Abstract
A combined working memory/repetition priming task was administered to 13 young (mean age 23) and 13 elderly (mean age 69) adults. Each participant memorized a sample target face at the beginning of a trial and then determined whether each of 13 serially presented test faces matched the sample target. In each trial, both the target and one particular distracter face were repeated during the test phase. Within-trial repetition priming effects indicated the contribution of implicit memory to task performance. Response times decreased as items were tested repeatedly within a trial, but this decrement was greater for distracters than for targets. Young and older participants were equally accurate at identifying targets, but elderly were slightly less accurate for distracters. Elderly participants showed repetition priming effects during the working memory task for both targets and distracters, while the young showed such effects only for distracters. The results suggest that active maintenance in working memory, but not inhibition or rejection of distracters, may suppress implicit memory systems.
Keywords: aging, repetition priming, working memory, implicit memory, explicit memory
Put yourself in the following everyday social situation that an older adult might face. You arrive at a place where there are many people you know well, such as a family gathering. You wish to track a particular individual (e.g., your daughter) in the crowd so as to engage her in conversation. This requires that you maintain the memory of her face as other equally familiar but distracting faces cross your line of sight in rapid fashion. Your search is successful when you locate the “target” face of your daughter and meet up with her. At some later time, however, your daughter’s face may be a “distracter” as you search for the face of another person you wish to speak with. And still later, you may once more wish to find your daughter, so her face again becomes a target whereas others are distracters.
As this little vignette illustrates, the ability to track in working memory equally familiar faces amidst other currently-irrelevant faces is an important tool in normal social interaction. The same ability for working memory is needed for goal-oriented behavior involving other everyday objects. Does this ability change with age? Many neural and behavioral studies indicate that although older adults can have recognition memory comparable to young adults for verbal materials, memory for visual objects such as faces may decline (Bartlett & Fulton, 1991; Grady et al, 1998; Leonards, Ibanez, & Giannakopoulos, 2002). However, little is known about how effectively older adults can dynamically track familiar objects (such as faces) in memory in order to satisfy a current goal (and thereby partially meet their need for remaining socially active).
Working Memory and Aging
The ability to recognize a recently experienced stimulus appears to be mediated by two functionally and neuroanatomically distinct memory systems (Squire, 1992). One, explicit recognition, is supported by mechanisms that result in the conscious awareness of having encountered the stimulus previously (Wagner, Gabrieli, and Verfaellie, 1997). The other, implicit memory, supports stimulus familiarity and facilitates performance in an unconscious manner (Wagner, Gabrieli, & Verfaellie, 1997). When an item is intentionally memorized so that it may be explicitly recognized in the near future, particularly when other distracting stimuli occur during the maintenance of the item in memory, the item is held in what frequently has been called working memory (Baddeley and Hitch, 1994).
It is well known that working memory declines with age (e. g. Dobbs & Rule, 1989), but there is no consensus on why these age-related deficits occur. Process-oriented views of working memory (Baddeley & Hitch, 1994; Atkinson & Shiffrin, 1971) have suggested possible changes in encoding (Haaland, Price, & Larue, 2003), retrieval (Rypma & D’Esposito, 2000), contextual maintenance (Braver, Barch, Keys, Carter, Cohen, Kaye, et al., 2001; Spencer & Raz, 1995), inhibitory (Hasher & Zacks, 1988), refresh (Johnson, Reeder, Raye, & Mitchell, 2002) and feature-binding (Mitchell, Johnson, Raye, Mather, & D’Esposito, 2000) processes. In addition, processing speed (Salthouse, 1996) and levels-of-processing (Morris, Gick, & Craik, 1988) accounts of the age-related working memory deficit have also been offered.
In most previous empirical tests of these theories, the target stimulus could often be identified either through explicit recognition or implicitly via familiarity with the item as one of the most recent items presented. Thus, in many well-known working memory tasks such as the n-back (Dobbs & Rule, 1989), delayed match-to-sample (e.g. Grady, McIntosh, Bookstein, Horwitz, Rapoport, & Haxby, 1998), and Sternberg memory search (Sternberg, 1966) paradigms, implicit memory could potentially support target recognition due to greater familiarity for the most recently or more frequently presented items, even under instructions designed to test explicit memory. Poorer performance by older adults in such tests, therefore, could result from a deficit in working memory, a deficit in the ability of implicit memory to support performance, or both. Older adults may be more likely to rely on familiarity when making memory judgments of faces (Bartlett & Fulton, 1991). Furthermore, repetition priming—a phenomenon in which the response to a particular stimulus is facilitated by prior exposure to that same stimulus and which might support the experience of stimulus familiarity—is impaired in healthy older adults under certain test conditions (e.g. Fleischman & Gabrieli, 1998). This further validates the concern that traditional working memory tests used in aging studies may not be pure measures of working memory function, particularly in older adults. These findings point to the need to investigate the effects of age on working memory performance using a method that dissociates working memory from stimulus familiarity mechanisms.
Examinations of Working Memory and Implicit Memory
Jiang, Haxby, Martin, Ungerleider, and Parasuraman (2000) recently developed such a task, based on a modification of an earlier animal paradigm of Miller and Desimone (1994). In this working memory task, individuals had to track repeated targets and distracters among equally familiar visual items. Participants studied a target face at the beginning of a trial and then were presented with 13 test faces. They had to decide whether each test face was the target or a non-target distracter. On every trial of 13 test faces, two test items—the target and one of the distracters—were repeated multiple times. Because the target and the repeated distracter were equally likely to occur when any given test face was presented, both were equally familiar, making it impossible to identify the target by familiarity alone. Thus, overall responses to targets and repeated distracters should reflect a pure measure of working memory uncontaminated by the potential contribution of stimulus familiarity. At the same time, response times to both the repeated targets and the repeated distracters became faster as the items were repeated within the trial, suggesting that this paradigm also allowed assessment of repetition priming effects.
Using event-related functional magnetic resonance imaging (fMRI) to record the local blood flow responses associated with the neural activity underlying each trial, Jiang et al (2000) found a dissociation between the neural mechanisms mediating working memory and repetition priming. Consistent with previous studies (reviewed in Smith & Jonides, 1999), activation within regions of frontal and insular cortex rose above baseline for target test faces and maintained the same level of activation from the first repetition of the test face to the last. Such a pattern of activity may reflect the active maintenance of the target item in working memory throughout the trial. In contrast, for both targets and repeated distracters, activity in ventral temporal regions peaked at the first test face presentation within a trial but declined with subsequent repetitions of the stimulus. Other studies of the neural correlates of repetition priming of visual objects have found similar decreases in neural responses of posterior brain regions (Buckner, Goodman, Burock, Rotte, Koutstaal, Schacter, Rosen, & Dale, 1998; Henson, Goshen-Gottstein, Ganel, Otten, Quayle, & Rugg, 2003; Koutstaal, Wagner, Rotte, Maril, Buckner, & Schacter, 2001; Vuilleumier, Henson, Driver, & Dolan, 2002).
The clear functional and neural dissociation of priming and working memory processes in this paradigm makes it an intriguing tool for investigating age-related declines in memory function. Accordingly, in the present study we compared the performance of young and older adults on this task to assess working memory performance while controlling for possible familiarity effects. The use of this task also allowed us to examine potential age-related changes in repetition priming for target and non-target stimuli separately.
Repetition Priming and Aging
Whether and to what extent repetition priming is age sensitive remains unclear. Some studies have reported age deficits, while others have not. In a review, Rybash (1996) suggested that older adults may be impaired in conceptual, but not perceptual, repetition priming tasks; however, Fleischman and Gabrieli (1998) argued against this view, noting that the performance of older adults on word-stem completion tasks, which presumably elicit perceptual priming effects, is often impaired, while performance on word association tasks is not. Fleischman and Gabrieli (1998) offered an alternate account in which older adults show reduced repetition priming effects specifically when the task requires the production rather than the identification of test items. This account explains much of the variability between studies, yet other repetition priming studies revealing age-related deficits have been reported even when test conditions required identification rather than production of test items. Wiggs and Martin (1994) found that young and older native English speakers who were not familiar with the Turkish language showed equivalent repetition priming for English words; however, the young demonstrated some priming for previously presented Turkish words, while the older group did not. Jiang, Greenwood, and Parasuraman (1999) found age-related priming reductions in the perceived direction of rotation of ambiguous three-dimensional figures. This finding was replicated with two-dimensional motion stimuli by Jiang, Luo, and Parasuraman (2002), confirming that priming on perceptual tasks using motion stimuli is impaired in healthy aging, even when the tasks involve simple identification of the direction of motion.
We also used the working memory task of Jiang et al. (2000) to examine another aspect of repetition priming – whether it remains stable across changes in behavioral context. There is virtually no research of which we are aware that has investigated the degree to which shifts in behavioral context, such as changes in task goals and stimulus-response relationships, mediate age effects on repetition priming. Although neuronal responses in posterior brain regions to particular stimuli are typically reduced upon repetition of that item, a phenomenon known as repetition suppression (Miller, Li & Desimone, 1993), this suppression may disappear when task goals shift. Such a shift can occur between working memory trials when a new object becomes the target stimulus. Jiang et al (2000) found that even though neural responses to a distracter face in ventral temporal cortex were reduced from the first appearance of that face within a trial to the last, when the same item appeared again as a repeated distracter in a new trial, the initial response to the distracter face returned to the same level at which it had started in the previous trial. Jiang et al (2000) dubbed this finding a “reset effect,” as the neural responses appeared to reset following a change from one trial to the next. Braver et al. (2001) have suggested that declines in cognitive function with healthy aging are marked by a specific deficit in the ability to process context. In their study, participants performed a version of the continuous performance task (CPT) in which they had to respond every time they saw an X in a stream of letters, but only when it was preceded by the letter A. Older adults were more likely than the young to make false alarms to X’s that were not preceded by A’s; however, the young had a higher hit rate for the target A-X pattern and were more likely than the old group to make false alarms to Y’s when they were followed by the letter A, which was the context for responding to the next letter. Given this evidence for age-related deficits in contextual processing, the reset effect, which reflects altered repetition priming resulting from a change in behavioral context, may also be affected by healthy aging.
In the present study we used the task developed by Jiang et al (2000) to examine working memory and repetition priming processes in young and older adults using a single test paradigm. The study was designed to answer the following questions: (1) Is working memory for faces comparable between young and older adults when, across a given trial, targets are no more familiar than one of the non-target, distracter faces, thus minimizing the contribution of familiarity with the target to working memory performance? (2) Does the degree to which repetition priming is preserved or impaired with aging on a target recognition task depend on whether the repeated item is a target or a distracter? (3) Can the reset effect, which has been observed in neural responses in ventral temporal regions, be observed using behavioral measures? (4) What is the effect of healthy aging on the reset effect?
Methods
Participants
Fifteen young participants were undergraduate and graduate students between the ages of 18 and 28 (mean age 22.9) recruited from The Catholic University of America community. Thirteen older participants between the ages of 65 and 84 (mean age 69) were recruited from the Washington, DC area via newspaper advertisement. All participants were unfamiliar with the task prior to testing and provided informed consent. Participants were tested for close-distance and far-distance visual acuity using the Rosenbaum and Snellen visual acuity tests. Although most participants scored 20/40 or better on both exams, two elderly participants scored above this criteria on the Rosenbaum exam. Given that data for both of these participants were consistent with data from the other elderly participants, we included this data in all statistical analyses reported below. All 15 young and 12 of 13 elderly participants reported being right-handed. Two young participants were excluded from all statistical analyses, one for a reported neuropsychological abnormality and one due to particularly poor performance in identifying target faces during the task. All elderly participants scored 27 or higher on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975). Table 1 provides demographic information for participants’ age, years of education, and scores from the vocabulary and Logical Memory subtests of the Wechsler Memory Scale (Russell, 1975; Wechsler, 1981).
Table 1. Demographics Information.
Demographics data and scores on neuropsychological tests obtained prior to testing
| Young | Elderly | |
|---|---|---|
| Age * | 22.8(3.2) | 69(5.4) |
| Number of Participants | 15 (13) | 13 (13) |
| Education | 16.7(2.6) | 15.2(3.2) |
| SES | 5.2(1.9) | 6.6(2.2) |
| MMSE | ----- | 28.8(1.1) |
| Wechsler Vocab | 59.8(11.2) | 53(15.1) |
| Wechsler Memory Scale -- Immediate | 12.5(2.6) | 13.2(3.8) |
| Wechsler Memory Scale -- Delayed | 12.1(3.2) | 12.0(3.6) |
An asterisk (*) denotes a significant difference between age groups, as determined by independent samples t-tests with an alpha level of .05. When appropriate, mean numbers are reported with standard error estimates in parentheses. Two participants were excluded from the young group, one for a reported neuropsychological abnormality and one due to excessively poor performance in identifying target faces (accuracy less than 65% for the first two target repetitions within a trial).
SES = socioeconomic status; MMSE = Mini-Mental State Exam.
Stimuli
All stimuli were presented on a high-resolution color monitor from a MacIntosh computer using Superlab presentation software (Haxby et al, 1993). The stimuli used in this experiment were 35 grayscale 7cm-×-8cm images of faces and 80 grayscale 7cm-×-8cm images of scrambled versions of the face stimuli, all presented against a black background.
Procedure
Participants were instructed to remember the sample target face presented at the beginning of each 30-second trial. Participants were to respond by pressing a button with their right index finger for each test face in the trial that matched the sample target face or by pressing another button with their left index finger for each test face that did not match the target face. Prior to performing the experimental runs, participants performed 1 run of practice trials to familiarize themselves with the stimuli and the task. Participants performed 12 runs of 14-item (1 study face + 13 test faces) trials, with each run containing six trials, for a total of 72 trials per participant across the entire experiment. One 30-second trial consisted of one sample target face followed by 13 test faces. Sample target faces appeared on the screen at the beginning of a trial for 3760 ms and were demarked by a 1-mm-thick white border. The subsequent test faces were presented for 1880 ms each, with no inter-stimulus interval. Within each 30-second trial, the target face and one particular distracter face were tested multiple times; all other distracters were only used once in each trial. Which face was the target and which particular distracter face was repeated varied from trial to trial. The number of times a repeated item appeared within a trial varied pseudo-randomly between two and five times. All responses were recorded on a Superlab response box.
Analysis
Both manual response time and accuracy were measured while the participants performed the working memory task. Unless otherwise stated, a 4×2×2 mixed measures analysis of variance (ANOVA) was used to analyze the data for each dependent measure separately, with order of appearance within a trial (4) and item type (2 – targets and distracters) as within-subjects variables and age category (2 – young and elderly) as a between-groups variable. When appropriate, the Greenhouse-Geisser correction was used to adjust the degrees of freedom and thereby compensate for potential violations of sphericity.
Results
Working Memory Accuracy
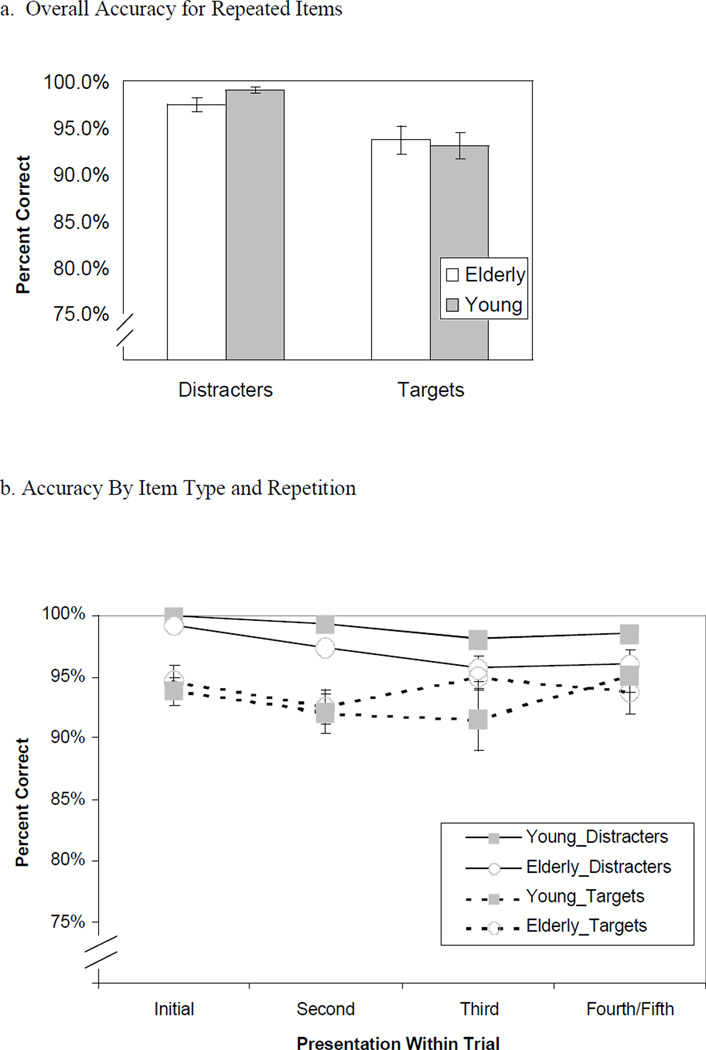
Response accuracy measures for all conditions were quite high (see figures 2a & 2b). To control for possible artificial constriction of variance due to ceiling effects, we performed an arc-sine transformation on these accuracy measures before submitting them to a 4×2×2 mixed measures ANOVA. Overall, elderly and young performed equally well on the task (marginal mean accuracy: young 96.1%, elderly 95.5%; F(1,24) = 2.12, p = .159, MSE = .179). Performance was better for distracter faces than for target faces (marginal mean accuracy: target 93.5%, distracter 98%; F(2,55) = 6.23, p < .01, MSE = .016). Furthermore, the age × item type (target/distracter) interaction was significant (F(1,24) = 5.96, p < .05); simple effects tests indicated that young participants performed better than the elderly for distracter faces (t(24) = −2.90, p < .01), but not for target faces (t(24) = .39, p = .70). Accuracy for a particular item (see figure 2b) declined after its initial presentation within a trial of test faces (marginal mean accuracy: initial appearance 96.8%, second 95.3%, third 95.0%, fourth/fifth 95.9%; F(1,24) = 52.84, p < .001, MSE = .033). This effect was significantly modulated by whether the test item was a target or a distracter (F(3,69) = 7.06, p < .001, MSE = .009). Simple effects tests indicated that performance declined linearly as distracter items were repeated, as indicated by a significant linear contrast (F(1,25) = 12.59, p < .005, MSE = .013); however, performance for targets did not decline linearly as the targets were repeated (linear contrast: F(1,25) = 2.95, p = .10, MSE = .008). No other interactions in this ANOVA were significant.
Figure 2. Accuracy for Repetitions of Items Within a Trial.
Accuracy of manual responses for repeated targets and distracters only, collapsing across all repetitions within a trial (figure 2a) and as a function of repetitions within a trial (figure 2b).
Repetition Priming
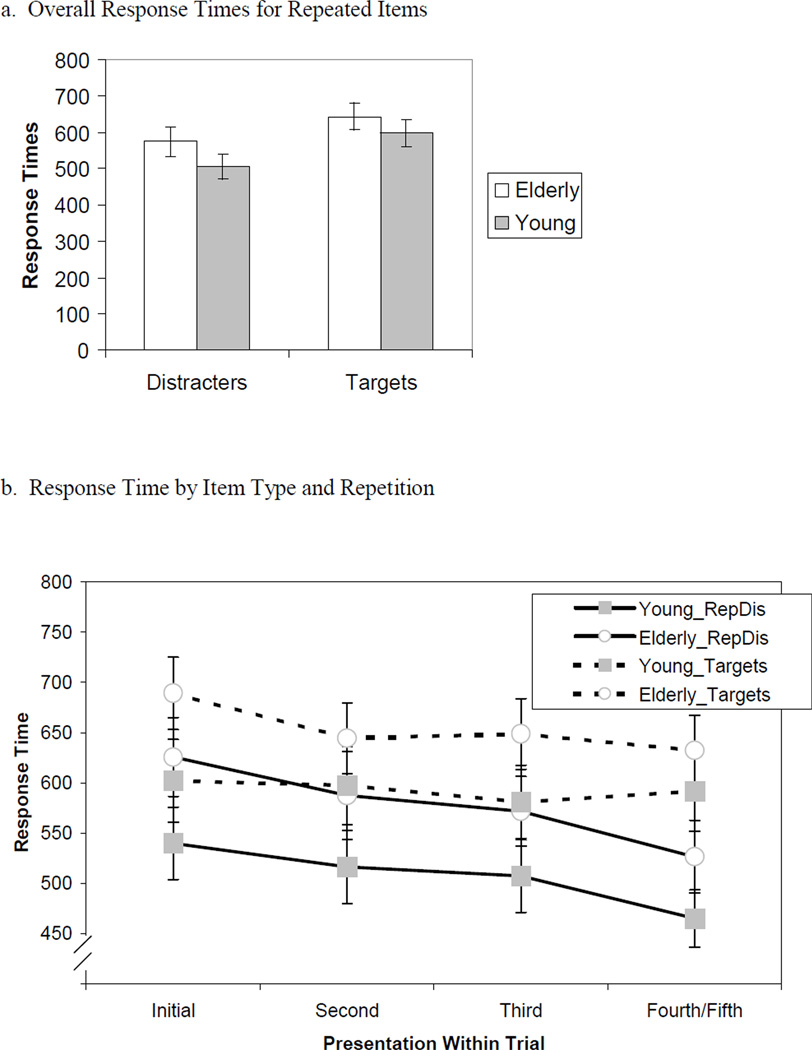
We performed a 4×2×2 mixed ANOVA on the reaction time data for the faces that were repeated within a 30-second trial. The mean data for within-trial repetition effects are presented in figures 3a and 3b. Overall, young participants responded somewhat more quickly than elderly (see figure 3a), although this difference was not statistically significant (marginal mean reaction times of 550msec and 616msec, respectively; F(1,24) = 1.72, p = .203, MSE = 125,940). Participants responded significantly more quickly to distracters than to target faces (marginal mean reaction times: distracters 542msec, targets 623msec; F(1,24) = 76.12, p < .001, MSE = 4,478); however, the effect of age on response time did not differ between target faces and distracter faces (F(1,24) = .31, p = .586).
Figure 3. Response Times for Repetitions of Items Within a Trial.
Manual response times for correct responses to repeated targets and distracters only, collapsing across all repetitions within a trial (figure 3a) and as a function of repetitions within a trial (figure 3b).
Repeating items within the same 30-second trial (see figure 3b) lead to significantly faster response times (marginal mean reaction times: initial appearance 614msec, second 586msec, third 577msec, fourth/fifth 554msec; F(2,51) = 38.95, p < .001, MSE = 4184). This repetition effect was greater for distracter faces than for target faces (F(2,53) = 11.49, p < .001, MSE = 890), and greater in elderly than in young (F(2,51) = 3.58, p < .05, MSE = 1169). The age × repetition × target interaction was not significant (F(2,53) = 1.03, p = .37, MSE = 890).
The “Reset” Effect
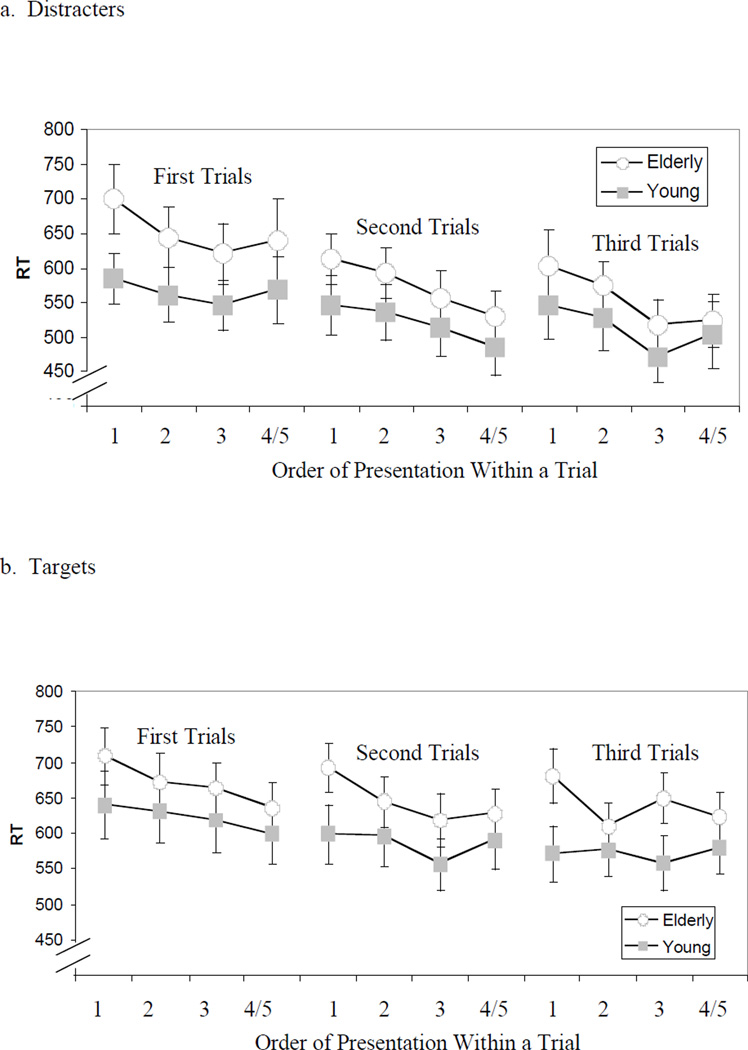
For each 30-second memory trial, the same target face repeated from one to five times. Because some of the targets and distracters were used as repeated items on multiple 30-second trials (between one and five trials), we were able to assess changes in response times to these items across trials in order to evaluate behaviorally the “reset” phenomenon that was observed in neural activation measures in a primate single-cell recording study (Miller & Desimone, 1994) and in our previous fMRI study (Jiang et al, 2000). A reset effect would be indicated by an increase in RT for the first within-trial presentation of an item compared to the last presentation in the previous trial; if the reset were complete, the increase would restore the RT to the level observed for the first item appearance in the previous trial. Figures 4a & 4b plot the across-trial repetition data for distracter and target items, respectively. A 3 (order of appearance) × 2 (target versus distracter) × 2 (age group) mixed measures ANOVA was run on the response times for the first test item in each trial. In terms of the order of appearance factor, a reset effect would be indicated by a lack of statistical significance (i.e. response times are not significantly different from one another). Across conditions and age groups, response times significantly decreased with each trial, F (2, 40) = 17.55, MSE = 3363, p < .001, implying there was no omnibus reset effect. However, the three-way interaction of order, target type, and age group was significant, F (2, 48) = 3.72, MSE = 2379, p < .05, indicating that a reset effect may have been dependent on age and target type. No other effects were significant (all p < .20) with the exception of the target factor, F (1,24) = 14.63, MSE = 6529, p < .005, which was not relevant to the question of the reset effect.
Figure 4. Response Times for Repetition of Items Across Trials.
Individual targets and distracters were used as repeated items in multiple trials to examine behavioral manifestations of the neural reset effect. Manual response times for correct responses to repeated distracters (figure 4a) and targets (figure 4b) only are plotted, showing repetition reductions both within an individual trial and response times in a later trial when that particular item was again repeatedly tested.
Post-hoc analyses were performed to further investigate the reset effect1. For repeated distracter items (figure 4a), comparison of the curves for the first and second trials revealed no apparent reset effect; however, a possible reset effect may have been masked by the substantial overall improvement in response time from trial 1 to trial 2, as indicated by a significant decrease in overall reaction time collapsing across repetitions from trial 1 to 2, paired samples t (25) = 6.11, p < .001. Comparison of trials 2 and 3 supports this masking notion, revealing a clear reset effect. Paired-sample t-tests for both the young (t(12) = −0.03, p = .98) and the elderly participants (t(12) = 0.52, p = .61) confirmed that response times for the initial appearance in trials 2 and 3 did not differ significantly in either age group (mean response times for young: first appearance of second trial 546msec, first appearance of third trial 546msec; mean response times for elderly: first appearance of second trial 614msec, first appearance of third trial 603msec).
Figure 4b presents a different picture for target items. The older group demonstrated a pronounced reset effect, with paired samples t-tests indicating that response times on the first appearance did not differ from the first appearance on the previous trial – (trial 1 vs. trial 2: t(12) = .90, p = .39; trial 2 vs. trial 3: t(12) = .62, p = .54). Elderly mean response times for the first target face of trials 1, 2, and 3 were 709msec, 692msec, and 681msec, respectively. Young participants, however, showed significant or nearly significant decreases in their response times for these same comparisons (first trial to second trial – t(12) = 2.83, p = .015; second trial to third trial – t(12) = 1.97, p = .072), suggesting that re-instating a particular item as a target after at least one trial in which a different item was the target face resulted in some cross-trial improvement for the young participants. In the young participants, mean response times for the first target face in each trial were 640msec, 598msec, and 571msec for blocks 1, 2, and 3, respectively.
Discussion
The present study used a single paradigm to examine the independent contribution of working memory and repetition priming processes to age-related changes in memory. The use of the repeated target/distracter face paradigm developed by Jiang et al. (2000) allowed an assessment of such effects while minimizing the contribution of familiarity with the target face to working memory performance. Young and older adults did not differ overall in their ability to maintain in working memory a representation of a target face over a 30-second interval and respond accurately to its subsequent presentations during that interval. There was a trend for older adults to reject distracter faces less efficiently than young adults. This trend might reflect reduced inhibition of irrelevant information sources with aging (Hasher & Zacks, 1988). However, both young and older adults had high accuracy rates (>90%), and the age effect was relatively small.
Evidence of repetition priming was observed for both young and older participants. In both groups, RTs were reduced by repetition of stimuli within a given trial. However, the magnitude of the reduction was greater for distracters than for targets. This finding is consistent with the observation that all participants were able to maintain an accurate representation of the target face over the 30-second duration of a trial. As discussed previously, this also accords with the previous fMRI study by Jiang et al. (2000) that target-related neural activation in frontal cortex was maintained at the same level during this interval. Hence participants were able to respond quickly and efficiently on the first presentation of the target, and subsequent presentations of the target within the trial yielded only minimal further facilitation. In fact, RT to targets showed a relatively flat pattern with within-trial repetition in the young participants (see Figure 3b). However, the neural activity in posterior regions associated with distracter items was shown by Jiang et al. (2000) to decline markedly within the trial, suggesting more efficient rejection of distracters with each repetition. In accordance with this previously observed pattern of neural activity, RT to distracters in the current study showed a large within-trial decrease with repetition in both the young and the old participants. This pattern of results argues against an account of priming effects based on the information availability model (Ostergaard, 1998). The information availability model predicts that any factor that makes a task more difficult should increase repetition priming effects. Both the accuracy and response data from the current task indicate that participants had more difficulty identifying target faces than rejecting distracter faces, yet priming was more prominent for the distracters. Given that distracters did not have to be explicitly recognized in order to be rejected as non-targets, it is likely that the demands for explicit recognition necessary to identify targets was the driving factor making target identification more difficult and simultaneously reducing within-trial repetition priming. Our results are more consistent with models of memory that posit distinct implicit and explicit memory systems (e.g. Squire, 1992).
Furthermore, it is interesting to note that both active maintenance of targets (Baddeley & Hitch, 1994) and inhibition of salient distracters (Hasher & Zacks, 1988) are functions that have been ascribed to working memory, but only active maintenance of targets interfered with within-trial repetition priming effects. This finding is consistent with previous suggestions that implicit memory and explicit, episodic memory systems can cause mutual interference (e.g. Wagner, Maril, & Schacter, 2000), but further extends these interference effects to working memory and distinguishes the contributions of storage and non-mnemonic executive processes of explicit memory to these interference effects.
This study was designed to investigate whether there is an age-related difference in repetition priming and if any such change depends on whether the repeated item is a target or a distracter. The age × repetition interaction was significant, indicating that older adults showed a greater reduction in RT with item repetition than the young. This finding is consistent with the proposal by Fleischman and Gabrieli (1998) that repetition priming effects are relatively spared for item identification tasks. Although the age × repetition × item type (target/distracter) interaction was not significant, it is apparent that the major difference between young and old participants was that whereas the old showed repetition-related reduction in RT for both distracters and targets, the young showed a reduction only for distracters (see Figure 3b). Exploratory simple effects tests support this notion – significant (p < .05) main effects of repetition were observed for both targets and distracters in elderly but only for distracters in the young. Therefore, it may be more accurate to conclude that repetition facilitation effects were more consistent across conditions in elderly, rather than larger overall in magnitude, as the significant age × repetition interaction might suggest. The lack of a significant three-way interaction may have been a type-II error due to the large between-subjects variability in response time (see figure 3) rather than the lack of a real effect. More importantly, the preserved repetition priming effects observed here are consistent with the proposal by Fleischman and Gabrieli (1998) that repetition priming for item recognition tasks is relatively spared with healthy aging. Because performance accuracy was relatively close to ceiling on this task, however, it is unclear whether repetition priming would continue to remain age-resistant under more demanding task conditions, such as those used by Wiggs and Martin (1994) and Jiang, Luo, and Parasuraman (2002), who found age deficits in repetition priming on item recognition tasks.
The results were somewhat mixed with respect to the “reset” effect, which has been observed in neural responses in ventral temporal regions in the previous fMRI study by Jiang et al. (2000). We asked whether such an effect could also be observed using behavioral (RT) measures and whether the effect was age sensitive. Both young and elderly showed some evidence of a behavioral reset effect for distracter items when comparing responses during the second trial with responses during the third trial. This observation must be tempered by the fact that no such effect was observed in either age group when comparing first trial and second trial performance; however, it is likely that a reset effect in this comparison may have been masked by the overall improvement in response time from the first to the second trial. In contrast with the results from distracter trials, older adults consistently exhibited a reset effect for targets, whereas young exhibited no evidence of this effect in either block transition. Thus, the results clearly suggest that the fMRI reset effect can be observed in behavioral measures, pointing to the functional significance of the fMRI findings.
Why was no such effect observed for targets in the young participants? In part, the lack of a reset effect could reflect the fact that young participants showed virtually no within-trial repetition priming for targets, a result we have previously interpreted as evidence for the sustained stability of the target working memory representation across the 30-second trial in these participants. It is also possible, however, that the reset effect for targets in the elderly is the only reason why we observed within-trial repetition priming effects for targets in elderly, given that there is no apparent reduction in response time after the first repetition within a trial (see figure 3). In either case, the age difference in the reset effect for targets is consistent with previous studies (Braver et al, 2001) that have noted age-related changes in performance across different task contexts for items maintained in working memory. If our age difference in the reset effect for targets reflects a true difference in cognitive processing, this finding suggests that mechanisms deployed to process the targets and the distracters were less distinguishable amongst elderly than amongst young. Such a pattern of findings is consistent with functional neuroimaging studies (e.g. Cabeza, 2002) implying that cognitive processes are less modular and less dissociable in elderly than in young adults. Future testing will be required to examine this possibility in relation to age differences in the reset effect.
The paradigm used here that was developed by Jiang et al (2000) holds promise as a method for assessing both repetition priming and working memory effects using identical stimuli and testing procedures. The current version of this paradigm hinted at age-related differences in the ability to reject distracter faces, but performance accuracy was too close to ceiling to draw definitive conclusions on this point. Simple modifications of the test procedure, such as reducing stimulus exposure time, adding a post-stimulus mask for test faces, or imposing a greater study-test delay, could be implemented in the future that would make the task more sensitive to differences in accuracy, reflecting efficiency of working memory systems.
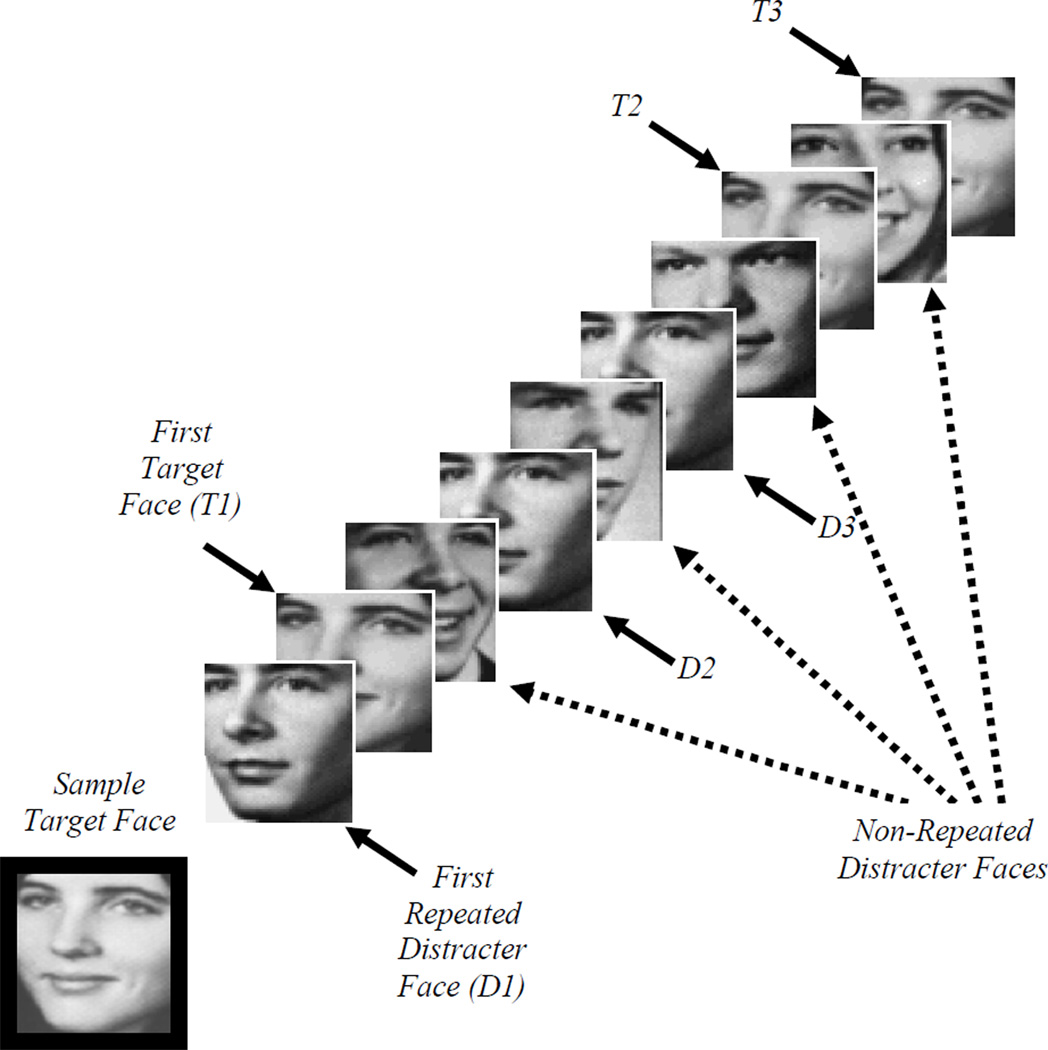
Figure 1. Task Schematic.
Task schematic of a single trial. Participants viewed a sample target face (outlined in black here) on each trial for 3.76 seconds, followed by a stream of 13 test faces that appeared for 1.88 seconds each. Target faces appeared in this stream of test faces between one and five times (three target repetitions are shown here). A single distracter face also appeared multiple times in each stream of test faces (three repetitions of the first distracter are shown here). All other distracters appeared only once per trial. Participants classified each test face as either a target or distracter by pushing one of two buttons on a button box.
Acknowledgements
The present study was supported by NIH Grants AG19653 to RP and AG000986 to YJ. We would like to thank M. Harris for her help during data collection, and A. Lawson for his comments on the manuscript.
Footnotes
We chose not to adopt a method of correcting alpha for multiple comparisons in this procedure. The purpose of these post-hoc tests was to address the question of whether a reset effect was present. Because a reset effect involves the absence of a statistically significant difference, our prediction was that the null hypothesis, rather than the experimental hypothesis, would be true based on previous work from our laboratory (Jiang et al, 2000). Lowering alpha for each comparison using a post-hoc correction, therefore, would actually increase the likelihood that a significant difference would not be observed for each individual comparison. Because this was our predicted result, in this case the net effect of traditional error correction methods would run counter to the theoretical motivation for correcting alpha in the general case.
References
- Atkinson RC, Shiffrin RM. The control of short-term memory. Scientific American. 1971;225:82–90. doi: 10.1038/scientificamerican0871-82. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Developments in the concept of working memory. Neuropsychology. 1994;8:485–493. [Google Scholar]
- Bartlett JC, Fulton A. Familiarity and recognition of faces in old age. Memory and Cognition. 1991;19:229–238. doi: 10.3758/bf03211147. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Janowsky JS, Taylor SF, Yesavage JA, Mumenthaler MS, Jagust WJ, Reed BR. Context processing in older adults: evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General. 2001;130:746–763. [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JD. Repetition priming in normal aging and Alzheimer's disease: a review of findings and theories. Psychology and Aging. 1998;13:88–119. doi: 10.1037//0882-7974.13.1.88. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age- related changes in regional cerebral blood flow during working memory for faces. NeuroImage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Price L, Larue A. What does the WMS-III tell us about memory changes with normal aging? Journal of the International Neuropsychological Society. 2003;9:89–96. doi: 10.1017/s1355617703910101. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Haxby JV, Parasuraman R, Lalonde F, Abboud H. SuperLab: General-purpose Macintosh software for human experimental psychology and psychological testing. Behavior Research Methods, Instruments, and Computers. 1993;25:400–405. [Google Scholar]
- Henson RN, Goshen-Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD. Electrophysiological and haemodynamic correlates of face perception, recognition and priming. Cerebral Cortex. 2003;13:793–805. doi: 10.1093/cercor/13.7.793. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Greenwood PM, Parasuraman R. Age-related reduction in 3-D visual motion priming. Psychology and Aging. 1999;14:619–626. doi: 10.1037//0882-7974.14.4.619. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Luo YJ, Parasuraman R. Priming of two-dimensional visual motion is reduced in older adults. Neuropsychology. 2002;16:140–145. doi: 10.1037//0894-4105.16.2.140. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Reeder JA, Raye CL, Mitchell KJ. Second thoughts versus second looks: an age-related deficit in reflectively refreshing just-activated information. Psychological Science. 2002;13:64–67. doi: 10.1111/1467-9280.00411. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Leonards U, Ibanez V, Giannakopoulos P. The role of stimulus type in age-related changes of visual working memory. Experimental Brain Research. 2002;146:172–183. doi: 10.1007/s00221-002-1175-9. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neuroscience. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D'Esposito M. Aging and reflective processes of working memory: binding and test load deficits. Psychology and Aging. 2000;15:527–541. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Morris RG, Gick ML, Craik FI. Processing resources and age differences in working memory. Memory and Cognition. 1988;16:362–366. doi: 10.3758/bf03197047. [DOI] [PubMed] [Google Scholar]
- Ostergaard AL. The effects on priming of word frequency, number of repetitions, and delay depend on the magnitude of priming. Memory and Cognition. 1998;26:40–60. doi: 10.3758/bf03211369. [DOI] [PubMed] [Google Scholar]
- Russell EW. A multiple scoring method for the assessment of complex memory functions. Journal of Consultation and Clinical Psychology. 1975;43:800–809. [Google Scholar]
- Rybash JM. Implicit memory and aging: A cognitive neuropsychological perspective. Developmental Neuropsychology. 1996;12:127–179. [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta- analysis. Psychology and Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RJ. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nature Neuroscience. 2002;5:491–499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Gabrieli JD, Verfaellie M. Dissociations between familiarity processes in explicit recognition and implicit perceptual memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:305–323. doi: 10.1037//0278-7393.23.2.305. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: when priming hinders new episodic learning. Journal of Cognitive Neuroscience. 2000;12(Supplement):52–60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Weschler adult intelligence scale -- revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wiggs CL, Martin A. Aging and feature-specific priming of familiar and novel stimuli. Psychology and Aging. 1994;9:578–588. doi: 10.1037//0882-7974.9.4.578. [DOI] [PubMed] [Google Scholar]