Abstract
Background
Long and short sleep duration are associated with increased risk for coronary heart disease (CHD) and cardiovascular disease (CVD); however, evidence is inconsistent. We sought to identify whether self-reported sleep duration and insomnia, based on a validated questionnaire, are associated with increased incident CHD and CVD among postmenopausal women.
Methods
Women's Health Initiative Observational Study Participants (N=86,329; 50–79 years) who reported on sleep at baseline were followed for incident CVD events. Associations of sleep duration and insomnia with incident CHD and CVD were evaluated using Cox proportional hazards models over 10.3 years.
Results
Women with high insomnia scores had elevated risk of CHD (38%) and CVD (27%) after adjustment for age and race, and in fully adjusted models (hazard ratio [HR]=1.19, 95% confidence interval [CI] 1.09–1.30; 1.11 95% CI 1.03–2.00). Shorter (≤5 hours) and longer (≥10 hours) sleep duration demonstrated significantly higher incident CHD (25%) and CVD (19%) in age- and race-adjusted models, but this was not significant in fully adjusted models. Formal tests for interaction indicated significant interactions between sleep duration and insomnia for risk of CHD (p<0.01) and CVD (p=0.02). Women with high insomnia scores and long sleep demonstrated the greatest risk of incident CHD compared to midrange sleep duration (HR=1.93, 95% CI 1.06—3.51) in fully adjusted models.
Conclusions
Sleep duration and insomnia are associated with CHD and CVD risk, and may interact to cause almost double the risk of CHD and CVD. Additional research is needed to understand how sleep quality modifies the association between prolonged sleep and cardiovascular outcomes.
Introduction
Large-scale epidemiological studies indicate that short and long habitual sleep duration is associated with increased risk of all-cause mortality.1–13 More recently, evidence shows that sleep duration may be associated with coronary heart disease (CHD) and cardiovascular disease (CVD),13 and it has been proposed that these associations may work through mechanisms involving hypertension,6,14,15 diabetes,16–19 and obesity.20,21 Existing findings regarding the association between sleep duration and CHD are inconsistent, especially by age group and gender, as well as by selected sleep and outcome variables.9,22–24
Insomnia is an underdiagnosed condition, and it is estimated that approximately one in three individuals suffer from insomnia symptoms at some point throughout adulthood.25,26 Insomnia may be a risk factor for CHD, and a recent meta-analysis indicated that insomnia was associated with a 45% increased risk of morbidity and/or mortality from cardiovascular disease.27 Peri- and postmenopausal women often experience increased problems with sleep, including increased insomnia, poor sleep quality, and increased sleep disturbance.28–31 Surveys have estimated that the prevalence of sleep disturbance among perimenopausal women ranges from 33% to 51% and that a larger portion of individuals suffering from insomnia are women.30,31 According to a recent meta-analysis conducted among 29 studies, women demonstrated an increased risk of insomnia compared to men (relative risk [RR]=1.41, 95% CI 1.28–1.55).32 Few studies have addressed associations between insomnia and CHD particularly among postmenopausal women, a subgroup that not only has an increased prevalence of insomnia, but also carries the greatest population burden of CHD.33 Furthermore, previous epidemiologic studies on this topic have not used a validated scale to measure insomnia or sleep disturbance. To our knowledge, this study is the first to investigate interactions of sleep duration with insomnia in relation to increased risk for CHD and CVD in postmenopausal women.
Study objectives were to (1) determine whether long and short self-reported sleep duration (at baseline) are independently associated with increased incident CHD or CVD among women ages 50–79 years; (2) investigate whether insomnia is independently associated with increased incident CHD or CVD; and (3) determine if an interaction exists between sleep duration and insomnia in associations with CHD and CVD. We used the Women's Health Initiative Observational Study, a prospective cohort study conducted among postmenopausal women, as our database.
Materials and Methods
Study participants
The Women's Health Initiative (WHI) is a multicenter national study that includes 161,809 postmenopausal women. This study utilized longitudinal data from the WHI observational study (WHI-OS) cohort, a multiethnic population comprised of 93,676 women. Participants were recruited between 1993 and 1998 from 40 clinical sites across the United States and followed through August 2009. Initial contact was through a mailed brochure followed by a telephone screening to determine eligibility. Women were eligible if they were 50–79 years of age, postmenopausal, willing and able to provide informed consent, and agreed to reside in the area for at least 3 years after enrollment. The WHI-OS included those women who were screened for the clinical trial and were ineligible or unwilling to be enrolled, but were still interested in actively participating in a research study. Additional details regarding recruitment, inclusion/exclusion criteria, protocols, and study design are described elsewhere.34–37 Our primary analysis was restricted to participants without CHD at baseline (N=86,329). All participants provided informed consent approved by the Institutional Review Board of the participating study sites.
Outcome assessment
Diagnosis and adjudication of outcomes for this study have been outlined in established protocols previously described.38,39 Outcomes were initially identified through annual follow-up contacts and then verified through medical records and death certificates. Our primary endpoint was incident CHD, defined as incident myocardial infarction (MI), CHD death, hospitalized angina, or coronary revascularization, which included coronary artery bypass graft (CABG) or percutaneous transluminal coronary angioplasty (PTCA). Participants were followed for first occurrence of a CHD outcome, and those individuals who did not develop CHD were censored at the date of death or last contact. This analysis also assessed outcomes for incident CVD, defined as total incident CHD or ischemic stroke. All analyses were conducted on a disease-free cohort, and thus baseline inclusion criteria (and total Ns for the two analyses) varied slightly.
Exposure assessment
Sleep duration
Sleep characteristics were evaluated using self-reported measures from the baseline screening visit. Sleep duration was measured in response to the question “About how many hours of sleep did you get on a typical night during the past 4 weeks?” Respondents chose from six categories, ≤5, 6, 7, 8, 9, or ≥10 hours. The variable was analyzed as a 5-level categorical variable, which used a reference category of 7–8 hours based on existing empirical evidence supporting this range as the ideal sleep duration for optimal health.9,13,22–24
Insomnia
The secondary exposure measure was assessed using the validated WHI Insomnia Rating Scale (WHIIRS), a 5-item instrument that measures perceived insomnia symptoms.40,41 Participants were asked, “During the last 4 weeks, how often have you been bothered by any of the following problems? (1) Trouble falling asleep, (2) Waking up several times per night, (3) Waking up earlier than you planned to,’ (4) Trouble getting back to sleep after you woke up too early,’ and (5) Overall, was your typical night's sleep during the past 4 weeks…” Responses were scored from 0–4. Question 1–4 responses included frequency options ranging from “No, not in the past 4 weeks” to “Yes, 5 or more times a week.” Response options to question 5 included “very sound or restful,” “sound or restful,” “average quality,” “restless,” and “very restless.” This measurement tool has high reliability, internal consistency, and good construct validity (Spearman R=0.96 for same-day administration; Cronbach α=0.78).40,42 The analysis was conducted using approximate quartiles of the WHIIRS. A score of 9 or greater has been shown to indicate high risk of insomnia and need for further clinical evaluation.42
Covariates
Demographics
All baseline demographic characteristics, including age, race/ethnicity, education, income, and marital status, were measured at baseline with a questionnaire. Race/ethnicity was categorized as White/Caucasian, Black/African American, Hispanic/Latina, Asian and Other. Education was classified as less than high school, high school graduate, some college, and college graduate. Annual household income was categorized into three groups: <$20,000, $20,000–<$50,000, and ≥$50,000. Marital status was dichotomized as married or marriage-like relationship, and widowed, divorced, separated or single.
Risk factors
Lifestyle risk factors, including smoking, alcohol consumption, and physical activity, were also measured through self-report at baseline. Smoking was operationalized as current, former, and nonsmoker. Alcohol consumption was measured as servings per week (6 oz of wine, 12 oz of beer, and 1.5 oz of liquor), and physical activity was measured as metabolic equivalent task [MET]-hours per week, based on nine questions related to expenditure of energy from recreational activity.43 Body Mass Index (BMI), systolic blood pressure, diastolic blood pressure, and all other physical measurements were assessed at the baseline visit (see Supplementary Methods). WHI participants were asked to bring all medications taken in the last two weeks to their baseline screening interview, and data was entered by trained clinical interviewers. Diabetes was defined by whether participants reported physician-diagnosed diabetes and were taking diabetes medications. Hyperlipidemia was defined by whether individuals reported that they were on lipid-lowering medications and had a physician diagnosis of high cholesterol. Use of hypnotics was also considered in the analysis utilizing reports from the medications database. The Charlson Comorbidity Index was used to assess comorbid conditions,44 and baseline depression was assessed using the Burnam screening algorithm, which consists of six items from the 20-item Center for the Epidemiological Studies of Depression (CES-D) and two items from the National Institute of Mental Health's Diagnostic Interview Schedule (DIS) (see Supplementary Methods).45 The Burnam algorithm uses these eight questions in a logistic regression model to generate a score between 0 and 1. Current depressive symptomatology is indicated by a Burnam cut point of 0.06. This instrument has shown good sensitivity (74%) and specificity (87%) for detecting depressive disorder (major depressive disorder and dysthymia) in both primary care and mental health settings among postmenopausal women.45
Statistical analysis
Survival analysis was conducted with sleep duration and insomnia as the primary exposure in Cox proportional hazards models of incident CHD and CVD. Person-years of follow-up for each participant were based on time from enrollment to either coronary event, loss to follow-up, death, or end of the study (August 2009). Crude event rates were calculated and compared across sleep duration categories. All models were assessed for the proportional hazards assumption.
Covariates that were considered from baseline data included age, race/ethnicity, education, income, marital status, BMI, waist-to-hip ratio, hypertension, diabetes, comorbidities, smoking, alcohol, physical activity, depression, use of hypnotics, hyperlipidemia, and hormone medication use. Due to the large numbers of variables in the model, we assessed multicollinearity by conducting Pearson's correlation coefficients to evaluate the presence of highly correlated variables (i.e.,>0.75). Potential confounders were identified based on previous findings from the current literature, and final parsimonious models included those covariates that are statistically and theoretically appropriate: age, race/ethnicity, education, income, smoking, alcohol, physical activity, depression, BMI, hypertension, diabetes, hyperlipidemia, and comorbidities.
Two-way interactions for sleep duration and age, insomnia and age, and insomnia and sleep duration were evaluated in the models, and stratified analyses were also conducted. There were statistical concerns around the “snoring” variable since more than 50% reported “don't know.” For this reason, we excluded this variable from the analysis and conducted a separate analysis to address this issue.46 All of the statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Table 1 presents the distribution of sleep duration among the 86,329 participants by demographic characteristics, risk factors, and sleep characteristics at baseline. Approximately 7.9% of participants (N=6,820) reported sleeping ≤5 hours per night (“short sleepers”), and 0.6% (N=472) reported sleeping ≥10 hours per night (“long sleepers”). Distribution of sleep time differed significantly by race group and socioeconomic status (SES). Participants with long and short sleep duration were more likely to be Black/African American and Hispanic/Latinos compared to midrange sleepers. Long and short sleepers tended to have a higher proportion in the lowest education and income category compared to midrange sleepers. Lifestyle and biological risk factors were significantly different by reported sleep duration. A U-shaped association was observed across sleep duration for biological risk factors, and short and long sleepers had higher prevalence of diabetes, elevated systolic and diastolic blood pressure, and depression. (Table 1)
Table 1.
Distribution of Baseline Demographics, Risk Factors, and Sleep Characteristics in the WHI Study Population (N=86,329) by Sleep Duration
| |
Sleep time hours/day |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | ≤5 h | 6 h | 7 h | 8 h | 9 h | ≥10 h | P-valuea | |
| N | 86,329 | 6820 | 23113 | 32643 | 19816 | 3465 | 472 | |
| Age (years) | 86,329 | 63.4 (7.7) | 63.3 (7.5) | 63.1 (7.3) | 63.7 (7.1) | 64.1 (7.2) | 63.2 (7.4) | <0.01 |
| Race (%) | 86,089 | |||||||
| White | 72,318 | 68.0 | 79.5 | 87.1 | 89.3 | 88.9 | 66.1 | <0.01 |
| Black/African American | 6657 | 17.7 | 10.3 | 5.7 | 4.9 | 5.9 | 16.7 | |
| Hispanic/Latino | 3259 | 6.2 | 4.2 | 3.3 | 3.1 | 3.0 | 15.0 | |
| Asian | 2528 | 5.5 | 4.3 | 2.6 | 1.5 | 1.1 | 1.3 | |
| Other | 1327 | 2.6 | 1.8 | 1.4 | 1.3 | 1.2 | 0.9 | |
| Education (%) | 85,644 | |||||||
| Less than high school graduate | 4112 | 9.0 | 5.2 | 3.8 | 4.3 | 4.9 | 18.3 | <0.01 |
| High school graduate | 13,708 | 19.8 | 16.7 | 15.0 | 15.4 | 16.4 | 15.1 | |
| Some college | 30,927 | 40.1 | 37.3 | 35.0 | 35.3 | 35.7 | 33.6 | |
| College graduate | 36,897 | 31.2 | 40.8 | 46.2 | 44.9 | 43.0 | 33.1 | |
| Income (% annual household) | 80,031 | |||||||
| <$20,000 | 11,922 | 24.8 | 16.6 | 12.5 | 13.5 | 15.1 | 35.2 | <0.01 |
| $20,000–$49,999 | 34,587 | 44.0 | 44.3 | 42.6 | 42.6 | 44.8 | 36.1 | |
| ≥$50,000 | 33,452 | 31.3 | 39.1 | 45.0 | 43.9 | 40.1 | 28.7 | |
| Marital status (%) | 85,915 | |||||||
| Married or marriage-like | 53,929 | 52.5 | 58.2 | 64.7 | 68.2 | 65.8 | 53.0 | <0.01 |
| Single/divorced/widowed | 31,986 | 47.5 | 41.8 | 35.3 | 31.8 | 34.2 | 47.0 | |
| Body Mass Index (kg/m2) | 85,316 | 28.3 (6.5) | 27.4 (5.9) | 26.7 (5.6) | 27.0 (5.6) | 27.3 (5.8) | 29.0 (6.5) | <0.01 |
| Waist-to-hip ratio | 85,850 | 0.82 (.08) | 0.81 (.08) | 0.80 (.08) | 0.80 (.08) | 0.81 (.08) | 0.83 (.08) | <0.01 |
| Systolic blood pressure (mm Hg) | 86,217 | 128.2 (18.1) | 126.7 (17.9) | 125.9 (17.8) | 126.7 (17.9) | 127.6 (17.8) | 130.5 (19.3) | 0.05 |
| Diastolic blood pressure (mm Hg) | 86,202 | 75.4 (9.6) | 74.9 (9.4) | 74.6 (9.2) | 74.9 (9.2) | 75.0 (9.1) | 76.2 (9.5) | 0.03 |
| Hyperlipidemia (%) | 84,507 | |||||||
| Yes | 7,920 | 10.1 | 9.7 | 9.0 | 9.1 | 10.4 | 11.6 | <0.01 |
| No | 76,587 | 89.9 | 90.3 | 91.0 | 91.0 | 89.6 | 88.5 | |
| Diabetes (%) | 86,225 | |||||||
| Yes | 2,942 | 5.7 | 3.5 | 2.8 | 3.0 | 3.5 | 4.7 | <0.01 |
| No | 83,283 | 94.3 | 96.5 | 97.2 | 97.0 | 96.5 | 95.3 | |
| Depression (% Short CES-D/DIS) | 84,376 | |||||||
| Yes | 9,219 | 25.0 | 13.6 | 8.1 | 7.3 | 11.2 | 25.9 | <0.01 |
| No | 75,157 | 75.1 | 86.4 | 91.9 | 92.7 | 88.8 | 74.1 | |
| Smoking (%) | 85,244 | |||||||
| Never smoker | 43,714 | 53.8 | 51.1 | 51.6 | 50.8 | 47.6 | 45.9 | <0.01 |
| Past smoker | 36,252 | 38.6 | 42.2 | 42.6 | 43.6 | 45.3 | 45.1 | |
| Current smoker | 5278 | 7.7 | 6.7 | 5.8 | 5.6 | 7.1 | 9.0 | |
| Alcohol intake (servings/wk) | 86,137 | 1.7 (4.3) | 2.3 (4.8) | 2.6 (5.2) | 3.0 (5.7) | 3.4 (6.1) | 2.7 (6.6) | <0.01 |
| Physical activity (MET-hrs/wk) | 85,533 | 12.2 (14.7) | 13.6 (14.6) | 14.3 (14.3) | 14.3 (14.4) | 13.3 (14.5) | 11.3 (14.0) | <0.01 |
| Reported snoring (%) | 86,003 | |||||||
| Frequent 5 or>days/week | 8,613 | 11.6 | 10.1 | 9.1 | 10.3 | 11.7 | 19.8 | <0.01 |
| Moderate 1 - 5 days/week | 13,723 | 13.3 | 15.1 | 16.3 | 17.2 | 17.1 | 15.5 | |
| No snoring | 19,871 | 21.2 | 21.6 | 24.0 | 24.1 | 23.6 | 18.9 | |
| Don't know | 43,796 | 53.9 | 53.2 | 50.7 | 48.4 | 47.6 | 45.9 | |
| WHIIRSb | 84,879 | 11.2 (5.5) | 7.8 (4.6) | 5.9 (3.8) | 5.0 (3.5) | 4.9 (3.5) | 6.0 (4.4) | <0.01 |
| Charlson comorbidity index (%>2) | 86,329 | |||||||
| >2 comorbid conditions | 4,607 | 7.5 | 5.7 | 4.7 | 5.0 | 6.3 | 8.1 | <0.01 |
| ≤2 comorbid conditions | 81,722 | 92.5 | 94.3 | 95.3 | 95.0 | 93.7 | 92.0 | |
P-values represent chi-square test for categorical variables and ANOVA for means.
WHIIRS is a measure of perceived insomnia symptoms (scored 0–20); higher score indicates more severe insomnia.
H, hours; Hg, Mercury; Kg, kilograms; M, meters; Mm, millimeters; WHI, Women's Health Initiative; WHIIRS, Women's Health Initiative Insomnia Rating Scale; Wk, week.
A total of 5,359 cases of incident CHD and 7,257 cases of incident CVD were observed over 881,888 and 867,513 person-years of follow-up, respectively (Table 2). U-shaped associations were observed between sleep duration and CHD and CVD (Table 2). Multivariate-adjusted Cox proportional hazards models assessed associations of sleep duration and insomnia with incident CHD and CVD (Tables 2 and 3). Compared to midrange sleep duration (7–8 hours), individuals with shorter (≤5 hours) and longer (≥10 hours) sleep demonstrated higher incident CHD after adjusting for age and race (HR=1.25, 95% CI 1.13–1.37; HR=1.43, 95% CI 1.03–1.99). This association was attenuated and no longer statistically significant in models adjusted for age, race, education, income, smoking, BMI, physical activity, alcohol, depression, diabetes, hypertension, hyperlipidemia, and comorbidities (HR=1.08, 95% CI 0.96–1.20; HR=1.33, 95% CI 0.94–1.88). Short and long sleep was significantly associated with CVD after adjusting for age and race (HR=1.19, 95% CI 1.10–1.30; HR=1.37, 95% CI 1.02–1.84). This association also was attenuated and no longer significant after further adjusting for the remaining aforementioned covariates (HR=1.06, 95% CI 0.96–1.16; HR=1.23, 95% CI 0.89–1.70).
Table 2.
Cox Proportional Hazards Models—Sleep Duration and Incident CHD and CVD Among WHI Participants
| |
|
|
|
|
Model 1a |
Model 2b |
||
|---|---|---|---|---|---|---|---|---|
| Cases | Person-yrs | Nc | HR | 95% CI | HR | 95% CI | ||
| CHD | Sleep time | |||||||
| ≤5 h | 479 | 64,942 | 6,820 | 1.25 | 1.13, 1.37 | 1.08 | 0.96, 1.20 | |
| 6 h | 1457 | 232,689 | 23,113 | 1.07 | 1.00, 1.14 | 1.00 | 0.94, 1.07 | |
| 7–8 h (ref) | 3166 | 544,730 | 52,459 | REF | REF | REF | REF | |
| 9 h | 221 | 35,208 | 3,465 | 1.01 | 0.88, 1.16 | 0.93 | 0.80, 1.08 | |
| ≥10 h | 36 | 4319 | 472 | 1.43 | 1.03, 1.99 | 1.33 | 0.94, 1.88 | |
| CVD | ≤5 h | 623 | 63,509 | 6,692 | 1.19 | 1.10, 1.30 | 1.06 | 0.96, 1.16 |
| 6 h | 1963 | 228,879 | 22,833 | 1.05 | 1.00, 1.11 | 1.00 | 0.95, 1.06 | |
| 7–8 h (ref) | 4316 | 536,556 | 51,911 | REF | REF | REF | REF | |
| 9 h | 310 | 34,447 | 3,414 | 1.04 | 0.93, 1.17 | 0.95 | 0.83, 1.08 | |
| ≥10 h | 45 | 4,122 | 454 | 1.37 | 1.02, 1.84 | 1.23 | 0.89, 1.70 | |
Model 1 adjusted for age and race.
Model 2 adjusted for age, race, education, income, smoking, BMI, physical activity, alcohol intake, depression, diabetes, high blood pressure, hyperlipidemia, comorbid conditions.
Total Ns may vary because analysis was conducted among a disease-free cohort.
CHD, Coronary Heart Disease: includes MI, CHD death, PTCA, CABG, or hospitalized angina; CI, Confidence Interval; CVD, Cardiovascular Disease: includes MI, CHD death, PTCA, CABG, hospitalized angina, or ischemic stroke; H, Hours; HR, Hazard ratio; Yr, years; WHI, Women's Health Initiative.
Table 3.
Cox Proportional Hazards Models—Insomnia and Risk of CHD and CVD Among WHI Participants
| |
|
|
|
|
Modela |
Modelb |
||
|---|---|---|---|---|---|---|---|---|
| Cases | Person-yr | N | HR | 95% CI | HR | 95% CI | ||
| CHD* | Insomnia§ | |||||||
| WHIIRS<3 (REF) | 812 | 172,539 | 16,661 | REF | REF | REF | REF | |
| 3 ≤WHIIRS<6 | 1,366 | 234,583 | 22,695 | 1.18 | 1.08, 1.29 | 1.11 | 1.01, 1.22 | |
| 6 ≤WHIIRS<9 | 1,244 | 202,468 | 19,759 | 1.21 | 1.11, 1.32 | 1.09 | 0.99, 1.20 | |
| WHIIRS ≥9 | 1,826 | 259,364 | 25,822 | 1.38 | 1.27, 1.49 | 1.19 | 1.08, 1.30 | |
| CVD‡ | WHIIRS<3 (REF) | 1,145 | 170,325 | 16,517 | REF | REF | REF | REF |
| 3 ≤WHIIRS<6 | 1,854 | 230,806 | 22,444 | 1.13 | 1.05, 1.22 | 1.09 | 1.00, 1.18 | |
| 6 ≤WHIIRS<9 | 1,721 | 199,034 | 19,529 | 1.18 | 1.09, 1.27 | 1.07 | 0.99, 1.16 | |
| WHIIRS≥9 | 2,400 | 254,780 | 25,463 | 1.27 | 1.19, 1.37 | 1.11 | 1.03, 2.00 | |
Model 1 adjusted for age and race.
Model 2 adjusted for age, race, education, income, smoking, BMI, physical activity, alcohol intake, depression, diabetes, high blood pressure, hyperlipidemia, comorbid conditions.
Based on WHIIRS.
CHD, Coronary Heart Disease: includes MI, CHD death, PTCA, CABG, or hospitalized angina; CI, Confidence Interval; CVD, Cardiovascular Disease: includes MI, CHD death, PTCA, CABG, hospitalized angina, or ischemic stroke; H, Hours; HR, Hazard ratio; Yr, years; WHI, Women's Health Initiative.
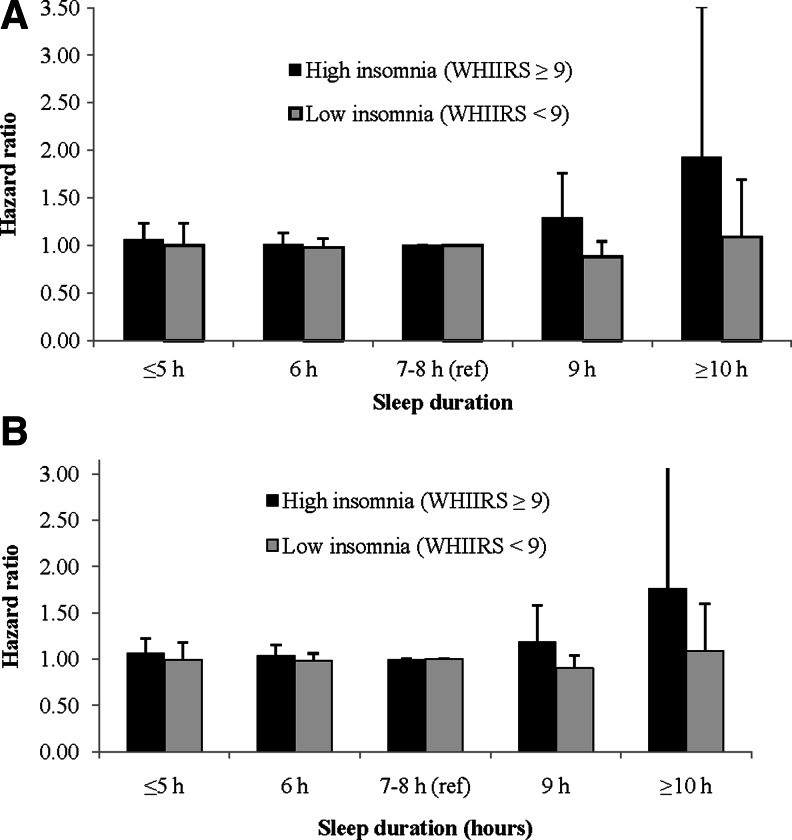
Women who had high insomnia scores (WHIIRS ≥9) demonstrated the greatest risk of CHD after adjusting for age and race (HR=1.38, 95% CI 1.27–1.49) and in models adjusted for confounders and mediators (HR=1.19, 95% CI 1.08–1.30) compared to the low insomnia women (Table 3). This association was significant for CVD in models adjusting for age and race (HR=1.27, 95% CI 1.19–1.37), and in fully adjusted models (HR=1.11, 95% CI 1.03–2.00). An interaction was observed between insomnia and sleep duration, which were modeled as categorical variables, for risk of CHD (p<0.01) and CVD (p=0.02). In an analysis stratified by insomnia level, among women with a high prevalence of insomnia (WHIIRS ≥9), long sleep duration (≥10 hours) demonstrated almost double the risk of incident CHD (HR=1.93, 95% CI 1.06–3.51) and increased risk of CVD (HR=1.76, 95% CI 0.99–3.12) compared to midrange sleep (7–8 hours) (Figure 1). This association did not persist among women with low levels of insomnia (WHIIRS< 9) in fully adjusted models.
FIG. 1.
(A) Sleep Duration and Incident CHD Stratified by Insomnia Levela. (B) Sleep Duration and Incident CVD Stratified by Insomnia Levela.
aModel adjusted for age, race, education, income, smoking, BMI, physical activity, alcohol intake, depression, diabetes, high blood pressure, cholesterol medication, comorbid conditions.
CHD, Coronary heart disease; Ref, reference; WHIIRS, Women's Health Initiative Insomnia Rating Scale; CVD, Cardiovascular disease
Discussion
This prospective cohort study provides additional evidence that short and long sleep duration are associated with modest increased risk of incident CHD and CVD in models adjusted for age and race, but not in fully adjusted models over an average of 10.3 years of follow-up (Table 2). Insomnia was significantly associated with CHD and CVD in models adjusted for age and race, which persisted in fully adjusted models. This study demonstrated a significant and strong association between long habitual sleep duration and incident CHD among postmenopausal women who report high levels of insomnia. When addressing these sleep characteristics in concert (WHIIRS ≥9 and sleep duration ≥10 hours), participants demonstrated almost double the risk for CHD (HR=1.93, 95% CI 1.06–3.51), suggesting characteristics of sleep quality may be an important factor contributing to the observed association between sleep duration and incident CHD and CVD reported in previous studies.
Our analysis extends those of previously reported findings on sleep duration, but also suggests that the impact of sleep duration on cardiac events may be due to comorbid conditions. A study conducted among 71,617 middle-aged women in the Nurses' Health Study showed increased risk for any coronary event was associated with both long (≥9 hours) and short (≤5 hours) sleep duration (RR=1.39, 95% CI 1.05–1.84; RR=1.37, 95% CI 1.02–1.85).9 Ayas and colleagues did not address insomnia in the analysis, and therefore whether insomnia was an effect modifier for the relationship between sleep duration and CHD in their study is unknown. A meta-analysis and systematic review among 15 cohort studies reported similar estimates, specifically reporting a U-shaped association between sleep duration and CHD and CVD outcomes.13 Findings indicated that both short and long sleep duration were significantly associated with an increased risk of CHD among women (RR=1.60, 95% CI 1.24–2.06; RR=1.43, 95% CI 1.09–1.89), respectively. Long sleep duration was a significant predictor of CVD (1.44, 95% CI 1.23–1.68), but the association for short sleep duration was not significant for the CVD outcome (RR=1.06, 95% CI 0.91–1.24). This meta-analysis did not report on how measures of sleep quality might modify the observed associations.
With regard to sleep disturbance and insomnia and CHD, our study aligns with previous reports conducted among middle-aged adults. Findings from the Whitehall II Study (N=10,264) recently reported an increased risk of CHD among men and women with “restless, disturbed nights” of sleep.47 Reported findings on the association between high levels of sleep disturbance and risk of CHD among women were slightly stronger than our adjusted estimates (RR=1.36, 95% CI 1.10–1.68).47 Contrary to our study, Vgontzas and colleagues (2010)48 investigated the joint effect of insomnia and short sleep duration among middle-aged adults in the Penn State Cohort. Their findings indicated that short sleep and insomnia was a significant predictor of mortality among men (OR=4.00 95% CI 1.14–13.99); however, this association was not significant among women in their study (OR=0.36 95% CI 0.03–4.33).
Results from a prospective community-based cohort of middle-aged Chinese adults (N=3430) demonstrated that frequent insomnia is associated with an increased risk of cardiovascular events (RR=1.78; 95% CI 1.03–3.08), as well as higher risk of all-cause mortality (RR=1.70; 95% CI 1.16–2.49) in fully adjusted models.24 A similar U-shaped association between sleep duration and cardiovascular events was also demonstrated in this study, and neither longer or shorter sleep duration was significant after adjusting for mediators and confounders. Chien and colleagues24 did address the joint analysis of insomnia and sleep duration, and their findings indicated an increased risk of CVD among those with frequent insomnia and long sleep duration (≥9 hours) compared to those sleeping 7–8 hours (HR=2.07, 95% CI 1.11-3.85). The estimate of effect in the WHI cohort was consistent with Chien and colleagues' study among middle-aged Chinese men and women. Our findings provide additional evidence that the joint effect between long sleep duration and insomnia, using a validated measure, results in almost a twofold increase in CHD, and suggest that this increased risk is generalizable to postmenopausal women.
Lastly, it has been previously demonstrated that use of hypnotics is significantly associated with mortality among postmenopausal women.49 Although we addressed the use of hypnotics as an important covariate in our analysis, it was not found to be a significant predictor of CHD or CVD in our final models. It is plausible, however, that nondifferential misclassification could have biased these results toward the null. Future investigations should therefore consider the impact of medication such as hypnotics on CVD outcomes.
A number of pathways, including SES, poor physical health, depression, and other underlying biological mechanisms, must be considered when interpreting our findings. Low SES is associated with a higher prevalence of obesity, mental illness, and heart disease.50–52 Sleep disturbances are also more prevalent among low SES individuals, and sleep quality and quantity may serve as a mediator between social and neighborhood characteristics and health outcomes.53–56 Although our analysis adjusted for education and income level, these measures do not completely account for the SES construct. Additional understanding of the biological underpinnings associated with socioeconomic pathways is needed.
Long sleep duration also may be an indicator of poor mental and physical health status. Previous large prospective studies have indicated that longer sleep duration is associated with all-cause mortality; however, findings from Gangwisch and colleagues indicated that these results were only significant among the elderly and not among middle-aged adults.7,12,57 Although a number of studies have investigated underlying mechanisms regarding short sleep duration and CVD,14,58–62 strong empirical evidence related to biological mechanisms involving long sleep duration is limited, and proposed mechanisms include underlying comorbid disease, depression, and poor sleep quality.53,63 Our study adjusted for a number of comorbid conditions in our fully adjusted models, which attenuated the estimate of effect suggesting underlying disease may account for some of the observed association. With regard to depression, the literature suggests that clinical depression is a predictor of cardiovascular disease among women64 and is associated with sleep disturbances.65 In this study, among women with a high insomnia level (WHIIRS ≥9), 20.8% were depressed compared with only 6.6% of those with a WHIIRS<9 (p<0.001), and it is likely that these differences in depression status may impact sleep characteristics. Although we adjusted for depression using the [CESD/DIS] measure, the Burnam screen is not a perfect measure of clinically diagnosed depression. Further investigation on sleep, depressive symptoms, and CVD among postmenopausal women is warranted.
To our knowledge, this study is the largest assessment of sleep duration and incident CHD and CVD outcomes among postmenopausal women to date. Another strength is that the CHD and CVD outcomes were assessed prospectively, which eliminates bias inherent in retrospective and cross-sectional analyses. In addition, all outcomes were adjudicated and verified through medical records and death certificates.
Nonetheless, this study has several limitations. First, since our study was conducted among postmenopausal women, we are not able to generalize these findings to men or younger women. The WHI study did not conduct assessments for obstructive sleep apnea or restless leg syndrome; because we could not adjust for these sleep disorders, it is possible that they have confounded the observed association.66–68 Also, self-reported sleep habits assessed by questionnaire are subject to some measurement error. Our study did not measure total time asleep, distinguish the time in bed from total sleep duration, or use other validated measures of sleep duration such as actigraphy. However, previous studies have reported a modest correlation between self-reported and objectively measured sleep.69 Furthermore, the WHIIRS has been previously validated against objective measures.40–42 Since our study measured the outcome prospectively, our subjective measure of sleep duration would likely lead to nondifferential misclassification with respect to the outcome, which would likely bias our estimates of effect toward the null.
Conclusion
A U-shaped association was observed for sleep duration and incident CHD and CVD, suggesting a modest increased risk for long and short sleepers. Women with high insomnia levels also demonstrated a modest increased risk for CHD and CVD. A significant interaction between sleep duration and insomnia was observed, and individuals with long sleep duration and a high level of insomnia demonstrated almost a twofold increased risk for CHD. Additional work is needed to understand how sleep quality modifies the association between prolonged sleep duration and CHD, as well as to investigate potential biological mechanisms underlying this association.
Funding Support
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services, through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Supplementary Material
Acknowledgments
Megan Sands-Lincoln had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We are grateful for the contributions of the WHI study investigators and participants.
WHI Investigators—Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. American Journal of Public Health and the Nation's Health. 1964;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kripke DF. Simons RN. Garfinkel L. Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Archives of General Psychiatry. 1979;36(1):103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 3.Wingard DL. Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6(2):102–107. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- 4.Pollak CP. Perlick D. Linsner JP. Wenston J. Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. Journal of Community Health. 1990;15(2):123–135. doi: 10.1007/BF01321316. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M. Wakai K. Kawamura T, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. Journal of Epidemiology/Japan Epidemiological Association. 2000;10(2):87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ. Redline S. Nieto FJ, et al. Association of usual sleep duration with hypertension: The Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 7.Kripke DF. Garfinkel L. Wingard DL. Klauber MR. Marler MR. Mortality associated with sleep duration and insomnia. Archives of General Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Heslop P. Smith GD. Metcalfe C. Macleod J. Hart C. Sleep duration and mortality: The effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Medicine. 2002;3(4):305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 9.Ayas NT. White DP. Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Archives of Internal Medicine. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 10.Tamakoshi A. Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27(1):51–54. [PubMed] [Google Scholar]
- 11.Amagai Y. Ishikawa S. Gotoh T, et al. Sleep duration and mortality in Japan: The Jichi Medical School Cohort Study. Journal of Epidemiology/Japan Epidemiological Association. 2004;14(4):124–128. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SR. Ayas NT. Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 13.Cappuccio FP. D'Elia L. Strazzullo P. Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangwisch JE. Heymsfield SB. Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: Analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 15.Cappuccio FP. Stranges S. Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: The Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaggi HK. Araujo AB. McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 17.Mallon L. Broman JE. Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: A 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28(11):2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 18.Ayas NT. White DP. Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 19.Gangwisch JE. Heymsfield SB. Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutson KL. Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Annals of the New York Academy of Sciences. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel SR. Blackwell T. Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32(12):1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qureshi AI. Giles WH. Croft JB. Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: A 10-year follow-up from NHANES I. Neurology. 1997;48(4):904–911. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 23.Meisinger C. Heier M. Lowel H. Schneider A. Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: The MONICA/KORA Augsburg cohort study. Sleep. 2007;30(9):1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chien KL. Chen PC. Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: Report from a community-based cohort. Sleep. 2010;33(2):177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth T. Insomnia: Definition, prevalence, etiology, and consequences. Journal of Clinical Sleep Medicine: Official Publication of the American Academy of Sleep Medicine. 2007;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- 26.Ohayon MM. Carskadon MA. Guilleminault C. Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 27.Sofi F. Cesari F. Casini A. Macchi C. Abbate R. Gensini GF. Insomnia and risk of cardiovascular disease: A meta-analysis. European Journal of Preventive Cardiology. 2012 Aug 31; doi: 10.1177/2047487312460020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Joffe H. Massler A. Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Seminars in Reproductive Medicine. 2010;28(5):404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson EO. Roth T. Schultz L. Breslau N. Epidemiology of DSM-IV insomnia in adolescence: Lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–256. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- 30.Shaver JL. Zenk SN. Sleep disturbance in menopause. Journal of Women's Health and Gender-based Medicine. 2000;9(2):109–118. doi: 10.1089/152460900318605. [DOI] [PubMed] [Google Scholar]
- 31.NIH State-of-the-Science Conference Statement on management of menopause-related symptoms. NIH Consensus and State-of-the-Science Statements. 2005;22(1):1–38. [PubMed] [Google Scholar]
- 32.Zhang B. Wing YK. Sex differences in insomnia: A meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 33.Mosca L. Benjamin EJ. Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the American Heart Association. Circulation. 2011;123(11):1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hays J. Hunt JR. Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 13(9 Suppl):S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 35.Langer RD. White E. Lewis CE. Kotchen JM. Hendrix SL. Trevisan M. The Women's Health Initiative Observational Study: Baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 36.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled Clinical Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 37.Rossouw JE. Hurd S. The Women's Health Initiative: Recruitment complete—looking back and looking forward. J Women's Health. 1999;8(1):3–5. doi: 10.1089/jwh.1999.8.3. [DOI] [PubMed] [Google Scholar]
- 38.Curb JD. McTiernan A. Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 39.Wassertheil-Smoller S. Hendrix SL. Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women's Health Initiative: A randomized trial. Journal of the American Medical Association. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 40.Levine DW. Dailey ME. Rockhill B. Tipping D. Naughton MJ. Shumaker SA. Validation of the Women's Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosomatic Medicine. 2005;67(1):98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 41.Levine DW. Kaplan RM. Kripke DF. Bowen DJ. Naughton MJ. Shumaker SA. Factor structure and measurement invariance of the Women's Health Initiative Insomnia Rating Scale. Psychological Assessment. 2003;15(2):123–136. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 42.Levine DW. Kripke DF. Kaplan RM, et al. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale. Psychological Assessment. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 43.Ainsworth BE. Haskell WL. Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Medicine and Science in Sports and Exercise. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 44.Charlson M. Szatrowski TP. Peterson J. Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 45.Tuunainen A. Langer RD. Klauber MR. Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103(2–3):261–270. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 46.Sands M LE. Lu B. Carskadon MA. Sharkey K. Stefanick M. Ockene J. Shah N. Hairston KG. Robinson J. Limacher M. Hale L. Eaton CB. Self-reported snoring and risk of cardiovascular disease among postmenopausal women (from the Women's Health Initiative) American Journal of Cardiology. 2013;111(4):540–6. doi: 10.1016/j.amjcard.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandola T. Ferrie JE. Perski A. Akbaraly T. Marmot MG. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: A prospective study from the Whitehall II cohort. Sleep. 2010;33(6):739–744. doi: 10.1093/sleep/33.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vgontzas AN. Liao D. Pejovic S. Calhaun S. Karataraki M. Busta M. Fernandez-Mendoza J. Bixler E. Insomnia with short sleep duration and mortality: The Penn State Cohort. Sleep. 2010;33(9):1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartz A. Ross JJ. Cohort study of the association of hypnotic use with mortality in postmenopausal women. BMJ Open. 2012;2(5) doi: 10.1136/bmjopen-2012-001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLaren L. Socioeconomic status and obesity. Epidemiologic Reviews. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 51.Kessler RC. Cleary PD. Social class and psychological distress. American Sociological Review. 1980;45(3):463–478. [PubMed] [Google Scholar]
- 52.Clark AM. DesMeules M. Luo W. Duncan AS. Wielgosz A. Socioeconomic status and cardiovascular disease: Risks and implications for care. Nature Reviews. Cardiology. 2009;6(11):712–722. doi: 10.1038/nrcardio.2009.163. [DOI] [PubMed] [Google Scholar]
- 53.Moore PJ. Adler NE. Williams DR. Jackson JS. Socioeconomic status and health: The role of sleep. Psychosomatic Medicine. 2002;64(2):337–344. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 54.Van Cauter E. Spiegel K. Sleep as a mediator of the relationship between socioeconomic status and health: A hypothesis. Annals of the New York Academy of Sciences. 1999;896:254–261. doi: 10.1111/j.1749-6632.1999.tb08120.x. [DOI] [PubMed] [Google Scholar]
- 55.Geroldi C. Frisoni GB. Rozzini R. De Leo D. Trabucchi M. Principal lifetime occupation and sleep quality in the elderly. Gerontology. 1996;42(3):163–169. doi: 10.1159/000213788. [DOI] [PubMed] [Google Scholar]
- 56.Hill TD. Burdette AM. Hale L. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health and Place. 2009;15(4):1006–1013. doi: 10.1016/j.healthplace.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Gangwisch JE. Heymsfield SB. Boden-Albala B, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31(8):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 58.Tochikubo O. Ikeda A. Miyajima E. Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27(6):1318–1324. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 59.Spiegel K. Leproult R. Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 60.Kato M. Phillips BG. Sigurdsson G. Narkiewicz K. Pesek CA. Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35(5):1173–1175. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 61.Meier-Ewert HK. Ridker PM. Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology 18. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 62.King CR. Knutson KL. Rathouz PJ. Sidney S. Liu K. Lauderdale DS. Short sleep duration and incident coronary artery calcification. Journal of the American Medical Association. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grandner MA. Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Medicine Reviews. 2007;11(5):341–360. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whang W. Kubzansky LD. Kawachi I, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: Results from the Nurses' Health Study. Journal of the American College of Cardiology. 2009;53(11):950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaussent I. Bouyer J. Ancelin ML, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34(8):1103–1110. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kripke DF. Jean-Louis G. Elliott JA, et al. Ethnicity, sleep, mood, and illumination in postmenopausal women. BMC Psychiatry. 2004;4:8. doi: 10.1186/1471-244X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Somers VK. White DP. Amin R, et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 68.Li Y WA. Chiuve S. Rimm E. Winkleman J. Gao X. Prospective study of restless leg syndrome and coronary heart disease among women. Circulation. 2012;126(14):1689–1694. doi: 10.1161/CIRCULATIONAHA.112.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lauderdale DS. Knutson KL. Yan LL. Liu K. Rathouz PJ. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.