Abstract
Listeriosis can lead to potentially lethal pulmonary complications in newborns and immune compromised patients, characterized by extensive permeability edema. Listeriolysin (LLO), the main virulence factor of Listeria monocytogenes, induces a dose-dependent hyperpermeability in monolayers of human lung microvascular endothelial cells in vitro. The permeability increasing activity of LLO, which is accompanied by an increased reactive oxygen species (ROS) generation, RhoA activation and myosin light chain (MLC) phosphorylation, can be completely inhibited by the protein kinase C (PKC) α/β inhibitor GÖ6976, indicating a crucial role for PKC in the induction of barrier dysfunction. The TNF-derived TIP peptide, which mimics the lectin-like domain of the cytokine, blunts LLO-induced hyperpermeability in vitro, upon inhibiting LLO-induced protein kinase C-α activation, ROS generation and MLC phosphorylation and upon restoring the RhoA/Rac 1 balance. These results indicate that the lectin-like domain of TNF has a potential therapeutic value in protecting from LLO-induced pulmonary endothelial hyperpermeability.
Keywords: Listeriolysin, Endothelial hyperpermeability, TNF, Protein kinase C, Reactive oxygen species
1. Introduction
Infections with the G+ bacterium Listeria monocytogenes mostly start as an oral infection caused by contaminated food and can cause severe lung complications in individuals having pre-disposing conditions, such as age, pregnancy, cancer, chronic diseases or organ transplantation (Vázquez-Boland et al., 2001; Ananthraman et al., 1983; Lerolle et al., 2002; Janssens et al., 2006). Moreover, airway infections with L. monocytogenes represent a serious problem in early onset neonatal listeriosis (Posfay-Barbe and Wald, 2004). Listeriosis-associated pulmonary complications can result in permeability edema, characterized by an extensive capillary endothelial hyperpermeability, that requires harsh therapeutic measures and often has a fatal outcome (Ananthraman et al., 1983). Since no standard therapy is currently available to treat pulmonary permeability edema, the need for novel substances that can improve oxygenation in these patients, by means of barrier restoration is of high clinical importance. The severity of permeability edema during listerial infection correlates with the presence of the most important virulence factor listeriolysin (LLO), which is a cholesterol-binding pore-forming toxin (Rose et al., 2001; Repp et al., 2002; Munder et al., 2005). Endothelial hyperpermeability can be caused by actin/myosin-driven contraction, which generates a contractile force that pulls VE-cadherin inward, thus forcing it to dissociate from its adjacent partner, as such producing interendothelial gaps (Vandenbroucke et al., 2008; Lucas et al., 2009). This contraction can be the result of myosin light chain (MLC) phosphorylation and is mediated by MLC kinase (MLCK) in a Ca2+/calmodulin-dependent manner. Both the Ras homologous GTP-binding proteins, RhoA and Rac1 play important roles in the regulation of cytoskeletal remodeling and EC barrier regulation (Birukova et al., 2005). The Rho A/Rho-associated kinase cascade may directly catalyze MLC phosphorylation, or alternatively act indirectly, via inactivation of MLC phosphatase (van Nieuw Amerongen et al., 2000) to induce cell contraction and endothelial barrier disruption. In turn, endothelial barrier enhancement is associated with Rac1-mediated formation of cortical F-actin, increased association of focal adhesion proteins, and enlargement of intercellular adherens junctions (Dudek and Garcia, 2001). Thus, a precise balance between RhoA- and Rac 1-mediated signaling is essential for endothelial barrier regulation. Apart from the RhoA/Rac1 balance, also reactive oxygen species (ROS) can lead to decreases in cortical actin banding, increased stress fibers, increased surface adhesiveness, as well as loss/disassembly of tight and adherens junctions in endothelial cells (Vandenbroucke et al., 2008). Several lines of evidence implicate the involvement of protein kinase C-α (PKC-α) in mediating the increased vascular endothelial permeability in response to oxidant stress (Mehta et al., 2001). During G+ bacterial infection-associated acute lung injury, the alveolar space, as well as the interstitium, are sites of intense inflammation where pro-inflammatory substances, such as TNF, are produced locally. Spatially distinct from its receptor binding sites, TNF carries a lectin-like domain, recognizing specific oligosaccharides, such as N,N’-diacetylchitobiose and branched trimannoses (Hession et al., 1987; Sherblom et al., 1988; Lucas et al., 1994). We have previously demonstrated that the TIP peptide, which mimics the lectin-like domain of TNF, activates sodium conductance in pulmonary microvascular endothelial cells, in a TNF receptor-independent manner (Hribar et al., 1999). Moreover recently, Vadász et al. (2008) have shown that the TIP peptide reduces barrier dysfunction in an isolated rabbit lung model of endotoxin/exotin-induced lung injury. The aim of this study was to better characterize the molecular mechanisms by which LLO reduces pulmonary endothelial permeability in vitro and to investigate whether the TNF-derived TIP peptide can interfere with these events.
2. Materials and methods
2.1. Antibodies and chemicals
Anti-MLC, anti-diphospho-MLC (Thr18/Ser19) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primary antibodies, generated against human antigens were from Cell Signaling Technology (Beverly, MA). Anti-mouse and anti-rabbit secondary antibodies conjugated to horse radish peroxidase were purchased from Sigma-Aldrich (St. Louis, MO). Enhanced chemiluminescence (ECL) detection kit was purchased from Pierce (Rockford, IL). Listeriolysin was purified as described previously (Darji et al., 1995).
2.2. Peptides
Human TNF TIP peptide, as acetate salt, was purchased from Bachem (Bubendorf, Switzerland). As a control peptide, a scrambled tip peptide was used, consisting of the same amino acid composition as the TIP peptide, but in a random order (Bachem, Bubendorf, Switzerland). The amino acid sequences were:
Human TIP (hTIP) peptide: CGPKETPEGAEAKPWYC
Human scrambled TIP peptide: CGTKPWELGPDEKPAYC
Both peptides are cyclic through CC oxidation.
2.3. Cells
Human lung microvascular endothelial cells (HL-MVEC) were isolated by Mrs. Connie Snead in our Institute and were grown in Endothelial Growth Medium-2-Microvessel (EGM-2MV) consisting of defined growth factors and supplemented with additional FBS up to 5% final concentration (Lonza). Cells were grown at 37 °C in 5% CO2 incubator and used from passage 2–6. Primary cultures of ovine pulmonary arterial endothelial cells (PAECs) were isolated by the explant technique as described previously (Wedgwood et al., 2003).
2.4. Purification of LLO
LLO was expressed and purified from the wild type L. innocua 6a strain, as described previously (Darji et al., 1995). 10 ml of an overnight bacterial culture grown at 37 °C in BHI broth was used to inoculate 1 l of the chemically defined minimal medium. Following 48 h incubation at 30 °C, bacteria were removed by centrifugation and the supernatant fluid was concentrated to 50 ml using a Millipore filtration apparatus with a cut-off point of 10 kDa. The crude supernatant of LLO was then batch absorbed for 60 min with Q-sepharose or SP-sepharose (Pharmacia, Freiburg, Germany) and pre-equilibrated with loading buffer (50 mM NaH2PO4, pH 6.2). The non-absorbed fraction was centrifuged and desalted by transferring through a super loop to a HiPrep(tm) 26/10 desalting column (Pharmacia, Freiburg, Germany) where loading buffer (50 mM NaH2PO4, pH 6.2) was used to elute the desalted fraction. This fraction is subsequently filtered through a Millipore filter (0.22 μM) and loaded onto a Resource-S column previously equilibrated with 50 mM NaH2PO4, pH 6.2. The pure toxin eluted reproducibly from the column at 0.21 to 0.28 M NaCl using elution buffer (50 mM NaH2PO4 1 M NaCl, pH 5.6). Fractions were collected and individually tested for hemolytic activity. Yields of the toxins range from 1 to 5 mg/l supernatant with a hemolytic activity (HU) of 20,000 HU/mg purified protein. One hemolytic unit (HU) is expressed as the amount of toxin required to lyse 50% of a 1% suspension of sheep erythrocytes. Protein desalting and purification processes were carried out using the high performance chromatography system ÄKTA explorer and UNICORN(tm) control system (Pharmacia, Freiburg, Germany). LLO showed a high purity on SDS–PAGE, efficiently recognised with LLO-specific antibodies, and exhibit haemolytic activity on sheep erythrocytes at pH 6.0. Protein concentrations were determined using a standard assay (Bio-Rad Protein Assay; Bio-Rad, Munich, Germany).
2.5. Measurement of transendothelial electrical resistance
Electrical cell-substrate impedance sensing (ECIS) system (1600R, Applied Biophysics, Troy, NY) was used to measure transendothelial electrical resistance (TER) with EC grown on gold microelectrodes. HL-MVEC were seeded as 75,000 cells/well in 300 μl 5% FBS complete EBM-2 medium (Lonza) onto an ECIS plate (8W10E) and cultured for 2–3 days. Confluence was assessed as minimum basal resistance of 2000 Ω for HL-MVEC. Resistance values from each microelectrode were normalized as the ratio of measured resistance to baseline resistance and plotted vs. time by Prism 3.0 software.
2.6. Western immunoblotting
After being stimulated, cells were washed with PBS and lysed with cell lysis buffer containing 10 mM Tris (pH 7.4), 1% Triton X-100, 0.5% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 0.2 mM EGTA, 0.2 mM vanadate, 0.2 mM PMSF, and 0.5% phosphatase inhibitor cocktail. Total cell lysates were cleared by centrifugation and boiled with the same amount of 3× SDS sample buffer for 5 min. Protein extracts were separated by 12% SDS–PAGE gel. Next the proteins were transferred to PVDF membranes (20 V for overnight) and subsequently blocked with 5% non-fat dry milk in PBST at room temperature for 1 h and then incubated at 4 °C overnight with specific primary antibodies of interest. After being washed three times for 5 min with PBST, the membrane was incubated with horseradish peroxidase-linked IgG secondary antibody at room temperature for 1 h, followed by three washes for 5 min with PBST. Immunoreactive proteins were detected using an enhanced chemiluminescent detection system according to the manufacturer's protocol (Pierce, Rockford, IL). The amount of detected proteins was analyzed using Image J software.
2.7. Measurement of ROS production in ovine PAEC
Sheep pulmonary artery endothelial cells were pretreated for 30 min with 50 μg/ml of TIP peptide or not, prior to the addition of 250 ng/ml LLO for 6 h. 20 μl of spin-trap stock solution consisting of CMH [1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl, 20 μM in DPBS+25 μM desferrioxamine (Sigma-Aldrich) and 5 μM diethyldithiocarbamate] and 2 μl of DMSO were added to each well for 30 min. On completion of incubation, the medium was removed and the adherent cells were trypsinized and pelleted at 500 g. The cell pellet was washed and suspended in a final volume of 35 μl of DPBS+ desferrioxamine and diethyldithiocarbamate, loaded into a 50 μl capillary tube and analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany) at 40-mW microwave power, 3000-mG modulation amplitude, and 100-kHz modulation frequency. EPR spectra were analyzed and measured for amplitude using ANALYSIS version 2.02 software (Magnettech).
2.8. Real-time RT-PCR for NADPH oxidase 4 (Nox 4)
Total RNA was extracted from cell lysate using the RNeasy® Plus Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA concentrations were determined by absorbance using a spectrophotometer (UV-1700; Shimadzu, Japan) at 260 nm. Aliquots of total RNA (1 μg) were reverse-transcribed using QuantiTect Reverse-Transcription Kit (Qiagen, Valencia, CA) following the manufacturer's protocol. Complementary DNA equivalent to 100 ng total RNA were subjected to real-time polymerase chain reaction (PCR) analysis using QuantiTect SYBR Green Kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. Oligonucleotide primer pairs were designed using Beacon Designer software (Premier Biosoft International, Palo Alto, CA). The primer sequences for NOX4 and housekeeping gene β-Actin were: NOX4 sense primer, 5′-TGGCTCTCCATGAATGTCCTGCTT-3′; Nox4 antisense primer, 5′-TGCTGAGGCTCTGCTTAGACACAA-3′; β-Actin sense primer, 5′-AATGTGGCCGAGGACTTTGATTGC-3′; β-Actin antisense primer, 5′-AGGATGGCAAGGGACTTCCTGTAA-3′. Each sample was run in triplicate. Thermal cycling and real-time detection were done with a Mx4000™ Multiplex Quantitative PCR System (Stratagene, La Jolla, CA): step 1) 95 °C for 15 min, step 2) 94 °C for 15 s followed by 50–60 °C for 60 s and then 72 °C for 30 s (repeated 40 times). Melting curve analysis was completed after each PCR reaction. Each gene's threshold cycle (Ct) value was normalized against the corresponding β-Actin Ct value to obtain a ΔCt value.
2.9. Rac1 and RhoA activation assays
RhoA and Rac1 activity of endothelial cells was determined using an absorbance-based G-LISA Rac1 and RhoA activation assay biochemistry kit, according to the manufacturer's instructions (Cytoskeleton, Inc., Denver, CO). Briefly, the endothelial cells were grown on 6-well cell culture plates to 50% confluence. After serum starvation for 24 h, cells were pretreated or not for 30 min with TIP peptide (50 μg/ml) or 8-pCPT-2′-O-Me-cAMP (100 μM). Subsequently, LLO (250 ng/ml) was added for 2 h, before harvesting cell lysates. Lysates were clarified by centrifugation at 4 °C (14,000×g, 2 min) and the protein concentration was determined. Equal amounts of lysates (1 μg/ml) were added to plates coated with a Rac or Rho–GTP-binding protein before incubation for 30 min at 4 °C. Subsequently, a primary antibody specific for Rac1 or RhoA was added and incubated for 45 min at room temperature. Finally, an HRP-conjugated secondary antibody was added and incubated for 45 min at room temperature. Absorbance was determined using the Bio-Tek microplate spectrophotometer.
2.10. Measurement of PKC-α activation in HL-MVEC
Unless otherwise indicated, all steps were conducted at 4 °C or on ice using chilled buffers. After incubation with LLO (250 ng/ml), in the presence or absence of TIP peptide (50 μg/ml), HL-MVEC were first washed twice with ice-cold phosphate buffered saline (PBS) and then incubated for 10 min with 1–2 ml isotonic MSE buffer [10 mM Tris–HCl, pH 7.5, 220 mM mannitol, 70 mM sucrose, 1 mM EGTA, 0.025% fatty acid-free bovine serum albumin (BSA)], 1.6 mM carnitine, 2 mM taurine, and 10 μg/ml each of aprotinin, leupeptin, and phenylmethylsulfonyl fluoride. Next the cells were collected using cell scrapers, transferred to glass tubes and subjected to homogenization using a motorized homogenizer. The homogenate was transferred to eppendorf tubes and centrifuged at 11,000 g for 10′ at 4 °C and the supernatant from this spin was then subjected to a 100,000 g centrifugation for 20′ at 4 °C in order to obtain the membrane fraction pellet. This pellet was then resuspended in 100 ul of MSE buffer. The protein concentrations of the resuspended and supernatant samples were determined using BCA™ Protein Assay Kit (Pierce, Rockford, IL). Same amounts of samples were placed in denaturing SDS sample buffer, boiled for 5 min and loaded onto a 7.5% polyacrylamide minigel (Bio-Rad Laboratories, Hercules, CA). Proteins were separated by electrophoresis at 75 V in stacking gel and 100 V in resolving gel. The gels were then transferred to nitrocellulose membranes in a transfer buffer (50 mmol/l Tris–HCl [pH 7.0], 380 mmol/l glycine, and 20% methanol). After being blocked for 1 h at room temperature in 1% BSA buffer dissolved in TTBS (TBS with 0.1% Tween 20), the membranes were incubated overnight with purified mouse anti-human PKCα antibodies (all from BD Transduction, Lexington, KY) and β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, nitrocellulose membranes were washed three times for 10 min each with TTBS and incubated with appropriate secondary antibodies (Amersham, Arlington Heights, IL). The luminescence detection of peroxidase was performed with the enhanced chemiluminescence system (ECL; Amersham, Arlington Heights, IL) according to the manufacturer's instructions and diagnostic films (Kodak, Rochester, NY) were exposed with the membranes. The blot densities were analyzed with NIH Imager (Scion, Frederick, MD).
2.11. Ca2+ influx measurements
Bovine aortic endothelial cells were transduced with adenovirus encoding the calcium-sensitive photoprotein aequorin and then seeded onto a 96 well plate. 24 h later the aequorin was activated by incubating the cells in Ca2+-free DMEM (Biosource, San Jose, CA) containing 5 mM coelenterazine (Sigma, St Louis, MO) for 30 min. Following this, loading media was replaced with phenol-free DMEM without coelenterazine. The cells were placed in a luminescence plate reader (Lumistar Galaxy) and challenged with LLO (250 ng/ml) as indicated. Aequorin-generated luminescence was recorded over time as previously demonstrated (Church and Fulton, 2006).
2.12. Statistical analysis
All data are summarized as means±SEM. Stimulated samples were compared with controls by unpaired Student's t-test. For multiple group comparisons, one-way ANOVA was used. P<0.05 was considered statistically significant.
3. Results
3.1. LLO alters RhoA and Rac1 activities and causes activation of MLC phosphorylation and hyperpermeability in HL-MVEC
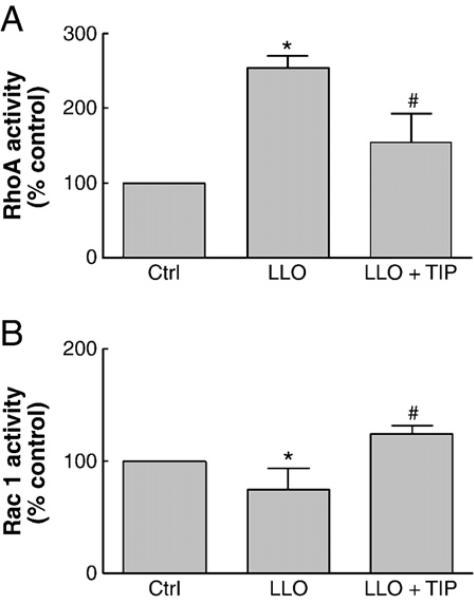
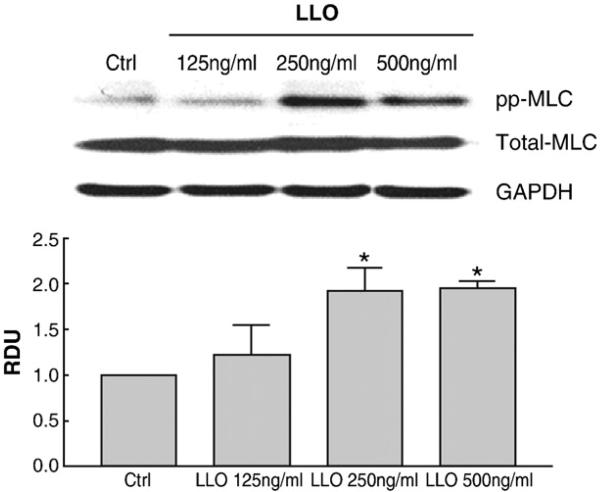
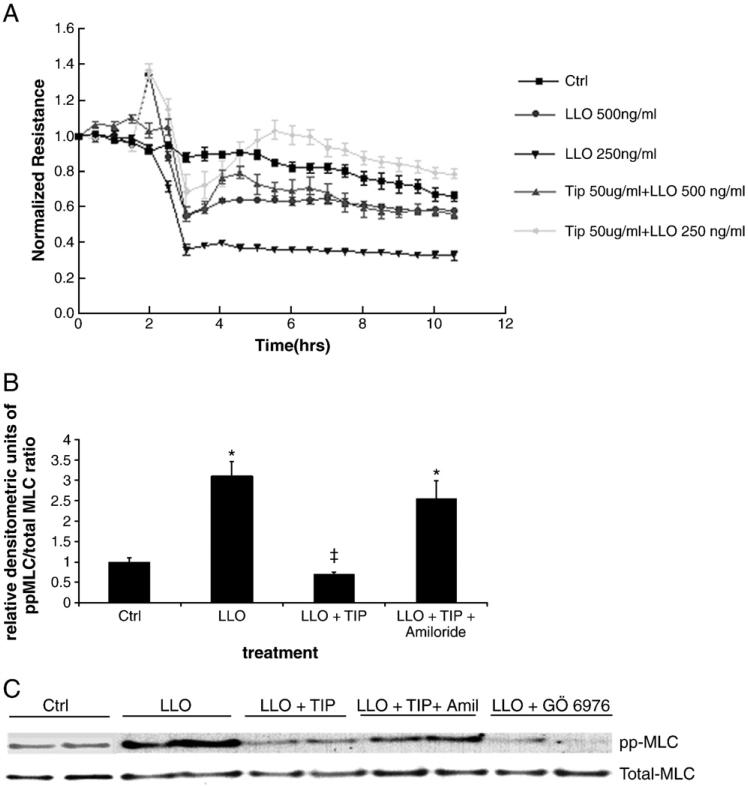
As shown in Catravas et al., this issue, we found that LLO induces a dose-dependent increase in permeability in monolayers of HL-MVEC, as measured by a decrease in transendothelial resistance in the electrical cell-substrate impedance sensing device (ECIS). In view of the importance of the activities of the disruptive RhoA and the protective Rac1 proteins in barrier integrity, we have investigated whether LLO is able to induce an imbalance in the activation status of these GTP-binding proteins. As shown in Fig. 1A and B, LLO induces an activation of RhoA and an inhibition of Rac1 activity within 2 h. In parallel with these changes, LLO induces a dose-dependent MLC phosphorylation within 2 h, as demonstrated in Fig. 2.
Fig. 1.
Effect of a 2 h treatment with LLO (250 ng/ml) on A. RhoA activity and B. Rac1 activity in HL-MVEC, both expressed as % of ctrl. Cells were pretreated for 30 min or not with TIP peptide (50 μg/ml). (n=4, *p<0.05 vs. ctrl; #p<0.05 vs. LLO).
Fig. 2.
LLO induces a dose-dependent increase in MLC phosphorylation upon a 2 h treatment in HL-MVEC, expressed as relative densitometric units (RDU) of the ppMLC/total MLC ratio (Western Blotting), normalized to the control cell signal. (n=3; *p<0.05 vs. ctrl).
3.2. LLO activates PKC-α, which mediates barrier dysfunction in HL-MVEC
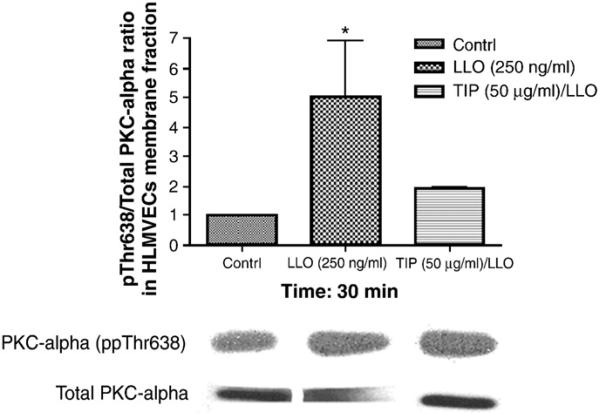
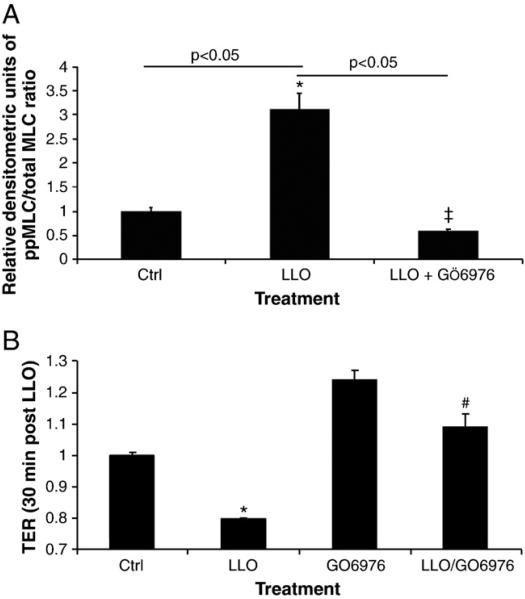
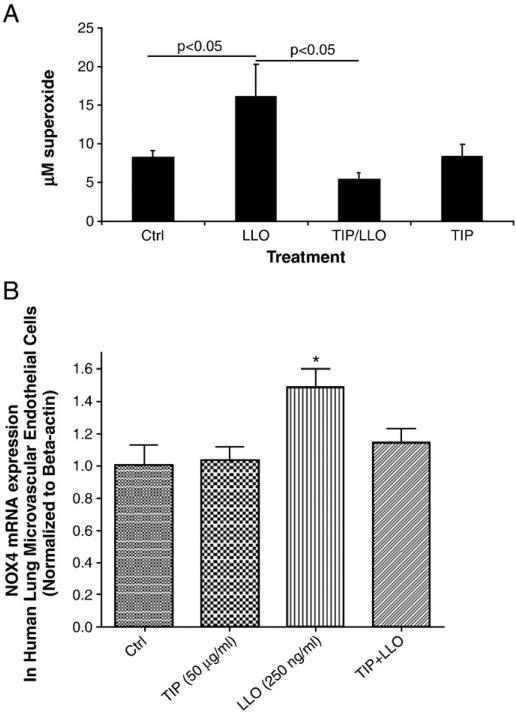
Since LLO induces a rapid Ca2+ influx in infected cells (Repp et al., 2002; Gekara et al., 2007), this can lead to the activation of Ca2+-dependent enzymes, such as the conventional PKC isoforms α, βI, βII and γ (Steinberg, 2008), which have been proposed to be implicated in endothelial permeability (Siflinger-Birnboim and Johnson, 2003). As shown in Fig. 3, LLO leads to a significant activation of PKC-α within 30 min, as measured by the ratio of pT638-PKC over total PKC-α in the membrane fraction of the cells. Treatment of HL-MVEC with GÖ6976 (1 μM), an inhibitor of the PKC-α and β isozymes, 30 min prior to applying LLO (250 ng/ml), attenuated LLO-induced MLC phosphorylation (Fig. 4A and blot in Fig. 6C) and blunted the decline in transendothelial resistance (TER) induced by LLO (Fig. 4B). Moreover, GÖ6976 treatment itself increased TER over basal levels in monolayers of HL-MVEC (Fig. 4B). Since PKC-α has been recently suggested to increase transcription of the ROS generating enzyme NADPH oxidase 4 (Nox4) in endothelial cells (Xu et al., 2008), we have subsequently investigated whether i) LLO induces ROS generation in endothelial cells and ii) whether LLO increases gene expression of Nox4. As shown in Fig. 5A, LLO significantly increases ROS generation in ovine pulmonary arterial endothelial cells, as measured by the electron paramagnetic resonance (EPR) technique. Moreover, as demonstrated in Fig. 5B, LLO (250 ng/ml, 6 h treatment) induces a significant increase in Nox4 mRNA, indicating that Nox4 is one of the sources for the increased LLO-induced ROS generation.
Fig. 3.
LLO (250 ng/ml) induces PKC-α activation within 30 min, which is blunted upon 30 min pretreatment with the TIP peptide in HL-MVEC (n=4; *p<0.05 vs. ctrl).
Fig. 4.
The PKC-α/βII inhibitor GÖ6976 (1 μM) significantly inhibits A. LLO-mediated MLC phosphorylation (expressed as relative densitometric units (RDU) of the ppMLC/total MLC ratio (Western Blotting), normalized to the control cell signal). n=3, *p<0.05 vs. ctrl; ‡p<0.05 vs. LLO and B. LLO-induced endothelial hyperpermeability (TER in ECIS) in HL-MVEC, upon 30 min preincubation. LLO: 250 ng/ml; (n=3; *p<0.05 vs. ctrl, #p<0.05 vs. LLO).
Fig. 6.
A. The TIP peptide (50 μg/ml) protects from LLO-induced hyperpermeability in HL-MVEC (TER measurements in ECIS, n=4; t test) analysis demonstrated that TER values in the TIP peptide/LLO groups were significantly different from the corresponding LLO groups between 3 and 10 h post LLO treatment (p<0.05). B. The TIP peptide (50 μg/ml) blunts LLO-induced MLC phosphorylation, measured as relative densitometric units (RDU) of the ppMLC/total MLC ratio, normalized to the control cell signal (2 h incubation, 250 ng/ml LLO) in an amiloride-sensitive (10 μM) manner. (n=3, *p<0.05 vs. ctrl; ‡p<0.05 vs. LLO). C. representative blot assessing total and ppMLC.
Fig. 5.
A. LLO (250 ng/ml; 6 h incubation) induces ROS generation in ovine PAEC, as measured by means of the EPR technique, and a 30 min pretreatment with the TIP peptide (50 μg/ml) inhibits this effect. The TIP peptide did not induce changes in basal ROS generation (n=6; *p<0.05 vs. ctrl; #p<0.05 vs. LLO). B. LLO treatment increases mRNA concentration of NADPH oxidase 4 in HL-MVEC, as measured by Real-Time RT-PCR and a 30 min pretreatment with the TIP peptide inhibits this activity. (n = 3; *p<0.05 vs. ctrl).
3.3. The TNF-derived TIP peptide inhibits LLO-mediated endothelial hyperpermeability
The TNF-derived TIP peptide (50 μg/ml), when applied 30 min prior to LLO, significantly inhibits LLO-mediated PKC-α activation (Fig. 3) as well as Nox4 activation (Fig. 5B), ROS generation (Fig. 5A), MLC phosphorylation (Fig. 6B) and endothelial hyperpermeability (TER in ECIS, Fig. 6A). The scrambled TIP peptide (50 μg/ml) failed to blunt LLO-induced MLC phosphorylation and hyperpermeability (data not shown). The TIP peptide (50 μg/ml) also restored the RhoA/Rac1 balance, when given as a 30 min pretreatment prior to LLO, as demonstrated in Fig. 1A and B, but had no effect on the activation status of these GTP-binding proteins by itself (data not shown).
Amiloride (10 μM), an inhibitor of the epithelial sodium channel, which is also expressed in endothelial cells (Kusche-Vihrog et al., 2008) and which was previously shown to blunt TIP peptide-mediated sodium uptake in pulmonary microvascular endothelial cells (Hribar et al., 1999), significantly diminished the inhibitory effect of the TIP peptide in LLO-mediated MLC phosphorylation (Fig. 6B, C).
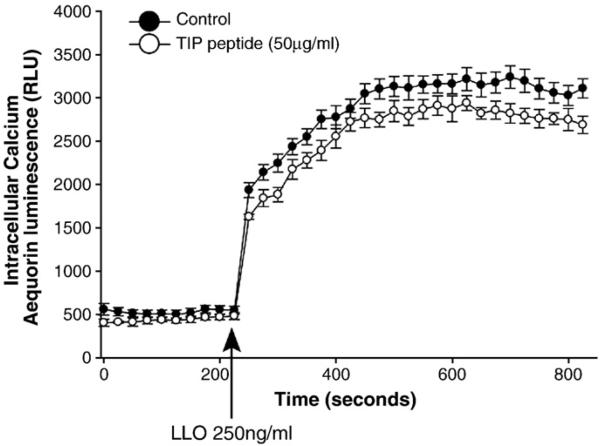
A potential mechanism by which the TIP peptide could interfere with PKC-α activation could be by inhibiting LLO-mediated Ca2+ influx. However, experiments assessing Ca2+-uptake in bovine aortic endothelial cells transduced with adenovirus encoding the calcium-sensitive photoprotein aequorin (Fig. 7) indicated that the TIP peptide only slightly attenuated LLO-mediated Ca2+ influx in these cells, which cannot explain its potent inhibitory effect on LLO-mediated PKC-α activation.
Fig. 7.
LLO induces a rapid Ca2+ influx in BAEC transduced adenovirus encoding the calcium-sensitive photoprotein aequorin (n=6, Church and Fulton, 2006).
4. Discussion
Listeriolysin O (LLO) is a member of the family of cholesterol-dependent cytolysins, which include pneumolysin and streptolysin and is the main virulence factor of L. monocytogenes (Munder et al., 2005). In view of its crucial role in bacterial virulence and its profound effects on the immune system of the host, LLO can therefore be considered as a model toxin for G+ infection-associated acute lung injury and permeability edema, which still result in high mortality. Our data demonstrate that LLO dramatically increases permeability in monolayers of HL-MVEC. The mechanism leading to LLO-mediated endothelial hyperpermeability appears to involve the activation of PKC-α, which occurs within 30 min, as well as the generation of ROS, the disturbance of the RhoA/Rac1 balance and the increase of MLC phosphorylation, all of which occur within hours. Recently, PKC has been proposed to be a central mediator of pulmonary endothelial permeability (Siflinger-Birnboim and Johnson, 2003). The conventional PKC isoforms can be activated upon increased Ca2+ influx (Steinberg, 2008), as can be caused by LLO. The source of the increased endothelial ROS generation upon LLO treatment is still unclear. Our data however suggest the implication of Nox4, the transcription of which was recently proposed to be activated by PKC-α (Xu et al., 2008), in LLO-induced ROS generation. However, we have also started to investigate other sources of superoxide generation, such as mitochondrial ROS and superoxide generated upon uncoupling of endothelial nitric oxide synthase (eNOS).
During pulmonary complications of listeriosis, LLO can induce a strong inflammatory response in the alveolar space, as well as in the interstitium (Munder et al., 2005). As such, pro-inflammatory substances such as TNF can be produced locally. TNF has previously been shown to directly promote edema formation, by means of decreasing transendothelial resistance (Petrache et al., 2003; Koss et al., 2006) and upon activating ROS generation in lung epithelial cells (Faggioni et al., 1994). However, in sharp contrast to its effects described above, TNF has also been shown to decrease edema formation in a rat pneumonia model (Rezaiguia et al., 1997). Our hypothesis is that two functionally distinct domains of the cytokine, i.e. the receptor binding sites vs. the lectin-like domain, account for the dichotomous activity of TNF in pulmonary edema (Elia et al., 2003; Braun et al., 2005). Our results presented here demonstrate that the TNF-derived TIP peptide, mimicking its lectin-like domain, apart from activating sodium uptake in endothelial and alveolar epithelial cells (Hribar et al., 1999; Fukuda et al., 2001), can also inhibit LLO-induced PKC-α activation (most likely not by interfering with LLO-mediated Ca2+ influx), as well as Nox4 transcription, ROS generation and endothelial hyperpermeability. Of note, Nox4 has recently been shown to be implicated in hypoxia-reoxygenation-induced ROS generation (Mittal et al., 2007) and also this effect can be blunted by the TIP peptide, as recently demonstrated by our group in a rat left lung isotransplantation model (Hamacher et al., in press). Intriguingly, amiloride, which inhibits TIP peptide-mediated Na+ uptake, also inhibits LLO-mediated MLC phosphorylation. Recently, the α subunit of the epithelial sodium channel, which has been shown to be crucial for the channel's activity, has been shown to be expressed in endothelial cells (Wang et al., 2009; Kusche-Vihrog et al., 2008). Together, these results thus suggest a link between Na+ uptake and regulation of permeability in endothelial cells, which requires further investigation. As such, the TIP peptide might represent an interesting candidate for the treatment of G+ infection-associated permeability edema, since it is able to restore both alveolar liquid clearance as well as endothelial barrier function.
5. Conclusion
Currently no standard therapy exists for permeability edema associated with G+ infection. Therefore, the search for candidate substances reducing endothelial hyperpermeability under these conditions remains important. The results from these studies extend the information about mechanisms of G+ exotoxin-induced pulmonary endothelial hyperpermeability and can thus contribute to the development of novel therapeutic strategies.
Acknowledgments
The authors would like to thank Dr. Supriya Sridmar for help with the figures. This work was supported in part by a BMBF-Clinical Research Group in Infectious Diseases grant to HH. ML. and TC. TC. is moreover supported by funds made available by the BMBF through NGFN-2. This work was partially supported by an industry grant from Apeptico, Vienna, Austria (RL., GY, and CX) and HL67841 (SMB). This work was also supported by a Programmatic Development award (to RL, SMB, JC, DF, and AV) and Seed Awards (to SK) from the Cardiovascular Discovery Institute of the Medical College of Georgia.
References
- Ananthraman A, Israel RH, Magnussen CR. Pleural-pulmonary aspects of Listeria monocytogenes infection. Respiration. 1983;44(2):153–157. doi: 10.1159/000194542. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J. Cell Physiol. 2005;204(3):934–947. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- Braun C, Hamacher J, Morel D, Wendel A, Lucas R. Dichotomal role of TNF in experimental pulmonary edema reabsorption. J. Immunol. 2005;175(5):3402–3408. doi: 10.4049/jimmunol.175.5.3402. [DOI] [PubMed] [Google Scholar]
- Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J. Biol. Chem. 2006;281(3):1477–1488. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- Darji A, Chakraborty T, Niebuhr K, Tsonis N, Wehland J, Weiss S. Hyperexpression of Listeriolysin in the non-pathogenic species Listeria innocua and high yield purification. J. Biotechnol. 1995;43(3):205–212. doi: 10.1016/0168-1656(95)00138-7. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Elia N, Tapponnier M, Matthay MA, Hamacher J, Pache JC, Brundler MA, Totsch M, De Baetselier P, Fransen L, Fukuda N, Morel DR, Lucas R. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor-alpha. Am. J. Respir Crit. Care Med. 2003;168(9):1043–1050. doi: 10.1164/rccm.200206-618OC. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Gatti S, Demitri MT, Delgado R, Echtenacher B, Gnocchi P, Heremans H, Ghezzi P. Role of xanthine oxidase and reactive oxygen intermediates in LPS- and TNF-induced pulmonary edema. J. Lab. Clin. Med. 1994;123(3):394–399. [PubMed] [Google Scholar]
- Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, Matthay MA. Mechanisms of TNF-stimulation of amiloride sensitive sodium transport across the alveolar epithelium in vivo and epithelial cells in vitro. Am. J. Physiol. 2001;280(6):L1258–L1265. doi: 10.1152/ajplung.2001.280.6.L1258. [DOI] [PubMed] [Google Scholar]
- Gekara NO, Westphal K, Ma B, Rohde M, Groebe L, Weiss S. The multiple mechanisms of Ca2+ signalling by listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Cell Microbiol. 2007;9(8):2008–2021. doi: 10.1111/j.1462-5822.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- Hamacher J, Stammberger U, Roux J, Kumar S, Schmid R, Fakin RA, Hossain H, Chakraborty T, Wendel A, Black SM, Lucas R. The lectin-like domain of TNF improves lung function after rat lung transplantation — potential role for a reduction in reactive oxygen species generation. Crit. Care Med. doi: 10.1097/CCM.0b013e3181cdf725. in press doi:10.1097/CCM.0b013e3181cdf725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hession C, Decker JM, Sherblom AP, Kumar S, Yue CC, Mattaliano RJ, Tizard R, Kawashima E, Schmeissner U, Heletky S, Muchmore AV. Uromodulin (Tamm–Horsfall glycoprotein): a renal ligand for lymphokines. Science. 1987;237(4821):1479–1484. doi: 10.1126/science.3498215. [DOI] [PubMed] [Google Scholar]
- Hribar M, Bloc A, van der Goot FG, Fransen L, De Baetselier P, Grau GE, Bluethmann H, Matthay MA, Dunant Y, Pugin J, Lucas R. The lectin-like domain of tumor necrosis factor-alpha increases membrane conductance in microvascular endothelial cells and peritoneal macrophages. Eur. J. Immunol. 1999;29(10):3105–3111. doi: 10.1002/(SICI)1521-4141(199910)29:10<3105::AID-IMMU3105>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Janssens W, Van Raemdonck D, Dupont L, Verleden GM. Listeria pleuritis 1 week after lung transplantation. J. Heart Lung Transplant. 2006;25(6):734–737. doi: 10.1016/j.healun.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Koss M, Pfeiffer GR, 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, Wang Q. Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. J. Immunol. 2006;176(2):1218–1227. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H. Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch. 2008;455(5):849–857. doi: 10.1007/s00424-007-0341-0. [DOI] [PubMed] [Google Scholar]
- Lerolle N, Zahar JR, Duboc V, Tissier F, Rabbat A. Pneumonia involving Legionella pneumophila and Listeria monocytogenes in an immunocompromised patient: an unusual coinfection. Respiration. 2002;69(4):359–361. doi: 10.1159/000063263. [DOI] [PubMed] [Google Scholar]
- Lucas R, Magez S, De Leys R, Fransen L, Scheerlinck JP, Rampelberg M, Sablon E, De Baetselier P. Mapping the lectin-like activity of tumor necrosis factor. Science. 1994;263(5148):814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- Lucas R, Verin AD, Black SE, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem. Pharm. 2009;77(12):1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 2001;276(25):22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hänze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ. Res. 2007;101(3):258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- Munder A, Zelmer A, Schmiedl A, Dittmar KE, Rohde M, Dorsch M, Otto K, Hedrich HJ, Tümmler B, Weiss S, Tschernig T. Murine pulmonary infection with Listeria monocytogenes: differential susceptibility of BALB/c, C57BL/6 and DBA/2 mice. Microbes Infect. 2005;7(4):600–611. doi: 10.1016/j.micinf.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am. J. Respir. Cell Mol. Biol. 2003;28(5):574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- Posfay-Barbe KM, Wald ER. Listeriosis. Pediatr. Rev. 2004;25(5):151–159. doi: 10.1542/pir.25-5-151. [DOI] [PubMed] [Google Scholar]
- Repp H, Pamukci Z, Koschinski A, Domann E, Darji A, Birringer J, Brockmeier D, Chakraborty T, Dreyer F. Listeriolysin of Listeria monocytogenes forms Ca2+-permeable pores leading to intracellular Ca2+ oscillations. Cell Microbiol. 2002;4(8):483–491. doi: 10.1046/j.1462-5822.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- Rezaiguia S, Garat C, Delclaux C, Meignan M, Fleury J, Legrand P, Matthay MA, Jayr C. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J. Clin. Invest. 1997;99(2):325–335. doi: 10.1172/JCI119161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose F, Zeller SA, Chakraborty T, Domann E, Machleidt T, Kronke M, Seeger W, Grimminger F, Sibelius U. Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors. Infect. Immun. 2001;69(2):897–905. doi: 10.1128/IAI.69.2.897-905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherblom AP, Decker JM, Muchmore AV. The lectin-like interaction between recombinant tumor necrosis factor and uromodulin. J. Biol. Chem. 1988;263(11):5418–5424. [PubMed] [Google Scholar]
- Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L435–L451. doi: 10.1152/ajplung.00106.2002. [DOI] [PubMed] [Google Scholar]
- Steinberg S. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadász I, Schermuly RT, Ghofrani HA, Rummel S, Wehner S, Mühldorfer I, Schäfer KP, Seeger W, Morty RE, Grimminger F, Weissmann N. The lectin-like domain of tumor necrosis factor-alpha improves alveolar fluid balance in injured isolated rabbit lungs. Crit. Care Med. 2008;36(5):1543–1550. doi: 10.1097/CCM.0b013e31816f485e. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler. Thromb. Vasc. Biol. 2000;20(12):E127–E133. doi: 10.1161/01.atv.20.12.e127. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann. N. Y. Acad. Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft Listeria pathogenesis and molecular virulence determinants. J. Clin. Microbiol. Rev. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Meng F, Mohan S, Champaneri B, Gu Y. Functional ENaC channels expressed in endothelial cells: a new candidate for mediating shear force. Microcirculation. 2009;16(3):276–287. doi: 10.1080/10739680802653150. [DOI] [PubMed] [Google Scholar]
- Wedgwood S, Mitchell CJ, Fineman JR, Black SM. Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284(4):L650–L662. doi: 10.1152/ajplung.00252.2002. [DOI] [PubMed] [Google Scholar]
- Xu H, Goettsch C, Xia N, Horke S, Morawietz H, Förstermann U, Li H. Differential roles of PKCalpha and PKCepsilon in controlling the gene expression of Nox4 in human endothelial cells. Free Radic. Biol. Med. 2008;44(8):1656–1667. doi: 10.1016/j.freeradbiomed.2008.01.023. [DOI] [PubMed] [Google Scholar]