Abstract
Objective
Abnormal prefrontal and subcortical activity during cognitive control tasks is identified in non-depressed adolescents with bipolar disorder (BD); however, little is known about the neural correlates of bipolar adolescents in a depressed state (BDd). We aimed to investigate baseline versus after-treatment patterns of neural activity underlying motor response and response inhibition in adolescents with BDd.
Methods
In this functional magnetic resonance imaging (fMRI) study, 10 adolescents with BDd relative to 10 age- and sex-matched healthy controls (HC) completed a well-validated go/no go block-design cognitive control task at baseline and after 6 weeks of naturalistic treatment. We used whole-brain analysis and controlled our results for multiple comparisons.
Results
There was significant improvement in depression scores (mean change: 57%±28). There was no behavioral difference in BDd baseline versus HC and after treatment. BDd adolescents relative to HC had higher baseline cortical, but not subcortical, neural activity (e.g., bilateral ventrolateral prefrontal during both the go [motor control] and the no go [response inhibition] conditions, and left superior temporal during the no go condition). However, after-treatment activity relative to baseline neural activity during response inhibition was significantly increased in subcortical (e.g., right hippocampus and left thalamus), but not cortical, regions. In addition, at baseline, lower left thalamus activity was correlated with higher depression scores.
Conclusions
Adolescents with BDd had baseline prefrontal and temporal hyperactivity underlying motor control and response inhibition that did not change after treatment in contrast to relatively decreased baseline subcortical activity underlying response inhibition associated with the depressive state that was increased after the treatment.
Introduction
Bipolar disorder (BD) is the fourth leading cause of disability among adolescents worldwide, and is associated with increased risk for suicide (Gore et al. 2011). A depressive episode (BDd) is the longest and most frequent manifestation of BD in adolescents (Birmaher et al. 2009; Chang 2009); however, there are very limited treatment options for BDd in adolescents, and neural correlates of its treatment are understudied.
Available studies report significant neurocognitive deficits (e.g., response inhibition, attention, working memory) (Pavuluri et al. 2006a,b; Joseph et al. 2008) and abnormal prefrontal and subcortical activity in response to cognitive control tasks in adolescents with non-depressed BD (Blumberg et al. 2003; Chang et al. 2004; Leibenluft et al. 2007; Passarotti et al. 2010; Pavuluri et al. 2010; Singh et al. 2010). Findings in adults with BD suggest that the depressed state may manifest different cognitive control deficits than do euthymic and hypomanic states (Malhi et al. 2007); however, no study has investigated neural correlates of cognitive control in BDd adolescents before and after treatment. A 3 year follow-up study suggested that BD in adolescents is associated with delay in neurocognition and long-term functional ability (Pavuluri et al. 2009); however, some of these neurocognitive and neural abnormalities identified in manic/hypomanic adolescents with BD improved after treatment (Pavuluri et al. 2010). Identifying differential patterns of functional abnormalities in cognitive control neural systems in adolescents with BDd relative to healthy controls may help facilitate understanding of the state-specific neural substrates of depression, and imaging the same subjects after treatment can provide insight about neural correlates of treatment. In this study, using a well-validated block-design go/no go cognitive control task (Singh et al. 2010; Pan et al. 2011), we aimed to investigate baseline versus after-treatment patterns of neural activity underlying motor response and response inhibition in BDd adolescents relative to healthy controls (HC). We hypothesized that BDd adolescents would have higher cortical (e.g., lateral prefrontal, cingulate, and temporal) and subcortical (e.g., dorsal striatum, thalamus, and hippocampus) activity relative to HC, and that these abnormal activities would be normalized after treatment.
Methods
Study design
BDd adolescents were scanned at baseline and after 6 weeks of naturalistic treatment, and HC adolescents were scanned at baseline while completing a block-design go/no go cognitive control task (e.g., a 5 minute 38 second block design with 120 letters in which subjects pressed a button to a visually presented letter stimulus in go trials, but avoided response to a non-target letter stimulus (the letter V) in no go trials. Go blocks included 20 go trials and no go blocks included 10 randomly distributed go and 10 no go trials, a well-validated measure of motor response and response inhibition (see Pan et al. 2011 for task details). All adolescents and their parents gave consent. The University of Pittsburgh Institutional Review Board approved the study and consent forms. All BDd adolescents met American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) criteria for BD I/II or research diagnostic criteria for BD-not otherwise specified (NOS) (American Psychiatric Association 2000; Axelson et al. 2006) and a current major depressive episode as determined by the parent and child Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL) (Kaufman et al. 1997) with score ≥40 on the Children's Depression Rating Scale-Revised (Poznanski and Mokros 1995) (CDRS-R) and <11 on the Young Mania Rating Scale (Young et al. 1978) (YMRS) on the day of functional magnetic resonance imaging (fMRI). In addition, we measured anxiety with a self-rated anxiety scale (Screen for Child Anxiety Related Emotional Disorders; SCARED) (Birmaher et al. 1997). Urine screenings to rule out pregnancy and drug use were performed before the scanning. We excluded BDd adolescents with psychotic disorders, pervasive developmental disorders, eating disorders, substance use disorders, learning disorders, and mental retardation. No personal or family psychiatric history was allowed for HC, and we excluded adolescents with any contraindications for fMRI.
Subjects
We included 10 right-handed (assessed using the Edinburgh Handedness Inventory (Oldfield 1971)) BDd adolescents ages 12–17 who had reached puberty (with a score ≥3 on Tanner's Pubertal Development Scale [Marshall and Tanner 1969]) and age- and sex-matched HC. BDd adolescents were allowed to be taking psychotropic medications (up to three nonstimulant medications; three adolescents with BDd were free of medications before treatment and all adolescents were on medications after 6 weeks of naturalistic treatment). Stimulants were held 24 hours before scanning (three BDd adolescents). All BDd adolescents received individual psychotherapy through their providers plus medication management during the 6 weeks of naturalistic treatment.
Functional imaging data acquisition
A Trio 3.0 Tesla scanner (Siemens, AG) was used, and anatomical images covering the entire brain were acquired using an axial 3D MPRAGE sequence, parallel to the AC–PC line (TE/TI/TR=3.29 ms/900 ms/2200 ms, flip angle=9, isotropic 1 mm3 voxel, 192 axial slices, matrix size=256×192). Blood oxygen level dependent (BOLD) functional images were acquired with a gradient-echo echo-planar imaging (EPI) sequence and covered 34 axial slices (3 mm thick, 0 mm gap) encompassing the entire cerebrum and the majority of the cerebellum (TR/TE=2000/25 msec, field of view=205 mm, matrix=64×64). Before collection of fMRI data for each subject, a reference EPI scan was acquired and inspected for artifacts and signal across the entire volume of acquisition.
Behavioral and imaging analysis
Repeated measures of analyses of variance (ANOVA) and t tests were used to examine the main effect of group on task performance accuracy—numbers of correct go and no go responses, omissions (misses for go stimuli), and commissions (incorrect button press for no go stimuli)—using SPSS 19 (SPSS, Inc., Chicago, IL). Imaging data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM5; London, United Kingdom). Data for each participant were first corrected for differences in acquisition time between slices; realigned using the first slice as a reference, and unwarped to correct static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. Movement cutoff was <2mm. Data were co-registered with the participant's anatomic image, segmented, normalized to a standard Montreal Neurological Institute (MNI) template, resampled to 3×3×3 mm3 voxels, and spatially smoothed with a Gaussian kernel of 6 mm full width at half-maximum. A first-level fixed-effect model was constructed for the two blocks (go and no go) entered as separate conditions in a block design in the design matrix. Movement parameters from the realignment stage were entered as covariates of no interest, to control for participant movement. Trials were modeled using the canonical hemodynamic response function. Two second-level random-effects group analyses were conducted on the t-contrast images generated in the previous single-subject analyses: first in a 2 (group: HC and BDd at baseline) by 2 (condition: go and no go) and second in a 2 (group: BDd at baseline and BDd after treatment) by 2 (condition: go and no go) repeated measures ANOVA covarying for age for each experiment (go and no go) to avoid any undetected age effects. First, a voxel-wise threshold of p<0.05 was used for whole-brain analyses. Second, a cluster-level false-positive detection rate of p<0.05 was maintained for whole-brain activity surviving the voxel-wise threshold of p<0.05 using small volume correction (SVC) with a regional anatomic mask from the Wake Forest University (WFU) PickAtlas for each whole-brain activity cluster ≥10 voxels and a cluster (k) extent empirically determined by Monte Carlo simulation implemented in AlphaSim (Pan et al. 2011). Peak BOLD signal changes were extracted from regions showing a significant group-by-condition interaction in the 2×2 analysis for each experiment. Post-hoc tests were performed on these extracted BOLD signal values to examine the extent to which pairwise between-group differences in activity contributed to the significant group-by-condition interactions in these analyses using independent and paired t tests and appropriate statistical thresholds (corrected p≤0.05/4=0.012) to control for multiple tests.
In exploratory analyses, we used repeated measures analysis, t test, or Pearson's correlational analysis (p≤0.05) appropriately to examine potential relationships among gender, severity and duration of depression, subtype of the BD, attention-deficit/hyperactivity disorder (ADHD) and anxiety disorders comorbidity, responders to treatment (e.g., Clinical GIobal Impressions-Severity (Spearing et al. 1997) ≤2 and ≥50% percentage reduction in CDRS-R), and psychotropic medications upon patterns of abnormal neural activity between the study groups. In addition, using regional interest of analysis (ROI), we explored neural activity differences between BDd after treatment and HC, focusing on the significant regions identified in this study with the whole-brain analysis of baseline BDd versus HC and BDd after treatment (e.g., ventrolateral prefrontal cortex (VLPFC), right hippocampus, and left thalamus).
Results
There were 10 BDd (3 BD type I, 4 BD type II, and 3 BD NOS; 8 females, mean age=15.6±0.9), and 10 HC adolescents (8 females, mean age=15.6±1.2). Nine out of 10 BDd adolescents had a first- or second-degree relative with BD and 3 BDd adolescents had comorbid ADHD (Table 1). All BDd adolescents received individual psychotherapy through their providers and four adolescents were started on a new medication (two with lamotrigine, one with quetiapine, and one with aripiprazole), five adolescents remained on the same medication combinations (one with lamotrigine and quetiapine, one with lamotrigine and aripiprazole, one with citalopram and aripiprazole, one with lithium and quetiapine, and one with lamotrigine and valproic acid and sertraline combination), but their doses were increased, and one adolescent remained on the same dose of the medication (lamotrigine).
Table 1.
Demographic and Clinical Variables of Adolescents with Bipolar Depression (BDd) and Healthy Controls (HC)
| BDd adolescents | HC | Significance | |
|---|---|---|---|
| BD subtypes | 3 BD type I, 4 BD type II, and 3 BD NOS |
N/A | N/A |
| Mean age (years) | 15.6±0.9 | 15.6±1.2 | − |
| Gender (females) | 80% | 80% | − |
| Race (White) | 70% | 70% | − |
| Family history of BD (%) | 90% | 0 | BDd>HC* |
| Comorbid ADHD* | 30% | 0 | BDd>HC* |
| Mean duration of the current depressive episode (weeks) | 5.5±4.5 | N/A | N/A |
| BDd baseline | BDd after treatment | |||
|---|---|---|---|---|
| Children's Depression Rating Scale-revised (CDRS-R) | 73.2±14 | 41.5±15.7 | 19.1±1.8 | BDd>HC* Baseline>after treatment* |
| Young Mania Rating Scale (YMRS) | 2.9±1.5 | 2.4±1.6 | 0.8±0.8 | − |
| Mean of the Clinical Global Impressions-Severity scores | 5.3±0.7 | 3.3±1.2 | 1 | BDd>HC* Baseline>after treatment* |
Indicates p≤0.05 between the groups.
BDd, bipolar depression; NOS, not otherwise specified; HC, healthy controls; BDd after treatment (6-weeks of naturalistic treatment); ADHD, attention-deficit/hyperactivity disorder.
Mean depression score (CDRS-R) was 73.2±14 at baseline and showed significant improvement after treatment (e.g., mean percentage change in depression [by taking into account the minimum CDRS score of 17] was 57%±28; F=32.8, p<0.0001). Six out of 10 adolescents were considered as responders after treatment. Baseline versus after-treatment scores were not significant; respectively, 30.9±14 and 24.3±13.6 for anxiety, and 2.9±1.5 and 2.4+1.65 for mania.
Task performance data
Between BDd adolescents at baseline and HC, there was no significant effect of group on task performance accuracy for percentage of inaccurate go responses (BDd: 13.5±8.6, HC: 13.7±4.9) and inaccurate no go responses (BDd: 23.1±11.6, HC: 16.2±7.5) or for reaction time in milliseconds for go responses (BDd: 372.2±25.1, HC: 387.9±55.5) and no go responses (BDd: 397.8±27, HC: 404.8±39.7). In addition, these measures in BDd adolescents after treatment (inaccurate go responses=18±18.9; inaccurate no go responses=23.1±12.5; reaction time in milliseconds for go responses=382.9±23.1; reaction time in milliseconds for no go=402.9±27.8) did not change relative to BDd at baseline.
Neuroimaging data
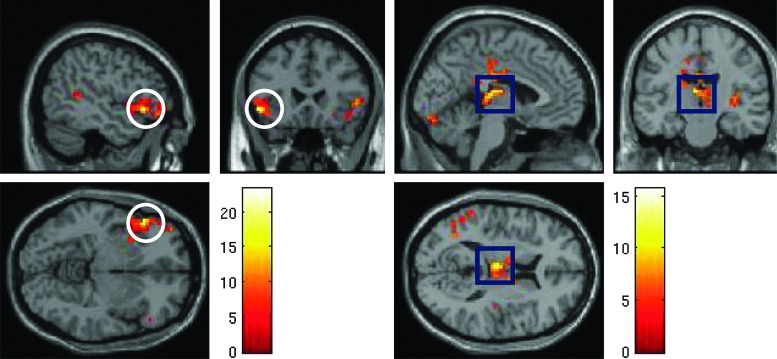
Between BDd at baseline and HC, group-by-condition analysis for the no go versus the go condition by employing whole-brain analysis showed that bilateral VLPFC (BA [Brodmann's Area] 47), left medial frontal cortex (BA 10), right inferior parietal cortex (BA 40), and left superior temporal cortex (BA 41) had significant activation (Table 2). Pairwise comparisons showed that significance between the groups was mainly the result of increased neural activity in BDd adolescents before treatment relative to HC in bilateral VLPFC during both the go and the no go conditions (Fig. 1) and in left superior temporal cortex during the no go condition.
Table 2.
Whole-Brain Analysis for Significant Neural Activities to the Go Motor Control and the Go/Nogo Response Inhibition Blocks in Adolescents with Bipolar Depression (BDd) at Baseline Versus Healthy Control (HC) and BDd After Treatment
| Neural region and Brodmann's area (BA) | Cl. size | Alpha Sim Cl. | MNI coordinates | Block | Post-hoc test | t | p | F | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BDd baseline versus HC | x | y | z | |||||||
| Left medial frontal (BA10) | 479 | 20 | −6 | 57 | 12 | Go | − | |||
| Go/No go | − | |||||||||
| Left ventrolateral prefrontal (BA 47) | 525 | 16 | −48 | 24 | −6 | Go | BDd>HC* | 3.10 | 0.006 | 9.48 |
| Go/No go | BDd>HC* | 4.66 | 0.0002 | 21.14 | ||||||
| Right ventrolateral prefrontal (BA 47) | 65 | 13 | 51 | 24 | 0 | Go | BDd>HC* | 2.86 | 0.010 | 8.08 |
| Go/No go | BDd>HC* | 3.46 | 0.003 | 11.78 | ||||||
| Right Inferior parietal (BA 40) | 73 | 32 | 69 | −30 | 24 | Go | − | |||
| Go/No go | − | |||||||||
| Left superior temporal (BA 41) | 1203 | 10 | −57 | −30 | 15 | Go | − | |||
| Go/No go | BDd>HC* | 3.34 | 0.004 | 11.01 | ||||||
| BDd baseline versus after treatment | ||||||||||
| Right medial frontal (BA 10) | 44 | 20 | 21 | 39 | −15 | Go | − | |||
| Go/No go | − | |||||||||
| Left posterior cingulate (BA 23) | 95 | 13 | −3 | −21 | 27 | Go | − | |||
| Go/No go | − | |||||||||
| Right inferior parietal (BA 40) | 66 | 32 | 63 | −39 | 39 | Go | − | |||
| Go/No go | − | |||||||||
| Left cerebellum | 191 | 38 | −33 | −60 | −6 | Go | − | |||
| Go/No go | − | |||||||||
| Right hippocampus | 99 | 13 | 15 | 3 | −21 | Go | ||||
| Go/No go | After>Baseline* | 3.73 | 0.002 | 13.65 | ||||||
| Left thalamus | 186 | 33 | −3 | −18 | 12 | Go | − | |||
| Go/No go | After>Baseline* | 3.27 | 0.004 | 10.53 | ||||||
p≤0.012 (=.05/4; corrected for pairwise comparisons). Cluster size corrected with AlphaSim.
BDd, bipolar depression; HC, healthy controls; BA, Brodmann's area; MNI, Montreal Neurological Institute; Cl, Cluster; BDd after treatment (6 weeks of naturalistic treatment).
FIG. 1.
Whole-brain analysis during the no go versus the go blocks of the go/no go block design task: Left ventrolateral prefrontal (VLPFC) activity (pictures in the left panel) in bipolar adolescents in a depressed state (BDd) versus healthy controls and left thalamus activity (pictures in the right panel) in BDd adolescents at baseline versus after 6 weeks of naturalistic treatment. A color version of this figure is available in the online article at www.liebertpub.com/jcap.
Between baseline and after treatment in BDd adolescents, group-by-condition analysis for the no go versus the go condition by employing whole-brain analysis showed that right medial frontal cortex (BA 10), left posterior cingulate cortex (BA 23), right inferior parietal cortex (BA 40), left cerebellum, right hippocampus, and left thalamus had significant activation (Table 2). Pairwise comparisons showed that significance between the groups was mainly the result of increased neural activity after treatment in BDd adolescents in the right hippocampus and left thalamus (Fig. 1).
Our exploratory analyses showed that baseline depression scores on CDRS-R were negatively correlated with the baseline left thalamus activity during the go (r=−0.69, p=0.027) and the no go (r=−0.88, p=0.001) conditions. Decrease in depression scores after treatment was positively correlated with left thalamus activity increase (r=0.85, p=0.032) during the no go condition only in treatment responders, not in non-responders. There were no other significant findings in our exploratory analysis including gender, severity and duration of depression, subtypes of BD, comorbid anxiety and ADHD, treatment responders versus non-responders, and medication effect on neural activity.
Our exploratory ROI analyses showed that adolescents with BDd after treatment relative to HC had higher left thalamus activity during the go (t=2.76, p=0.013) and no go (t=4.1, p=0.001), higher right hippocampus activity during the go (t=2.45, p=0.025) and no go (t=3.52, p=0.002), and higher left VLPFC (BA 47) during the no go (t=3.62, p=0.002) conditions.
Discussion
This first cognitive control fMRI study in adolescents with BDd showed that BDd adolescents relative to HC had higher cortical neural activity at baseline (e.g., bilateral VLPFC during both the go and the no go conditions and left superior temporal during the no go condition); however, there was no change after 6 weeks of naturalistic treatment, suggesting that cortical hyperactivity to cognitive tasks previously identified in non-depressed BD adolescents (Chang et al. 2004; Singh et al. 2010) may be a trait abnormality. However, in contrast to our hypothesis, subcortical neural activity at baseline was not different relative to HC, but showed increased activity after treatment relative to baseline in the right hippocampus and left thalamus. Our result suggested that depression was associated with a relative decrease in subcortical activity during response inhibition in BDd adolescents that was also supported by 1) our inverse correlation finding of lower baseline left thalamus activity with higher baseline depression scores in all BDd adolescents, and 2) positive correlation finding of increased left thalamus activity with improvement in depression scores over follow-up in those who responded to the treatment. In summary, our cognitive control fMRI study showed that BDd in adolescents was associated with baseline cortical hyperactivity underlying motor control and response inhibition that did not change with treatment, and disrupted subcortical activity underlying response inhibition that was associated with the depressed state (e.g., decreased activity associated with the severity of baseline depression and increased activity after the treatment).
Our results of no behavioral difference in depressed group relative to HC, but abnormal neural activity underlying cognitive control in cortical (lateral prefrontal for executing inhibitory control processes and temporal for attentional processing) and subcortical (hippocampus [Fusar-Poli et al. 2009], an important region for emotion processing in addition to memory encoding, retention, and retrieval; and thalamus [Li et al. 2008], a major relay center that sends inhibitory signals to striatum and motor cortex to stop prepotent responses) are similar to the majority of available studies in non-depressed BD adolescents (Blumberg et al. 2003; Chang et al. 2004; Leibenluft et al. 2007; Passarotti et al. 2010; Pavuluri et al. 2010; Singh et al. 2010). In addition, increased activity of cortical and/or subcortical regions during response inhibition (e.g., no go condition) in this study in BDd relative to HC was similar to the studies in adolescents with non-depressed BD (Blumberg et al. 2003; Chang et al. 2004; Singh et al. 2010). However, there are other fMRI studies with opposite findings such as hypoactivity of prefrontal and subcortical neural activity during response inhibition in adolescents with BD (Leibenluft et al. 2007; Passarotti et al. 2010) and in euthymic/manic adults with BD (Altshuler et al. 2005; Kaladjian et al. 2009; Townsend et al. 2012). Different findings in the abovementioned studies could be the result of different clinical profiles of the subjects (sex, acute mood state, and comorbidity) and different tasks (e.g., response inhibition, interference) and designs (e.g., block, event-related).
There is little known about the trait (e.g., mood independent) versus state (e.g., mood state specific) neural abnormalities in BD adolescents, but this is very critical information that can potentially improve clarifying diagnoses and identifying neural targets for interventions (Diler 2011). There are few pharmaco-imaging studies in BD adolescents and directions of neural change in manic/hypomanic BD adolescents during a response inhibition task that were in contrast (e.g., increased prefrontal and decreased subcortical activity after treatment) (Pavuluri et al. 2010) to our findings. In parallel to the fMRI study that reported high subcortical activity in BD youth who were not in depression as compared to HC (Blumberg et al. 2003), our study provided promising findings that BDd adolescents might have a relative reduction in left thalamus activity associated with depression (mainly during the no go condition) and that the left thalamus activity increased and became detectable after the treatment of depression. The thalamus is an important region subserving neural networks underlying external and internal emotional control as well as cognitive control processes (Basso et al. 2005; Strakowski et al. 2011; Fleck et al. 2012). It acts as a relay between several subcortical areas and cortex and plays an important role in processing sensory information and regulating states of sleep and wakefulness (Li et al. 2008; Altshuler and Townsend 2012). Given its central role, it was not surprising that abnormal thalamus activity was associated with ADHD, depression (Greicius et al. 2007), and BD (Blumberg et al. 2003) and few available studies reported normalization (e.g., increase) of thalamus activity after treatment of ADHD (Rubia et al. 2011) and depression (Anand et al. 2005, 2007). Our exploratory analysis suggested that improvement in depression scores was correlated with an increase in left thalamus activity in those who responded to treatment; however, we need larger studies with longer duration in treatment to draw a better conclusion about depression-related changes in thalamus activity in adolescents with BD.
In this study, we identified increased VLPFC and temporal cortical activity in BDd relative to HC during response inhibition, but did not find depression- or treatment-related activity changes. Similar to our findings, the only published fMRI study in BDd adolescents used an emotion processing task, and did not include HC, but reported no change in prefrontal activity after treatment (Chang et al. 2008) suggesting that prefrontal abnormality in BD adolescents identified during depression may persist after the treatment (Singh et al. 2010). Our findings of similar behavioral response (e.g., accuracy and reaction time) between BDd adolescents and HC might be the result of more extensive recruitment of VLPFC in BDd adolescents to compensate for prefrontal dysfunction. Others have proposed that impaired VLPFC activation in subjects with BD might be a trait abnormality in BD (Strakowski et al. 2012) and potentially reflected an increased cortical control over a hyperactive emotion processing system that could still be detected during non-emotional cognitive control paradigms (Singh et al. 2010). Previous cognitive control studies with BD youth reported both increased (Leibenluft et al. 2007; Singh et al. 2010) and decreased (Passarotti et al. 2010) activity in lateral prefrontal cortex, suggesting an impaired recruitment of this region to exert response inhibition and attentional control. Our study adds to the accumulating literature in BD that ventrolateral prefrontal activity was impaired in BDd adolescents; however, we need larger longitudinal fMRI studies with connectivity analysis of this network to better understand its trait versus state network abnormalities.
Limitations
There are limitations to our study. Some adolescents might have delayed treatment response and neural changes; however, our naturalistic study for 6 weeks was similar to an open-label treatment study in bipolar depression in youth (Patel et al. 2006), and decrease in depression scores and percent response rates were comparable to those in the previous studies (DelBello et al. 2009). Also, our small sample size possibly limited us to identify neural activity differences between those who had higher versus those who had lower reduction in depression scores after treatment and between subtypes of BD. Studies in adults suggested potential clinical and neuroimaging differences between BD I and II (Summers et al. 2006; Baek et al. 2011), and a study in youth suggested neural differences during emotion processing between those with BD I and BD NOS (Ladouceur et al. 2011). Our negative findings between BD I (n=3), II (n=4), and NOS (n=3) could be the result of our small sample size and developmental progression in categorical BD diagnosis in adolescents (e.g., >20% of youth with BD II became BD I over a 4 year follow up (Birmaher et al. 2009), and >40% of youth with BD NOS became BD I or II [Axelson et al. 2011]). We had predominantly female adolescents with relatively low ADHD comorbidity, and they were taking psychotropic medications during scanning. However, our sample characteristics were similar to the only randomized treatment study in bipolar depression (DelBello et al. 2009). Although we did not find any differences in those BDd adolescents with (n=3) and without ADHD (n=7), it is important to consider that ADHD was associated with decreased prefrontal and increased subcortical activity in earlier cognitive control fMRI studies and that ADHD and BD can result in greater variation in patterns of prefrontal and subcortical activations than each disorder alone (Adler et al. 2005; Passarotti et al. 2010). We did not scan HC subjects for the second time and could not control our results for practice effects with repeated scans that limit our ability to draw a better conclusion about treatment related changes. We included BDd adolescents with and without medications; however, available studies in adults and adolescents suggested that medication treatment is not likely to cause new abnormalities, but may result in amelioration of neural activity abnormalities found in BD and may cause type II error (Hafeman et al. 2012). Although event-related designs and analyses might be able to extract individual cognitive processes subserved by activated brain regions, we used block design go/no go task, similar to studies in adolescents with UDd (Pan et al. 2011) and euthymic BD (Singh et al. 2010), to examine a combination of cognitive control processes that included response inhibition in addition to sustained attention, target detection, and rule maintenance over a sustained period of time to capitalize on a higher proportion of no go trials (Singh et al. 2010; Pan et al. 2011).
Conclusions
Our study indicates that adolescents with BD during acute depression had cortical hyperactivity underlying motor control and response inhibition that did not change with treatment, whereas they had relatively decreased subcortical activity during response inhibition that was associated with the depressive state and increased with treatment. Examining patterns of neural activity changes after treatment can help identify state markers and predictors of treatment response of depression in BD adolescents. We need larger longitudinal studies in BDd children and adolescents investigating the effects of specific types of medications and therapies on cortical and subcortical neural activity and connectivity.
Clinical Significance
Adolescents with BDd can successfully perform response inhibition and motor control, but need to recruit more neural regions relative to controls to meet the demand of the cognitive task. Abnormal activation in prefrontal/temporal and subcortical regions is postulated to underlie impaired cognitive/attention control and impulsivity, respectively, that are commonly reported in adolescents with BD; however, in contrast to a relative decrease in subcortical activity at baseline that was increased after treatment, prefrontal/temporal hyperactivity did not change after the treatment of depression.
Disclosures
Dr. Ladouceur (K01 MH083001) and Dr. Pan (K23 MH082884) received funding from the National Institute of Mental Health (NIMH). Dr. Birmaher receives royalties from Random House Inc. and Lippincott Williams and Wilkins. Other authors reported no conflict of interest.
References
- Adler CM. Delbello MP. Mills NP. Schmithorst V. Holland S. Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord. 2005;7:577–588. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- Altshuler LL. Bookheimer SY. Townsend J. Proenza MA. Eisenberger N. Sabb F. Mintz J. Cohen MS. Blunted activation in orbitofrontal cortex during mania: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Altshuler LL. Townsend JD. Functional brain imaging in bipolar disorder. In: Strakowski SM, editor. The Bipolar Brain. New York: Oxford University Presss; 2012. pp. 53–78. [Google Scholar]
- American Psychiatric Association. Text Revision. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anand A. Li Y. Wang Y. Gardner K. Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: An FMRI study. J Neuropsychiatry Clin Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A. Li Y. Wang Y. Wu J. Gao S. Bukhari L. Mathews VP. Kalnin A. Lowe MJ. Antidepressant effect on connectivity of the mood-regulating circuit: An FMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Axelson D. Birmaher B. Strober M. Gill MK. Valeri S. Chiappetta L. Ryan N. Leonard H. Hunt J. Iyengar S. Bridge J. Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Axelson DA. Birmaher B. Strober MA. Goldstein BI. Ha W. Gill MK. Goldstein TR. Yen S. Hower H. Hunt JI. Liao F. Iyengar S. Dickstein D. Kim E. Ryan ND. Frankel E. Keller MB. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50:1001–1016. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA. Uhlrich D. Bickford ME. Cortical function: A view from the thalamus. Neuron. 2005;45:485–488. doi: 10.1016/j.neuron.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Baek JH. Park DY. Choi J. Kim JS. Choi JS. Ha K. Kwon JS. Lee D. Hong KS. Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. J Affect Disord. 2011;131:59–67. doi: 10.1016/j.jad.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Axelson D. Goldstein B. Strober M. Gill MK. Hunt J. Houck P. Ha W. Iyengar S. Kim E. Yen S. Hower H. Esposito–Smythers C. Goldstein T. Ryan N. Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B. Khetarpal S. Brent D. Cully M. Balach L. Kaufman J. Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Blumberg HP. Martin A. Kaufman J. Leung HC. Skudlarski P. Lacadie C. Fulbright RK. Gore JC. Charney DS. Krystal JH. Peterson B. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Chang K. Challenges in the diagnosis and treatment of pediatric bipolar depression. Dialogues Clini Neurosci. 2009;11:73–80. doi: 10.31887/DCNS.2009.11.1/kchang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. Adleman NE. Dienes K. Simeonova DI. Menon V. Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chang KD. Wagner C. Garrett A. Howe M. Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal–amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disord. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Chang K. Welge JA. Adler CM. Rana M. Howe M. Bryan H. Vogel D. Sampang S. Delgado SV. Sorter M. Strakowski SM. A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disord. 2009;11:483–493. doi: 10.1111/j.1399-5618.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- Diler R. Neuroimaging can help identify biomarkers of early onset bipolar disorder. Bull Clin Psychopharmacol. 2011;22:1–4. [Google Scholar]
- Fleck DE. Eliassen JC. Durling M. Lamy M. Adler CM. DelBello MP. Shear PK. Cerullo MA. Lee JH. Strakowski SM. Functional MRI of sustained attention in bipolar mania. Mol Psychiatry. 2012;17:325–336. doi: 10.1038/mp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar–Poli P. Placentino A. Carletti F. Landi P. Allen P. Surguladze S. Benedetti F. Abbamonte M. Gasparotti R. Barale F. Perez J. McGuire P. Politi P. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gore FM. Bloem PJN. Patton GC. Ferguson J. Joseph Vr. Coffey C. Sawyer SM. Mathers CD. Global burden of disease in young people aged 10–24 years: A systematic analysis. Lancet. 2011;377:2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- Greicius MD. Flores BH. Menon V. Glover GH. Solvason HB. Kenna H. Reiss AL. Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM. Chang KD. Garrett AS. Sanders EM. Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Joseph MF. Frazier TW. Youngstrom EA. Soares JC. A quantitative and qualitative review of neurocognitive performance in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2008;18:595–605. doi: 10.1089/cap.2008.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladjian A. Jeanningros R. Azorin JM. Nazarian B. Roth M. Mazzola–Pomietto P. Reduced brain activation in euthymic bipolar patients during response inhibition: An event–related fMRI study. Psychiatry Res. 2009;173:45–51. doi: 10.1016/j.pscychresns.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD. Farchione T. Diwadkar V. Pruitt P. Radwan J. Axelson DA. Birmaher B. Phillips ML. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1275–1289. doi: 10.1016/j.jaac.2011.09.023. e1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E. Rich BA. Vinton DT. Nelson EE. Fromm SJ. Berghorst LH. Joshi P. Robb A. Schachar RJ. Dickstein DP. McClure EB. Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Li CS. Yan P. Sinha R. Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41:1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS. Ivanovski B. Hadzi–Pavlovic D. Mitchell PB. Vieta E. Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007;9:114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Marshall WA. Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edingurgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pan LA. Batezati–Alves SC. Almeida JR. Segreti A. Akkal D. Hassel S. Lakdawala S. Brent DA. Phillips ML. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2011;50:602–611. doi: 10.1016/j.jaac.2011.03.018. e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM. Sweeney JA. Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NC. DelBello MP. Bryan HS. Adler CM. Kowatch RA. Stanford K. Strakowski SM. Open-label lithium for the treatment of adolescents with bipolar depression. J Am Acad Child Adolesc Psychiatry. 2006;45:289–297. doi: 10.1097/01.chi.0000194569.70912.a7. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. O'Connor MM. Harral EM. Moss M. Sweeney JA. Impact of neurocognitive function on academic difficulties in pediatric bipolar disorder: A clinical translation. Biol Psychiatry. 2006a;60:951–956. doi: 10.1016/j.biopsych.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Passarotti AM. Harral EM. Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry. 2010;71:1526–1534. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN. Schenkel LS. Aryal S. Harral EM. Hill SK. Herbener ES. Sweeney JA. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006b;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. West A. Hill SK. Jindal K. Sweeney JA. Neurocognitive function in pediatric bipolar disorder: 3-year follow-up shows cognitive development lagging behind healthy youths. J Am Acad Child Adolescent Psychiatry. 2009;48:299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E. Mokros H. Los Angeles: Western Psychological Services; 1995. Children's Depression Rating Scale-Revised. [Google Scholar]
- Rubia K. Halari R. Mohammad AM. Taylor E. Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70:255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK. Chang KD. Mazaika P. Garrett A. Adleman N. Kelley R. Howe M. Reiss A. Neural correlates of response inhibition in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20:15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearing MK. Post RM. Leverich GS. Brandt D. Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Strakowski SM. Adler CM. Almeida J. Altshuler LL. Blumberg HP. Chang KD. Delbello MP. Frangou S. McIntosh A. Phillips ML. Sussman JE. Townsend JD. The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM. Eliassen JC. Lamy M. Cerullo MA. Allendorfer JB. Madore M. Lee JH. Welge JA. DelBello MP. Fleck DE. Adler CM. Functional magnetic resonance imaging brain activation in bipolar mania: Evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. Papadopoulou K. Bruno S. Cipolotti L. Ron MA. Bipolar I and bipolar II disorder: Cognition and emotion processing. Psychol Med. 2006;36:1799–1809. doi: 10.1017/S0033291706008804. [DOI] [PubMed] [Google Scholar]
- Townsend JD. Bookheimer SY. Foland–Ross LC. Moody TD. Eisenberger NI. Fischer JS. Cohen MS. Sugar CA. Altshuler LL. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–450. doi: 10.1111/j.1399-5618.2012.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC. Biggs JT. Ziegler VE. Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]