio L, Vanelli A, Gasparrini B, Gandolfi F. Culture conditions and signalling networks promoting the establishment of cell lines from parthenogenetic and biparental pig embryos. Stem cell reviews. 2010;6:484–495. doi: 10.1007/s12015-010-9153-2. [DOI] [PubMed] [Google Scholar]
- Buehr M, McLaren A. An electrophoretically detectable modification of glucosephosphate isomerase in mouse spermatozoa. Journal of reproduction and fertility. 1981;63:169–173. doi: 10.1530/jrf.0.0630169. [DOI] [PubMed] [Google Scholar]
- Butler JE, Anderson GB, BonDurant RH, Pashen RL, Penedo MC. Production of ovine chimeras by inner cell mass transplantation. Journal of animal science. 1987;65:317–324. doi: 10.2527/jas1987.651317x. [DOI] [PubMed] [Google Scholar]
- Chan AW, Dominko T, Luetjens CM, Neuber E, Martinovich C, Hewitson L, Simerly CR, Schatten GP. Clonal propagation of primate offspring by embryo splitting. Science. 2000;287:317–319. doi: 10.1126/science.287.5451.317. [DOI] [PubMed] [Google Scholar]
- Chen LR, Shiue YL, Bertolini L, Medrano JF, BonDurant RH, Anderson GB. Establishment of pluripotent cell lines from porcine preimplantation embryos. Theriogenology. 1999;52:195–212. doi: 10.1016/S0093-691X(99)00122-3. [DOI] [PubMed] [Google Scholar]
- Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE., Jr Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes & development. 1994;8:2466–2477. doi: 10.1101/gad.8.20.2466. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de Leon FA, Robl JM. Transgenic bovine chimeric offspring produced from somatic cell-derived stem-like cells. Nature biotechnology. 1998;16:642–646. doi: 10.1038/nbt0798-642. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. The Journal of clinical investigation. 2010;120:995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattena M, Chessa B, Lacerenza D, Accardo C, Pilichi S, Mara L, Chessa F, Vincenti L, Cappai P. Isolation, culture, and characterization of embryonic cell lines from vitrified sheep blastocysts. Molecular reproduction and development. 2006;73:31–39. doi: 10.1002/mrd.20378. [DOI] [PubMed] [Google Scholar]
- De Moor G, De Bock G, Noens L, De Bie S. A new case of human chimerism detected after pregnancy: 46, XY karyotype in the lymphocytes of a woman. Acta clinica Belgica. 1988;43:231–235. doi: 10.1080/17843286.1988.11717936. [DOI] [PubMed] [Google Scholar]
- Demers SP, Desmarais JA, Vincent P, Smith LC. Rat blastocyst-derived stem cells are precursors of embryonic and extraembryonic lineages. Biology of reproduction. 2011;84:1128–1138. doi: 10.1095/biolreprod.109.082792. [DOI] [PubMed] [Google Scholar]
- Duncan SA, Nagy A, Chan W. Murine gastrulation requires HNF-4 regulated gene expression in the visceral endoderm: tetraploid rescue of Hnf-4(−/−) embryos. Development. 1997;124:279–287. doi: 10.1242/dev.124.2.279. [DOI] [PubMed] [Google Scholar]
- Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–2037. [PubMed] [Google Scholar]
- Ezashi T, Telugu BP, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10993–10998. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehilly CB, Willadsen SM, Dain AR, Tucker EM. Cytogenetic and blood group studies of sheep/goat chimaeras. Journal of reproduction and fertility. 1985;74:215–221. doi: 10.1530/jrf.0.0740215. [DOI] [PubMed] [Google Scholar]
- Fehilly CB, Willadsen SM, Tucker EM. Interspecific chimaerism between sheep and goat. Nature. 1984;307:634–636. doi: 10.1038/307634a0. [DOI] [PubMed] [Google Scholar]
- Fujishiro SH, Nakano K, Mizukami Y, Azami T, Arai Y, Matsunari H, Ishino R, Nishimura T, Watanabe M, Abe T, Furukawa Y, Umeyama K, Yamanaka S, Ema M, Nagashima H, Hanazono Y. Generation of Naive-Like Porcine-Induced Pluripotent Stem Cells Capable of Contributing to Embryonic and Fetal Development. Stem cells and development. 2012 doi: 10.1089/scd.2012.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill HS, Nelson JL. Naturally acquired microchimerism. The International journal of developmental biology. 2010;54:531–543. doi: 10.1387/ijdb.082767hg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL. Mouse chimeras obtained by the injection of cells into the blastocyst. Nature. 1968;220:596–597. doi: 10.1038/220596a0. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Munro AJ. Successful construction of chimaeric rabbit. Nature. 1974;250:146–147. doi: 10.1038/250146a0. [DOI] [PubMed] [Google Scholar]
- Gearhart J, Oster-Granite ML. Reproduction in a population of chimeric mice: relationship of chromosomal sex to functional germ cells and proportions of chimeric components in several tissues. Biology of reproduction. 1981;24:713–722. doi: 10.1095/biolreprod24.4.713. [DOI] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Han J, Ding F, Cao S, Lim SS, Dai Y, Zhang R, Zhang Y, Lim B, Li N. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell research. 2011;21:1509–1512. doi: 10.1038/cr.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Ding G, Lin J, Zhang M, Shi L, Lv W, Yang H, Xiao H, Pei G, Li Y, Wu J, Li J. Different developmental potential of pluripotent stem cells generated by different reprogramming strategies. Journal of molecular cell biology. 2011;3:197–199. doi: 10.1093/jmcb/mjr012. [DOI] [PubMed] [Google Scholar]
- Kara RJ, Bolli P, Karakikes I, Matsunaga I, Tripodi J, Tanweer O, Altman P, Shachter NS, Nakano A, Najfeld V, Chaudhry HW. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circulation research. 2012;110:82–93. doi: 10.1161/CIRCRESAHA.111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosrotehrani K, Bianchi DW. Multi-lineage potential of fetal cells in maternal tissue: a legacy in reverse. Journal of cell science. 2005;118:1559–1563. doi: 10.1242/jcs.02332. [DOI] [PubMed] [Google Scholar]
- Koopmans M, Kremer Hovinga IC, Baelde HJ, Harvey MS, de Heer E, Bruijn JA, Bajema IM. Chimerism occurs in thyroid, lung, skin and lymph nodes of women with sons. Journal of reproductive immunology. 2008;78:68–75. doi: 10.1016/j.jri.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kumar De A, Malakar D, Akshey YS, Jena MK, Dutta R. Isolation and characterization of embryonic stem cell-like cells from in vitro produced goat (Capra hircus) embryos. Animal biotechnology. 2011;22:181–196. doi: 10.1080/10495398.2011.622189. [DOI] [PubMed] [Google Scholar]
- Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, Pera MF, Ying QL. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Cui C, Chen S, Ren J, Chen J, Gao Y, Li H, Jia N, Cheng L, Xiao H, Xiao L. Generation of induced pluripotent stem cell lines from adult rat cells. Cell stem cell. 2009;4:11–15. doi: 10.1016/j.stem.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Amano T, Zhang J, Chen YE, Tian XC. Acceptance of embryonic stem cells by a wide developmental range of mouse tetraploid embryos. Biology of reproduction. 2010;83:177–184. doi: 10.1095/biolreprod.110.084707. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H, Jiang W, Cai J, Liu M, Cui K, Qu X, Xiang T, Lu D, Chi X, Gao G, Ji W, Ding M, Deng H. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell stem cell. 2008;3:587–590. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Liu J, Balehosur D, Murray B, Kelly JM, Sumer H, Verma PJ. Generation and characterization of reprogrammed sheep induced pluripotent stem cells. Theriogenology. 2012;77:338–346 e331. doi: 10.1016/j.theriogenology.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Malan V, Vekemans M, Turleau C. Chimera and other fertilization errors. Clinical genetics. 2006;70:363–373. doi: 10.1111/j.1399-0004.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- Malaver-Ortega LF, Sumer H, Liu J, Verma PJ. The state of the art for pluripotent stem cells derivation in domestic ungulates. Theriogenology. 2012 doi: 10.1016/j.theriogenology.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Mayer JF, Jr, Fritz HI. The culture of preimplantation rat embryos and the production of allophenic rats. Journal of reproduction and fertility. 1974;39:1–9. doi: 10.1530/jrf.0.0390001. [DOI] [PubMed] [Google Scholar]
- McLaren A. Genetics of the early mouse embryo. Annual review of genetics. 1976a;10:361–388. doi: 10.1146/annurev.ge.10.120176.002045. [DOI] [PubMed] [Google Scholar]
- McLaren A. Mammalian Chimaeras. Cambridge: Cambridge University Press; 1976b. [Google Scholar]
- Mintz B. Experimental Study of the Developing Mammalian Egg: Removal of the Zona Pellucida. Science. 1962;138:594–595. doi: 10.1126/science.138.3540.594. [DOI] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Advances in biochemical engineering/biotechnology. 2009;114:185–199. doi: 10.1007/10_2008_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima H, Giannakis C, Ashman RJ, Nottle MB. Sex differentiation and germ cell production in chimeric pigs produced by inner cell mass injection into blastocysts. Biology of reproduction. 2004;70:702–707. doi: 10.1095/biolreprod.103.022681. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Markkula M, Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell stem cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Notarianni E, Galli C, Laurie S, Moor RM, Evans MJ. Derivation of pluripotent, embryonic cell lines from the pig and sheep. Journal of reproduction and fertility Supplement. 1991;43:255–260. [PubMed] [Google Scholar]
- O’Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, Roberts IA, Fisk NM. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- Ohta H, Sakaide Y, Wakayama T. Generation of mice derived from embryonic stem cells using blastocysts of different developmental ages. Reproduction. 2008;136:581–587. doi: 10.1530/REP-08-0184. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes & development. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar SS, Malakar D, De AK, Akshey YS. Stem cell-like outgrowths from in vitro fertilized goat blastocysts. Indian journal of experimental biology. 2009;47:635–642. [PubMed] [Google Scholar]
- Picard L, Chartrain I, King WA, Betteridge KJ. Production of chimaeric bovine embryos and calves by aggregation of inner cell masses with morulae. Molecular reproduction and development. 1990;27:295–304. doi: 10.1002/mrd.1080270404. [DOI] [PubMed] [Google Scholar]
- Polzin VJ, Anderson DL, Anderson GB, BonDurant RH, Butler JE, Pashen RL, Penedo MC, Rowe JD. Production of sheep-goat chimeras by inner cell mass transplantation. Journal of animal science. 1987;65:325–330. doi: 10.2527/jas1987.651325x. [DOI] [PubMed] [Google Scholar]
- Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Rossant J, Lis WT. The possible dual origin of the ectoderm of the chorion in the mouse embryo. Developmental biology. 1979;70:249–254. doi: 10.1016/0012-1606(79)90021-6. [DOI] [PubMed] [Google Scholar]
- Rossant J, Vijh M, Siracusa LD, Chapman VM. Identification of embryonic cell lineages in histological sections of M. musculus in-equilibrium M. caroli chimaeras. Journal of embryology and experimental morphology. 1983;73:179–191. [PubMed] [Google Scholar]
- Roth TL, Anderson GB, Bon Durant RH, Pashen RL. Survival of sheep × goat hybrid inner cell masses after injection into ovine embryos. Biology of reproduction. 1989;41:675–682. doi: 10.1095/biolreprod41.4.675. [DOI] [PubMed] [Google Scholar]
- Saito SSN, Niemann H. Bovine embryonic stem cell-like cell lines cultured over several passages. Roux’s Arch Dev Biol. 1992:134–141. doi: 10.1007/BF00188711. [DOI] [PubMed] [Google Scholar]
- Stice SL, Strelchenko NS, Keefer CL, Matthews L. Pluripotent bovine embryonic cell lines direct embryonic development following nuclear transfer. Biology of reproduction. 1996;54:100–110. doi: 10.1095/biolreprod54.1.100. [DOI] [PubMed] [Google Scholar]
- Strain L, Dean JC, Hamilton MP, Bonthron DT. A true hermaphrodite chimera resulting from embryo amalgamation after in vitro fertilization. The New England journal of medicine. 1998;338:166–169. doi: 10.1056/NEJM199801153380305. [DOI] [PubMed] [Google Scholar]
- Strain L, Warner JP, Johnston T, Bonthron DT. A human parthenogenetic chimaera. Nature genetics. 1995;11:164–169. doi: 10.1038/ng1095-164. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Ramsey C, Ma H, Lee HS, Penedo MC, Mitalipov S. Generation of chimeric rhesus monkeys. Cell. 2012;148:285–295. doi: 10.1016/j.cell.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tam PP, Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155–6163. doi: 10.1242/dev.00893. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK. Mouse chimaeras developed from fused eggs. Nature. 1961;190:857–860. doi: 10.1038/190857a0. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK. Mouse chimaeras revisited: recollections and reflections. The International journal of developmental biology. 1998;42:903–908. [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Tucker EM, Moor RM, Rowson LE. Tetraparental sheep chimaeras induced by blastomere transplantation. Changes in blood type with age. Immunology. 1974;26:613–621. [PMC free article] [PubMed] [Google Scholar]
- Vassiliev I, Vassilieva S, Beebe LF, Harrison SJ, McIlfatrick SM, Nottle MB. In vitro and in vivo characterization of putative porcine embryonic stem cells. Cellular reprogramming. 2010;12:223–230. doi: 10.1089/cell.2009.0053. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- Wells DN, Misica PM, Day TA, Tervit HR. Production of cloned lambs from an established embryonic cell line: a comparison between in vivo- and in vitro-matured cytoplasts. Biology of reproduction. 1997;57:385–393. doi: 10.1095/biolreprod57.2.385. [DOI] [PubMed] [Google Scholar]
- West FD, Terlouw SL, Kwon DJ, Mumaw JL, Dhara SK, Hasneen K, Dobrinsky JR, Stice SL. Porcine induced pluripotent stem cells produce chimeric offspring. Stem cells and development. 2010;19:1211–1220. doi: 10.1089/scd.2009.0458. [DOI] [PubMed] [Google Scholar]
- Wheeler MB. Development and validation of swine embryonic stem cells: a review. Reproduction, fertility, and development. 1994;6:563–568. doi: 10.1071/rd9940563. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S. Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biology of reproduction. 2004;71:486–493. doi: 10.1095/biolreprod.103.025932. [DOI] [PubMed] [Google Scholar]
- Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, Rao L, Li H, Gu Y, Dai H, Zhu H, Teng X, Cheng L, Xiao L. Generation of pig induced pluripotent stem cells with a drug-inducible system. Journal of molecular cell biology. 2009;1:46–54. doi: 10.1093/jmcb/mjp003. [DOI] [PubMed] [Google Scholar]
- Yu N, Kruskall MS, Yunis JJ, Knoll JH, Uhl L, Alosco S, Ohashi M, Clavijo O, Husain Z, Yunis EJ. Disputed maternity leading to identification of tetragametic chimerism. The New England journal of medicine. 2002;346:1545–1552. doi: 10.1056/NEJMoa013452. [DOI] [PubMed] [Google Scholar]
- Zeng XX, Tan KH, Yeo A, Sasajala P, Tan X, Xiao ZC, Dawe G, Udolph G. Pregnancy-associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem cells and development. 2010;19:1819–1830. doi: 10.1089/scd.2010.0046. [DOI] [PubMed] [Google Scholar]