Abstract
Background
Our objective was to provide a contemporary analysis of the prevalence, types, and impact of advance health care directives in critically ill cancer patients.
Methods
We retrospectively reviewed all intensive care unit (ICU) admissions (January 1, 2006 to April 25, 2008) at an oncologic center and identified all patients who completed a living will (LW), or health care proxy (HCP), or neither prior to ICU admission. Demographics, clinical data, end-of-life (EOL) parameters and outcomes were compared among three groups: LWs, HCPs, and no LW or HCP.
Results
Of 1,333 ICU admissions, 1,121 patients (84%) were included for analysis: 176 patients (15.7%) had LW, 534 (47.6%) had HCP and 411 (36.7%) had no LW or HCP. Patients with LW were significantly more likely to be older and white as compared to patients with HCP alone, or no LW or HCP. There were no significant demographic differences between patients with HCP or no LW or HCP. Patients with HCP alone, or no LW or HCP, were significantly more likely to have Medicaid than patients with LW. There were no differences noted in ICU care, EOL management, or outcomes among the three groups.
Conclusions
The prevalence of LWs in patients admitted to our oncologic ICU is low. More than half of the remaining patients had designated HCPs. Older age and white race were associated with the presence of LWs. However, the presence of LWs or HCPs did not influence ICU care, EOL management or outcomes at our institution.
Introduction
Americans hope that their physicians and designated proxies will ensure that their wishes are followed at the end of their lives.1–4 The Patient Self-Determination Act (PSDA) passed by Congress in 19905,6 provided a national framework for two types of advance health care directives to enhance end-of-life (EOL) care for incapacitated patients; the living will and the health care proxy. To date, despite the PSDA and a myriad of broad-based EOL planning initiatives, less than a third of Americans have completed advance health care directives.1
The dearth of EOL care planning in America has been identified as one of the barriers to optimizing EOL care and may be a major contributor to the use of overly aggressive and costly hospital resources at the EOL.7 This is especially relevant to intensive care units (ICU) in the United States where 22% of deaths in the hospital involve an ICU admission.8 Many intensivists consider EOL care to be one of the most challenging and time consuming elements of their critical care medicine (CCM) practice.9
Despite the soaring numbers of new patients annually diagnosed with cancer in the United States,10 their frequent need for ICU care,11,12 and their associated low survival rates,13 there are few reports of EOL planning in critically ill patients with cancer.14,15 Kish and colleagues15 of the University of Texas M.D. Anderson Cancer Center analyzed data from 872 cancer patients admitted to their ICU between 1994 and 1996 and found that only 27% of patients had a written living will; moreover, compliance with the provisions of these advance directives was poor. The purpose of our study was to provide a contemporary analysis of the prevalence, types, impact, and timing of both living wills and health care proxies in patients admitted to an oncologic ICU.
Methods
Study design
This study was a 28-month retrospective comparative analysis of patients with and without advance directives documented in the medical record. The study was granted a waiver of authorization by the Institutional Review Board (Project Approval Number WA 0171-08).
Using hospital and ICU databases, we reviewed the electronic medical records (EMR) of all patients admitted to the closed medical-surgical ICU of Memorial Sloan-Kettering Cancer Center from January 1, 2006 through April 25, 2008. At our institution, ICU attending approval is required for all ICU admissions, discharges, or rejections. As we are managing an oncologic ICU in a tertiary care cancer facility, our ICU triage process is focused primarily upon the perceived reversibility of the patient's acute critical illness, rather than the type, extent, duration, or aggressiveness of their malignancy.16,17
We extracted advance health care documents from the “Advance Directives” section of the EMR. Four types of documents were identified; a living will (LW), a combined/hybrid health care proxy-living will (HCP-LW), a Health Care Proxy (HCP), and a do-not-resuscitate (DNR) order. For the purpose of this study, “LW” represents patients with written instructions that limit care at EOL (LW, combined/hybrid HCP-LW, or patients with both a HCP and LW); “HCP” represents a Health care proxy or agent, or a durable power of attorney for health care.
Patients were included in the LW and HCP categories only if the advance health care documents were dated prior to the time of ICU admission. Whenever duplicate HCP or LW documents were found, we used the document that was dated closest to the date of ICU admission. Patients were excluded from analysis if their documents were not dated, if their LWs requested that there be no limitations of care, or if DNR orders were issued prior to ICU admission since the DNR may have influenced the application of aggressive ICU procedures or EOL care. If a patient had more than one ICU admission, we included only the first ICU admission for analysis.
Demographic and clinical data were extracted from the EMR. The demographic data included age, gender, race (whites and minorities/other), insurer (Medicare, Medicaid/none, and Commercial), marital status (married, divorced/separated, single, widowed), and religion (Christian, Jewish, Roman Catholic, and other/unknown). Clinical parameters tracked on ICU admission included service type (medical or surgical), mortality prediction score (Mortality Probability Model at ICU admission V2) (MPM0-II) and cancer classification.
Cancer diagnoses were grouped as follows: thoracic, gastrointestinal, genitourinary, head and neck, hematologic, hematopoietic stem cell transplantation (HSCT), miscellaneous, and no cancer. If a patient had more than one cancer diagnosis, we selected the most active. When the cancer was remote and considered nonactive by the oncologic team, and the ICU admission was not related in any way to the prior cancer, we selected “no cancer.” If a patient had a HSCT as the most recent cancer therapy, then HSCT was listed as the cancer diagnosis. However, if a patient had a HSCT in the past, but had a relapse of the primary cancer posttransplant, then the cancer was listed as the active diagnosis.
ICU-specific clinical data included use of mechanical ventilation and vasopressor agents at any time during the ICU stay, airway management (intubation or tracheostomy), renal support (hemodialysis and continuous renal replacement therapy), and other interventions (endoscopy, percutaneous insertion of gastrostomy or jejunostomy feeding tubes, chemotherapy administration, and intrahospital transports). Outcome data included length of stay (LOS) (pre-ICU, ICU, and post-ICU) and mortality (ICU and hospital). EOL data included performance of cardiopulmonary resuscitation (CPR), writing a DNR order during the ICU stay, and the implementation of no escalation of care and/or withdrawal of life-sustaining therapy.
Statistical analyses
Fisher's exact test and the Wilcoxon rank sum test were used for univariate comparisons of covariate differences between patients with LW, HCP, or no LW or HCP. Variables significant in the univariate analyses were then entered into multivariate polychotomous logistic regression model to determine the set of factors that independently associated with differences among the three groups (LW, HCP, or no LW or HCP). Odds ratio, 95% confidence interval, and p values were reported. We calculated the time period between the dates of the LW or HCP and the ICU admission using three intervals chosen a priori: 3 months, 3–6 months, and greater than 6 months prior to ICU admission. When a LW patient had both LW and HCP documents that were dated differently, we used the date on the LW as most representative of intent. The Cochran-Armitage trend test was used to examine the trends in binominal proportions in procedures performed upon or during ICU admission as well as end of life care relative to the timing of LW and HCP, respectively. In addition, the Cochran-Armitage trend test was also used to compare the timing of LW vs HCP relative to the three time intervals.
We also applied logistic regression to adjust for any significant demographic differences when comparing individual ICU or EOL parameters among the LW, HCP and no LW or HCP groups. The statistical package SAS (9.1) (SAS Institute Inc., Cary, NC) was used to generate the test statistics and build the regression model. A p value <0.05 was considered significant.
Results
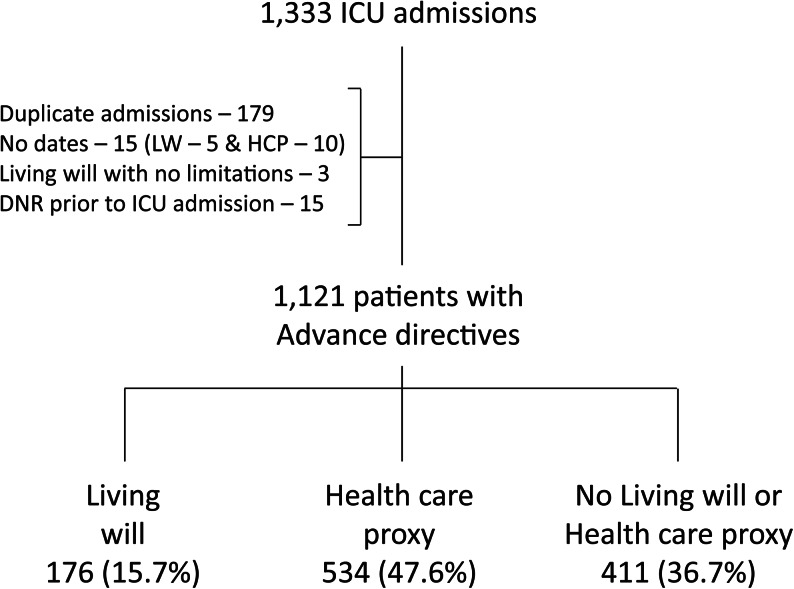
During the study period, 1333 patients were admitted to our ICU. Of these, 212 (16%) patients were excluded from analysis (Fig. 1), resulting in 1121 evaluable patients: 176 patients (15.7%) had an LW, 534 (47.6%) had an HCP, and 411 (36.7%) had no LW or HCP.
FIG. 1.
Flow chart of intensive care unit (ICU) patients with advance directives. After exclusion of 212 (16%) patients, there were 1121 ICU patients for analysis. A minority (176, 15.7%) had a living will. Of these, 137 (78%) had a combined/hybrid LW-health care proxy (HCP), 34 (19%) had separate LW and HCP documents (29 were dated within 30 days of each other), and 5 (3%) patients had a LW alone.
Univariate analysis comparing demographic data among the three groups (LW, HCP and no LW or HCP) were significant only for age, race, insurance, and service type (Table 1). The presence of a LW was significantly more common in the older, white, and surgical patients, and less common in Medicaid patients (Table 1). Using multivariate analysis when we compared patients with LW versus HCP, patients who were older and white were more likely to have LW. Additionally, patients who had commercial insurance were more likely to have LW; and patients who had Medicaid were less likely to have LW (Tables 1 and 2). Similarly, when we compared patients with LW versus no LW or HCP, patients who were older and white were more likely to have LW; and patients with Medicaid were less likely to have LW. In addition, patients admitted under a surgical service were more likely to have LW than patients admitted from a medical service. In contrast, there were no significant demographic differences observed when comparing patients with HCP vs no LW or HCP (Tables 1 and 2).
Table 1.
Demographics of Patients with Living Wills, Health Care Proxies or with no Living Will or Health Care Proxy
| Living will n = 176 | Health care proxy n = 534 | No living will or health care proxy n = 411 | |
|---|---|---|---|
| Age on ICU admissiona | |||
| Age y (mean ± SD) | 64.5 (±11.7) | 61.0 (±14.9) | 61.0 (±15.5) |
| Gender | |||
| Male | 115 (65.3%) | 323 (60.5%) | 234 (56.9%) |
| Racea | |||
| White | 162 (92.0%) | 412 (77.2%) | 306 (74.5%) |
| Minorities/others | 14 (8.0%) | 119 (22.8%) | 105 (25.5%) |
| Insurancea | |||
| Commercial | 82 (46.6%) | 214 (40.1%) | 183 (44.5%) |
| Medicare | 92 (52.3%) | 270 (50.6%) | 188 (45.7%) |
| Medicaid/none | 2 (1.1%) | 50 (9.3%) | 40 (9.8%) |
| Marriage status | |||
| Divorced | 16 (9.1%) | 35 (6.6%) | 21 (5.1%) |
| Married | 122 (69.3%) | 353 (66.1%) | 279 (67.9%) |
| Single | 23 (13.1%) | 94 (17.6%) | 78 (19.0%) |
| Widowed | 15 (8.5%) | 52 (9.7%) | 33 (8.0%) |
| Religion | |||
| Roman Catholic | 76 (43.2%) | 240 (44.9%) | 154 (37.5%) |
| Christian | 29 (16.5%) | 99 (18.9%) | 80 (19.5%) |
| Jewish | 30 (17.1%) | 95 (17.8%) | 78 (18.9%) |
| Other/Unknown | 41 (23.2%) | 100 (18.7%) | 99 (24.1%) |
| Admitting servicea | |||
| Medicine | 83 (47.2%) | 301 (56.4%) | 256 (62.3%) |
| Surgery | 93 (52.8%) | 233 (43.6%) | 155 (37.7%) |
| MPM II score on ICU admission | |||
| MPM II % (mean ± SD) | 46 (±26) | 48 (±26) | 48 (±28) |
p values <0.05 in univariate analysis. See Table 2 for multivariate model. Race: Minorities/others include Asian/Indian, Black, Hispanic and other. Christian includes Baptist, Christian, Christian Orthodox, Episcopalian, Greek Orthodox, Jehovah's Witness, Lutheran, Methodist, Mormon, Pentecostal, Presbyterian, Protestant, Russian Orthodox, Seventh Day Adventist, Unitarian, and Quaker). Other/unknown includes Buddhist, Hindu, Moslem, other, and unknown.
ICU, intensive care unit, MPM II, Mortality Probability Model version II.
Table 2.
Multivariate Polychotomous Logistic Regression Comparing the Three Study Groups
| |
Living will vs. health care proxy |
Living will vs. no living will or health care proxy |
Health care proxy vs. no living will or health care proxy |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| Age on ICU admission | 1.3a | 1.06–1.5 | 0.01 | 1.2a | 1.03–1.43 | 0.03 | 0.9a | 0.8–1.1 | 0.71 |
| Raceb | <0.01 | <0.01 | 0.25 | ||||||
| White | 2.7 | 1.5–5.0 | 3.2 | 1.77–5.88 | 1.2 | 0.9–1.6 | |||
| Minorities/others | 1.0 | 1.0 | 1.0 | ||||||
| Insurance | |||||||||
| Medicaid/none | 0.1 | 0.03–0.7 | 0.008 | 0.2 | 0.04–0.07 | 0.004 | 1.1 | 0.7–1.8 | 0.56 |
| Medicare | 0.6 | 0.4–0.9 | 0.02 | 0.7 | 0.43–1.15 | 0.17 | 1.2 | 0.8–1.8 | 0.21 |
| Commercial | 1.0 | 1.0 | 1.0 | ||||||
| Admitting service | 0.08 | <0.01 | 0.1 | ||||||
| Medicine/others | 1.3 | 0.9–1.9 | 0.6 | 0.40–0.84 | 0.8 | 0.6–1.1 | |||
| Surgery | 1.0 | 1.0 | 1.0 | ||||||
Proportional change in OR for each 10 year increase in age. bMinorities/others include Asian/Indian, Black, Hispanic and other.
OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
There were no statistically significant univariate differences in any other demographic (Table 1), clinical, procedural, or therapeutic parameters on ICU admission (Tables 1 and 3) or during the ICU stay (Table 4). Finally, there were no differences among the three groups in approach to EOL, and ICU and hospital LOS or mortality (Table 5).
Table 3.
Cancer Diagnoses
| Cancer diagnosis | Living will n = 176 | Health care proxy n = 534 | No living will or health care proxy n = 411 |
|---|---|---|---|
| Thoracic | 14 (8.0%) | 57 (10.7%) | 50 (12.2%) |
| Gastrointestinal | 49 (27.8%) | 131 (24.5%) | 88 (21.4%) |
| Genitourinary | 27 (15.3%) | 79 (14.9%) | 66 (16.1%) |
| Head and Neck | 9 (5.1%) | 46 (8.6%) | 23 (5.6%) |
| Hematologic | 35 (19.9%) | 77 (14.4%) | 86 (20.9%) |
| HSCT | 19 (10.8%) | 44 (8.2%) | 24 (5.8%) |
| Miscellaneous | 19 (10.8%) | 85 (15.9%) | 57 (13.9%) |
| No cancer | 4 (2.3%) | 15 (2.8%) | 17 (4.1%) |
Thoracic (lung, mediastinal, thymoma, and mesothelioma); gastrointestinal (esophageal, gastric, small bowel, colorectal, pancreas, hepato-biliary and peritoneal); genitourinary (renal, bladder, urothelial, prostate, testicular, ovarian, uterine, cervical and vaginal); Head and neck (oral cavity, neck, sinuses, thyroid and parathyroid); hematologica (leukemia, lymphoma, myelodysplastic syndrome, multiple myeloma and amyloidosis); HSCT, hematopoietic stem cell transplantation (allogeneic or autologous); miscellaneous (breast, central nervous system, sarcoma, human immunodeficiency virus (HIV)-related, and unknown primary).
Table 4.
Procedures and Therapies Performed upon or during ICU Admission
| Living will n = 176 | Health care proxy n = 534 | No living will or health care Proxy n = 411 | |
|---|---|---|---|
| Mechanical ventilation | |||
| MV in ICU | 109 (61.9%) | 313 (58.6%) | 231 (56.2%) |
| MV on ICU discharge | 16 (11.4%) (n = 140) | 53 (12.5%) (n = 425) | 40 (12.4%) (n = 323) |
| Vasopressors | |||
| Vasopressors in ICU | 75 (42.6%) | 249 (46.6%) | 180 (43.8%) |
| Airways | |||
| Intubation (pre- or post-ICU admission) | 60 (34.1%) | 188 (35.2%) | 123 (30.0%) |
| Intubation in ICU (post-ICU admission) | 55 (31.3%) | 177 (33.2%) | 116 (28.2%) |
| Tracheostomy (includes pre- and post-ICU admission)a | 24 (13.6%) | 81 (15.2%) | 47 (11.4%) |
| Days in ICU to tracheostomy (mean ± SD) | 11.4 ± 6.3 | 10 ± 6.2 | 10 ± 6.3 |
| Renal support | |||
| Hemodialysis/CRRT | 19 (10.8%) | 49 (9.2%) | 41 (10%) |
| Others | |||
| Endoscopy | 8 (4.5%) | 28 (5.2%) | 19 (4.6%) |
| PEG/PEJ | 3 (2.0%) | 20 (3.8%) | 11 (2.7%) |
| Chemotherapy | 7 (4.0%) | 24 (4.5%) | 22 (5.3%) |
| Intrahospital transports/patient (mean ± SD) | 2.1 ± 1.5 (n = 84) | 2.2 ± 2.3 (n = 259) | 1.9 ± 1.4 (n = 193) |
The HCP group had 5 tracheostomies pre-ICU; there were no differences in percentages of tracheostomies done after ICU admission in all three groups.
ICU, intensive care unit; MV, mechanical ventilation; VP, vasopressors; CRRT, continuous renal replacement therapy; REG, percutaneous endoscopic gastrostomy; PEJ, percutaneous endoscopic jejunostomy.
Table 5.
End-of-Life Care in the Intensive Care Unit
| Living will n = 176 | Health care proxy n = 534 | No living will or health care proxy n = 411 | |
|---|---|---|---|
| End of life | |||
| Cardiopulmonary resuscitation | 5 (2.8%) | 33 (6.2%) | 24 (5.8%) |
| DNR in ICU | 50 (28.4%) | 124 (23.2%) | 98 (23.8%) |
| No escalation of care | 10 (5.7%) | 27 (5.1%) | 20 (4.8%) |
| Withdrawal of life-sustaining therapy | 16 (9.1%) | 45 (8.4%) | 28 (6.8%) |
| Length of stay (mean ± SD) | |||
| Pre-ICU | 10 ± 13.8 (n = 135) | 9.7 ± 11.4 (n = 417) | 8.0 ± 16.6 (n = 296) |
| ICU | 7.8 ± 7.7 | 7.6 ± 9.0 | 6.2 ± 7.3 |
| Post-ICU | 13.8 ± 15.0 (n = 140) | 15.5 ± 18.9 (n = 425) | 14.8 ± 18.5 (n = 323) |
| Hospital total | 26.5 ± 22.9 | 26.0 ± 26.1 | 24.0 ± 26.5 |
| Mortality | |||
| ICU | 36 (20.5%) | 109 (20.4%) | 88 (21.4%) |
| Hospital | 62 (35.2%) | 173 (32.4%) | 146 (35.5%) |
DNR, do not resuscitate; ICU, intensive care unit.
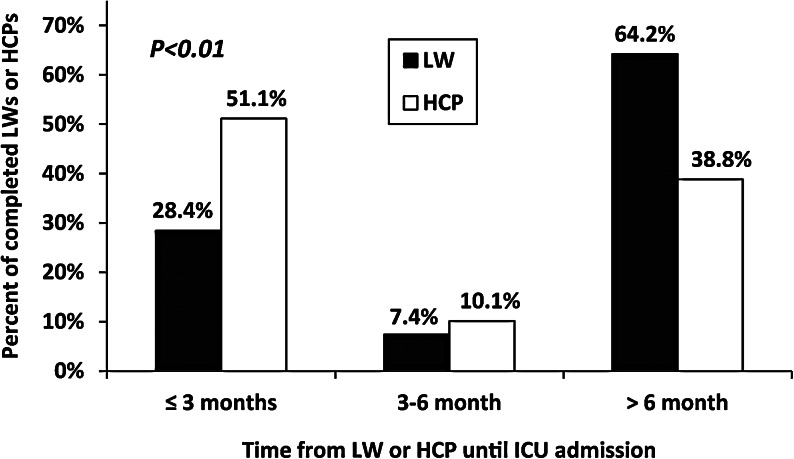
We observed a statistically significant inverse relationship between the time intervals between the dating of the LWs and HCPs and ICU admission. HCPs were dated closer to the time of ICU admission than the LWs (p < 0.01; Fig. 2). However, the differences in time from the dating of the LW or HCP to ICU admission did not impact ICU procedures, EOL care or outcomes (data not shown).
FIG. 2.
Differences in time intervals. The time intevals between writing the living will (LW) or health care proxy (HCP) and intensive care unit (ICU) admission are inversely related. HCPs were predominantly written within 6 months of ICU admission, in contrast to LWs, which were mostly written greater than 6 months prior to ICU admission.
The striking similarity in ICU or EOL parameters obviated the need for a formal post hoc power analysis. Furthermore, the lack of significant differences in the ICU or EOL parameters among the LW, HCP, and no LW or HCP groups was confirmed after adjusting for race, age and type of insurance by logistic regression.
Discussion
Our study, the first analysis of advance directives in an oncologic ICU since the mid-1990s,14,15 shows a continuing low rate of LWs in this population. Low frequencies of LWs have also been documented in patients admitted to adult ICUs (0% to 13%)18–20 and in units for the chronically critically ill (16%–38%).21,22 These low LW rates are not unexpected, especially in the United States, given the paucity of advance care planning in America,1 whether among the healthy, the elderly, the hospitalized,23 those undergoing major surgical procedures,24 or those with cancer.25,26 In contrast to the low frequency of LWs, we found a much higher percentage of patients with HCPs. Our contemporary analysis replicates prior studies1,14,15,18,27 in both oncologic and medical-surgical critically ill populations, which shows that the presence of LWs has no impact on ICU care patterns, EOL management, and outcomes.
Demographic and societal factors play, albeit inconsistent, roles in the writing and compliance of LWs.1,28 We found a significantly higher frequency of LWs with advancing age. Older patients tend to address EOL issues more often than younger patients possibly as a result of their greater life experience, exposure to illness, and estate planning.29 Like others,1 we also found that whites were more apt to have LWs than minorities. This correlated with the better education, socioeconomics, and physician and attorney relationships observed in whites than minorities.30 African Americans, in far greater percentages than whites, often express the wish to preserve life at any cost, thus limiting their desire to draft LWs.31 We also found that Medicaid patients had a lower frequency of LWs than patients covered by other insurers. This finding may reflect the more limited access of Medicaid recipients to health care professionals and their perceptions that LWs negatively influence quality of care.1
The failure of LWs to exert an effect on ICU and EOL care has been well explored.1,32–34 LWs are not written with the prescience required to guide ICU triage decisions and the nuances of ICU care.1,35 The lack of efficacy of LWs is commonly attributed to their confusing and legalistic writing style.1,36 The LW may also not be perceived as being a valid tool for limiting ICU care until the relevant parties have studied it. Even then, they must agree that the patient's clinical status meets the conditions of hopelessness as set forth in the typical LW.1,4,37,38 Achieving this consensus is challenging, because a universal perception of hopelessness does not exist, and the concept of an “abbreviated” ICU trial (5–7 days) for critically ill patients with cancer is just entering the CCM lexicon.17 Concomitantly, proxies do not have much faith in physicians' prognostication abilities29,30,39,40 and may disagree among themselves or with their physicians even when faced with irrefutable evidence that death is imminent.41,42 Finally, there is no legal accountability if a LW is ignored.
To our knowledge, we are the first to address the impact of HCPs on ICU care. Our data shows that having a HCP alone did not have any effect on ICU procedures, EOL actions or ICU or hospital outcomes as compared to the no LW or HCP group. This data is not surprising as having a LW with written directives limiting care also did not exert any impact. The HCP process assumes that a high level of shared understanding exists between the HCP and the patient. Ideally this results in an agreement in EOL values that permits the HCP to suspend his or her own judgement and implement the patient's wishes. In reality, communications between the patient and the proxy regarding the patient's wishes are commonly suboptimal.43,44 Even with excellent discussions, the proxy may choose to disregard earlier agreements and project their own, or the physician's, attitudes onto ICU decisions and EOL care rather than the patient's.1
A greater percentage of HCPs were completed closer to ICU admission than LWs. We can only speculate that as patients became sicker, they felt the need to identify a HCP; in contrast, healthier patients were more likely to address the writing of LWs at a greater time interval prior to becoming critically ill. Despite the differences in time periods between HCPs and LWs and ICU admission, the similarities in ICU care patterns, EOL management and outcomes in all three study groups (LW, HCP, or no LW or HCP) suggests that a common denominator exists within each group.45 We believe that whether the patient's wishes were expressed in writing or orally, explicitly, generally, or not at all, with a designated proxy or not, the suddenness of a loved one's clinical deterioration may not give the proxy, or others not formally appointed, much time to mentally and emotionally prepare for this experience and process the issues at hand.46,47 Thus, the ICU and EOL management and outcomes are the same for patients in each of the three study groups.
Our study has several limitations. First, we cannot comment on the frequency and efficacy of LWs or HCPs in patients who were never admitted to the ICU as this was beyond our scope. Second, the retrospective nature of our study precluded a determination of whether the ICU team was actually aware of the presence of the LWs or HCPs during the ICU stay.20 Third, we had no method of assessing whether there were “unofficial” surrogates in the no LW or HCP group. Finally, our findings were confined to critically ill cancer patients potentially limiting their generalizability to critically ill patients without cancer.
In conclusion, we found a low prevalence of LWs and a stronger presence of HCPs among patients admitted to our oncologic ICU. Older age and white race were demographic factors associated with the presence of LWs. Medicaid patients, a small percentage of our study population, were more likely to have HCPs, or no LWs or HCPs, than to have LWs. The existence of LWs or HCPs did not have any more impact on ICU management, EOL care, or patient outcomes than the absence of LWs or HCPs. In our opinion, the next generation LW should contain unambiguous language that applies selectivity to ICU admission,48 and documents preferences for resuscitation and provision of high-quality palliative care at EOL.4,17,49,50 Additionally, we believe that ICUs should incorporate programs on advance care planning for HCPs to assist them in making decisions about EOL care for their loved ones during critical illnesses.1,51 Further research is necessary to evaluate if the current AD system can be enhanced to ensure compliance with patients' wishes, or whether an entirely new approach to EOL is required.
Acknowledgments
We acknowledge Christie Galarza, Nicola Phillips-Tate, Kermitt Ramirez, Khy Huynh, and Ana Dabo-Trubelja, M.D., Department of Anesthesiology and Critical Care Medicine, and Elaine Ciccaroni, Medical Graphics, Memorial Sloan-Kettering Cancer Center, New York, New York, for their participation in data gathering and preliminary analysis and preparation of the figures and tables.
Supported by Department of Anesthesiology and Critical Care Medicine, Memorial Sloan-Kettering Cancer Center, New York, New York.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Advance directives and advance care planning: Report to Congress August 2008. U.S.Department of Health and Human Services Assistant Secretary for Planning and Evaluation Office of Disability Aging and Long-Term Planning. http://aspe.hhs.gov/daltcp/reports/2008/ADCongRpt.pdf. [Sep 1;2010 ]. http://aspe.hhs.gov/daltcp/reports/2008/ADCongRpt.pdf
- 2.Beckstrand RL. Callister LC. Kirchhoff KT. Providing a “good death”: Critical care nurses' suggestions for improving end-of-life care. Am J Crit Care. 2006;15:38–45. [PubMed] [Google Scholar]
- 3.Teno JM. Clarridge BR. Casey V. Welch LC. Wetle T. Shield R. Mor V. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 4.Kass-Bartelmes BL. Hughes R. Advance care planning: Preferences for care at the end of life. AHRQ; 2009. [Sep 1;2010 ]. [PubMed] [Google Scholar]
- 5.Federal Patient Self-Determination Act Final Regulations. www.denbar.org/docs/psda.pdf?ID=1816. [Sep 1;2010 ]. www.denbar.org/docs/psda.pdf?ID=1816
- 6.American Cancer Society: The Patient Self-Determination Act (PSDA) www.cancer.org/docroot/home/index.asp?level=0. [Sep 1;2010 ]. www.cancer.org/docroot/home/index.asp?level=0
- 7.Critical Care Societies Collaborative: An open letter to President Obama. www.sccm.org/SiteCollectionDocuments/CCSC%20letter%20on%20EOL%20care%208-19-09.pdf. [Sep 1;2010 ]. www.sccm.org/SiteCollectionDocuments/CCSC%20letter%20on%20EOL%20care%208-19-09.pdf
- 8.Angus DC. Barnato AE. Linde-Zwirble WT. Weissfeld LA. Watson RS. Rickert T. Rubenfeld GD Robert Wood Johnson Foundation ICU End-Of-Life Peer Group. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 9.Truog RD. Campbell ML. Curtis JR. Haas CE. Luce JM. Rubenfeld GD. Rushton CH. Kaufman DC American Academy of Critical Care Medicine. Recommendations for end-of-life care in the intensive care unit: A consensus statement by the American College of Critical Care Medicine. Crit Care Med. 2008;36:953–963. doi: 10.1097/CCM.0B013E3181659096. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics: National Vital Statistics Reports, Deaths: Final Data for 2006. www.cdc.gov/NCHS/data/nvsr/nvsr57/nvsr57_14.pdf. [Sep 1;2010 ]. www.cdc.gov/NCHS/data/nvsr/nvsr57/nvsr57_14.pdf
- 11.Williams MD. Braun LA. Cooper LM. Johnston J. Weiss RV. Qualy RL. Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: Analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8:R291–R298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taccone FS. Artigas AA. Sprung CL. Moreno R. Sakr Y. Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13:R15. doi: 10.1186/cc7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caruso P. Ferreira AC. Laurienzo CE. Titton LN. Terabe DS. Carnieli DS. Deheinzelin D. Short- and long-term survival of patients with metastatic solid cancer admitted to the intensive care unit: Prognostic factors. Eur J Cancer Care (Engl) 2010;19:260–266. doi: 10.1111/j.1365-2354.2008.01031.x. [DOI] [PubMed] [Google Scholar]
- 14.Ewer MS. Taubert JK. Advance directives in the intensive care unit of a tertiary cancer center. Cancer. 1995;76:1268–1274. doi: 10.1002/1097-0142(19951001)76:7<1268::aid-cncr2820760726>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Kish WS. Martin CG. Shaw AD. Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29:2294–2298. doi: 10.1097/00003246-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Massion PB. Dive AM. Doyen C. Bulpa P. Jamart J. Bosly A. Installé E. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit Care Med. 2002;30:2260–2270. doi: 10.1097/00003246-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Lecuyer L. Chevret S. Thiery G. Darmon M. Schlemmer B. Azoulay E. The ICU trial: A new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35:808–814. doi: 10.1097/01.CCM.0000256846.27192.7A. [DOI] [PubMed] [Google Scholar]
- 18.Goodman MD. Tarnoff M. Slotman GJ. Effect of advance directives on the management of elderly critically ill patients. Crit Care Med. 1998;26:701–704. doi: 10.1097/00003246-199804000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Esteban A. Gordo F. Solsona JF. Alía I. Caballero J. Bouza C. Alcalá-Zamora J. Cook DJ. Sanchez JM. Abizanda R. Miró G. Fernández Del Cabo MJ. de Miguel E. Santos JA. Balerdi B. Withdrawing and withholding life support in the intensive care unit: A Spanish prospective multi-centre observational study. Intensive Care Med. 2001;27:1744–1749. doi: 10.1007/s00134-001-1111-7. [DOI] [PubMed] [Google Scholar]
- 20.Kavic SM. Atweh N. Possenti PP. Ivy ME. The role of advance directives and family in end-of-life decisions in critical care units. Conn Med. 2003;67:531–534. [PubMed] [Google Scholar]
- 21.Camhi SL. Mercado AF. Morrison RS. Du Q. Platt DM. August GI. Nelson JE. Deciding in the dark: Advance directives and continuation of treatment in chronic critical illness. Crit Care Med. 2009;37:919–925. doi: 10.1097/CCM.0b013e31819613ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley CG. Lipson AR. Daly BJ. Douglas SL. Use of advance directives in the chronically critically ill. JONAS Healthc Law Ethics Regul. 2006;8:42–47. doi: 10.1097/00128488-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Morrell ED. Brown BP. Qi R. Drabiak K. Helft PR. The do-not-resuscitate order: Associations with advance directives, physician specialty and documentation of discussion 15 years after the Patient Self-Determination Act. J Med Ethics. 2008;34:642–647. doi: 10.1136/jme.2007.022517. [DOI] [PubMed] [Google Scholar]
- 24.Yang AD. Bentrem DJ. Pappas SG. Amundsen E. Ward JE. Ujiki MB. Angelos P. Advance directive use among patients undergoing high-risk operations. Am J Surg. 2004;188:98–101. doi: 10.1016/j.amjsurg.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 25.Sahm S. Will R. Hommel G. Attitudes towards and barriers to writing advance directives amongst cancer patients, healthy controls, and medical staff. J Med Ethics. 2005;31:437–440. doi: 10.1136/jme.2004.009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dow LA. Matsuyama RK. Ramakrishnan V. Kuhn L. Lamont EB. Lyckholm L. Smith TJ. Paradoxes in Advance Care Planning: The complex relationship of oncology patients, their physicians, and advance medical directives. J Clin Oncol. 2010;28:299–304. doi: 10.1200/JCO.2009.24.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Covinsky KE. Fuller JD. Yaffe K. Johnston CB. Hamel MB. Lynn J. Teno JM. Phillips RS. Communication and decision-making in seriously ill patients: Findings of the SUPPORT project. The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(5 Suppl):S187–S193. doi: 10.1111/j.1532-5415.2000.tb03131.x. [DOI] [PubMed] [Google Scholar]
- 28.Phelps AC. Maciejewski PK. Nilsson M. Balboni TA. Wright AA. Paulk ME. Trice E. Schrag D. Peteet JR. Block SD. Prigerson HG. Religious coping and use of intensive life-prolonging care near death in patients with advanced cancer. JAMA. 2009;301:1140–1147. doi: 10.1001/jama.2009.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.http://people-press.org/report/266/strong-public-support-for-right-to-die. [Sep 1;2010 ]. http://people-press.org/report/266/strong-public-support-for-right-to-die
- 30.Thorevska N. Tilluckdharry L. Tickoo S. Havasi A. Amoateng-Adjepong Y. Manthous CA. Patients' understanding of advance directives and cardiopulmonary resuscitation. J Crit Care. 2005;20:26–34. doi: 10.1016/j.jcrc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Loggers ET. Maciejewski PK. Paulk E. DeSanto-Madeya S. Nilsson M. Viswanath K. Wright AA. Balboni TA. Temel J. Stieglitz H. Block S. Prigerson HG. Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J Clin Oncol. 2009;27:5559–5564. doi: 10.1200/JCO.2009.22.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Will your advanced directive be followed? The Robert Powell Center for Medical Ethics. National Right to Life Committee; Washington, D.C.: 2007. [Sep 1;2010 ]. [Google Scholar]
- 33.Perkins HS. Controlling death: The false promise of advance directives. Ann Intern Med. 2007;147:51–57. doi: 10.7326/0003-4819-147-1-200707030-00008. [DOI] [PubMed] [Google Scholar]
- 34.Fagerlin A. Schneider CE. Enough. The failure of the living will. Hastings Cent Rep. 2004;34:30–42. [PubMed] [Google Scholar]
- 35.Nishimura A. Mueller PS. Evenson LK. Downer LL. Bowron CT. Thieke MP. Wrobleski DM. Crowley ME. Patients who complete advance directives and what they prefer. Mayo Clin Proc. 2007;82:1480–1486. doi: 10.1016/S0025-6196(11)61091-4. [DOI] [PubMed] [Google Scholar]
- 36.Thompson T. Barbour R. Schwartz L. Adherence to advance directives in critical care decision making: Vignette study. BMJ. 2003;327:1011. doi: 10.1136/bmj.327.7422.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teno JM. Stevens M. Spernak S. Lynn J. Role of written advance directives in decision making: Insights from qualitative and quantitative data. J Gen Intern Med. 1998;13:439–446. doi: 10.1046/j.1525-1497.1998.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cesta MA. Cardenas-Turanzas M. Wakefield C. Price KJ. Nates JL. Life-supportive therapy withdrawal and length of stay in a large oncologic intensive care unit at the end of life. J Palliat Med. 2009;12:713–718. doi: 10.1089/jpm.2009.0045. [DOI] [PubMed] [Google Scholar]
- 39.Zier LS. Burack JH. Micco G. Chipman AK. Frank JA. Luce JM. White DB. Doubt and belief in physicians' ability to prognosticate during critical illness: The perspective of surrogate decision makers. Crit Care Med. 2008;36:2341–2347. doi: 10.1097/CCM.0b013e318180ddf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upadya A. Muralidharan V. Thorevska N, et al. Patient, physician, and family member understanding of living wills. Am J Respir Crit Care Med. 2002;166(11):1430–1435. doi: 10.1164/rccm.200206-503OC. [DOI] [PubMed] [Google Scholar]
- 41.Levin TT. Li Y. Weiner JS. Amoateng-Adjepong Y. Manthous CA. How do-not-resuscitate orders are utilized in cancer patients: Timing relative to death and communication-training implications. Palliat Support Care. 2008;6:341–348. doi: 10.1017/S1478951508000540. [DOI] [PubMed] [Google Scholar]
- 42.Hill QA. Intensify, resuscitate or palliate: Decision making in the critically ill patient with haematological malignancy. Blood Rev. 2009;24:17–25. doi: 10.1016/j.blre.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Malcomson H. Bisbee S. Perspectives of healthy elders on advance care planning. J Am Acad Nurse Pract. 2009;21:18–23. doi: 10.1111/j.1745-7599.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 44.Buckey JW. Abell N. Life-sustaining treatment decisions: A social work response to meet needs of health care surrogates. J Soc Work End Life Palliat Care. 2010;6:27–50. doi: 10.1080/15524256.2010.489221. [DOI] [PubMed] [Google Scholar]
- 45.Baumrucker SJ. Durable power of attorney versus the advance directive: Who wins, who suffers? Am J Hosp Palliat Care. 2007;24:68–73. doi: 10.1177/1049909106297068. [DOI] [PubMed] [Google Scholar]
- 46.Handy CM. Sulmasy DP. Merkel CK. Ury WA. The surrogate's experience in authorizing a do not resuscitate order. Palliat Support Care. 2008;6:13–19. doi: 10.1017/S1478951508000035. [DOI] [PubMed] [Google Scholar]
- 47.Boyd EA. Lo B. Evans LR. Malvar G. Apatira L. Luce JM. White DB. "It's not just what the doctor tells me:" Factors that influence surrogate decision-makers' perceptions of prognosis. Crit Care Med. 2010;38:1270–1275. doi: 10.1097/CCM.0b013e3181d8a217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darmon M. Azoulay E. Critical care management of cancer patients: Cause for optimism and need for objectivity. Curr Opin Oncol. 2009;21:318–326. doi: 10.1097/CCO.0b013e32832b68b6. [DOI] [PubMed] [Google Scholar]
- 49.Tulsky JA. Beyond advance directives: importance of communication skills at the end of life. JAMA. 2005;294:359–365. doi: 10.1001/jama.294.3.359. [DOI] [PubMed] [Google Scholar]
- 50.Lo B. Steinbrook R. Resuscitating advance directives. Arch Intern Med. 2004;164:1501–1506. doi: 10.1001/archinte.164.14.1501. [DOI] [PubMed] [Google Scholar]
- 51.Detering KM. Hancock AD. Reade MC. Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]