Abstract
In this work, we focus on the methods for controlling cell function with ferromagnetic disk-shaped particles. We will first review the history of magnetically assisted modulation of cell behavior and applications of magnetic particles for studying physical properties of a cell. Then, we consider the biological applications of the microdisks such as the method for induction of cancer cell apoptosis, controlled drug release, hyperthermia and MRI imaging.
Index Terms: biomagnetics, ferromagnetic disks, magneto-mechanical cell actuation, hyperthermia, MRI
I. Brief historic introduction
Mechanosensitivity of eukaryotic cells is responsible for such fundamental physiological processes as hearing, proprioception, touch, and control of cell turgor [1]. The idea of using magnetic particles for studying the properties of biological cells was proposed almost a hundred years ago by Heilbronn and Seifriz [2–3]. Later in 1950, Crick and Hughes quantified the elastic modulus of the cytoplasm by tracking the movement of different types of iron oxide particles inside cells [4]. Their work served as a basis for multiple publications to appear in the next several decades which were mainly dealing with using magnetic particles for characterization of cell rheological properties [5–8]. Meanwhile, the rapid expansion of consumer electronics and the development of the world’s power grid in the latter part of the 20th century, eventually led to the discussions about the potential adverse effects of continuous exposure of weak electromagnetic fields on a human body. In 1991, Adair stated that such fields could not have any biological effects on cells due to their extremely low magnitudes (~50 µT) and frequency (~tens of Hz) [9]. However, as it was soon pointed out by Kirschvink [10], this statement was clearly in contradiction with such a well-known phenomenon as the ability of honeybees to detect the Earth’s magnetic field [11] with the latter being comparable in magnitude to the fields under consideration. A number of studies demonstrated that the dance language and the recruitment behavior of honeybees are mediated by fluctuations in the geomagnetic field [12–13]. This was attributed to the presence of magnetoreceptors in the animal body, particularly, the biomineralized magnetite particles [14]. Taking this as a starting point, Kirschvink predicted that the torque exerted on the cell through magnetic particles in a weak extremely low frequency magnetic field (tens of Hz) would be sufficient for opening a single mechanosensitive channel in response to the membrane deformation [10]. For the first time, Kirschvink proposed the mechanism for manipulation of mechanosensitive ion channels by magnetically controlling the movement of biomineralized magnetite particles attached to the cytoskeletal filament anchors. This originated the idea of directly applying magnetic materials for controlling cell function.
In 1993, Wang et al applied ferromagnetic RGD (Arg-Gly-Asp) peptide coated microbeads for mechanical stimulation of integrin receptors responsible for cell adhesion to the substrate [15]. Pommerenke et al (1996) later demonstrated that this method of cell activation results in the intracellular calcium response [16]. This work is especially interesting in the context of using magnetomechanical actuation for triggering cell apoptosis since calcium is known to be implicated in multiple steps of this process. It is also interesting to note that already in 1931 Robert Chambers reported on a systematic study on the effects of mechanical stimulation in different parts of a living cell [17]. He demonstrated that mechanical disturbance of the cell membrane may cause the eventual cell death, depending on the exact location of cell probing. His work, even being purely descriptive, appears to be one of the earliest experimental proofs that the cells have characteristic mechanosensitive properties which are tied with the mechanisms governing cell death.
Currently, two-pore domain inward-rectifying potassium channels TREK and TRAAK are among the most widely studied mechanosensitive channels present in cell membrane [18–20]. Hughes et al were the first ones to apply magnetic particles (Fe3O4 and CrO2) for selective targeting of particular locations of the TREK-1 channel [21]. They inserted an engineered poly-histidine tag(a sequence of six histidines) into the external loop of the channel protein and then inserted the tagged channel clone into the membrane of COS-7 cells. This cell type does not express ion channels similar to TREK-1 which makes it an ideal model system for independent studies of this channel. Magnetic particles were attached to the poly-histidine sequence via either Nickel-NTA linker or anti-histidine antibody, both with high affinity to the tag, for selective activation of the channel. The results of this work suggest that the TREK-1 channel can be activated by very low forces on the order of several pN when they are applied directly to the channel. Furthermore, the non-specific deformations of the cell membrane do not cause any noticeable response in terms of cell current.
The majority of biological applications of magnetic materials developed to date utilize chemically synthesized superparamagnetic particles on the order of tens of nanometers [22–24]. While these particles do not interact with each other, their typically small magnitude of magnetization calls for very strong magnetic fields required for their detection and manipulation. Ferromagnetic particles offer the advantage of higher magnetization levels but their uncontrollable agglomeration due to fringe magnetic fields poses a severe practical limitation. On the other hand, two-dimensional ferromagnetic structures with spin-vortex ground state have zero net magnetization in the absence of the external magnetic field which eliminates the agglomeration problem. The particle aspect ratio plays crucial role in formation of the spin vortex structure. Compared to wet chemistry synthesis, lithographic methods allow for tightly controlled fabrication of particles with virtually any size, shape and composition. Physical vapor deposition, compatible with most types of photoresists, offers a wide choice of materials available for magnetic particle fabrication. In our work, we employ lithographically defined permalloy disks with ~1 µm diameter for controlling cell function. This paper is organized as follows –following a short historical introduction the section II focuses on the applications of microdisks for targeted destruction of cancer cells by means of induction of apoptosis. Then in the Section III we review an optimized method for controlling the dynamic behavior of the disks by using two orthogonal magnetic fields and demonstrate its advantages for magnetically driven drug release. Finally, in Sections IV and V we demonstrate the feasibility of using the microdisks as multifunctional platform for induced drug release, in hyperthermia and magnetic resonance imaging.
II. Magneto-mechanical induction of programmed cancer cell death
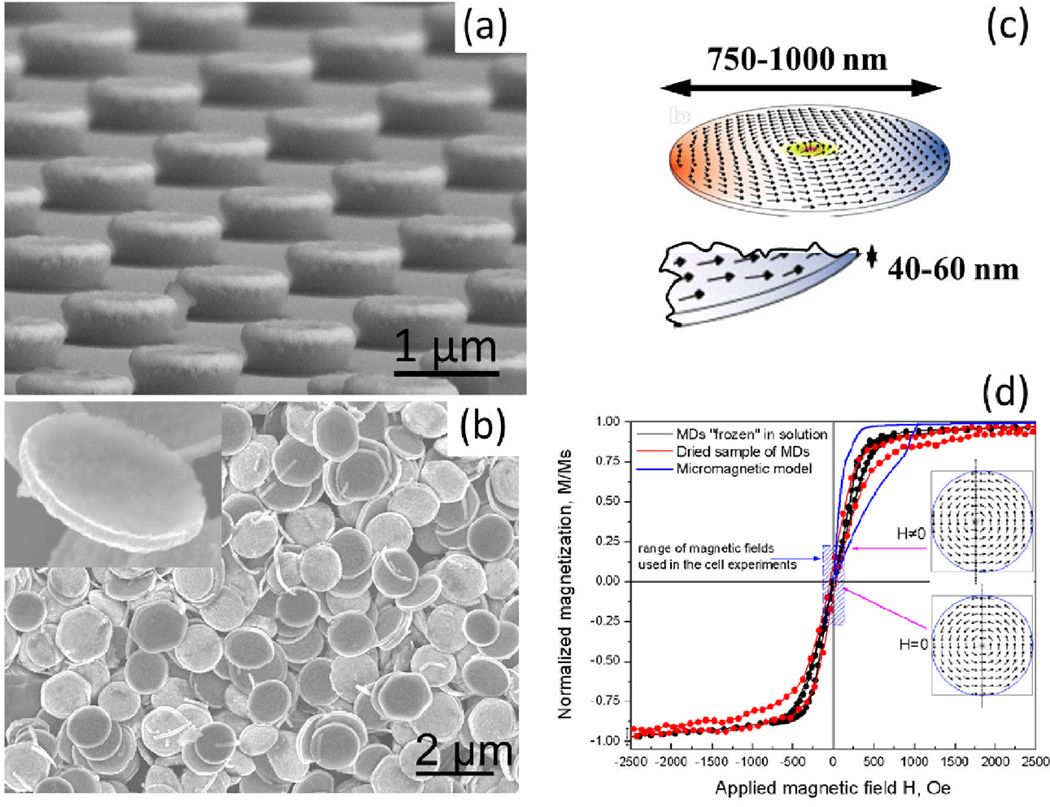
Apoptosis or programmed cell death is a process maintaining the tissue homeostasis and controlling the removal of cells during inflammation [25]. Disruption of regular apoptotic pathways is responsible for the uncontrollable proliferation of cancer cells leading to tumor growth. Our group has recently demonstrated a new method for triggering cancer cell apoptosis by magnetomechanical stimulation of cell membrane with ferromagnetic disks. The method for disks fabrication is described in [26–27]. Briefly, the disks were fabricated by photolithographic patterning of a negative photoresist to obtain 1 µm diameter pillars which were then coated with 60 nm layer of permalloy by electron beam evaporation. To facilitate the biofunctionalization of the disks, they were additionally coated with 5 nm layers of gold on each side. The particles were released from the photoresist by lift-off. Figure 1 shows the scanning electron micrographs of the disks before and after the release from the patterned photoresist.
Figure 1.
SEM micrographs of (a) ferromagnetic microdisks deposited on the patterned photoresist and (b) the microdisks after photoresist lift off. The inset shows a single microdisk. (c) Simulated spin vortex arrangement in a microdisk. (d) A room temperature magnetic hysteresis loop for a dried sample (red symbols) measured with VSM and MDs in acetone solution cooled in presence of 50 Oe magnetic field below freezing point of −95°C (black symbols). The data agree with the concept of magnetization reversal due to nucleation and annihilation of the magnetic vortices. In zero field (remanent state) the vortex core is in the center of each disk, whereas under magnetic field application the vortex shifts (reversibly) to develop the magnetization component parallel to the field (see two representative spin structures shown in the insert). The dashed blue area depicts the magnetic field range used in the cell experiments. (Adapted from [26, 28].
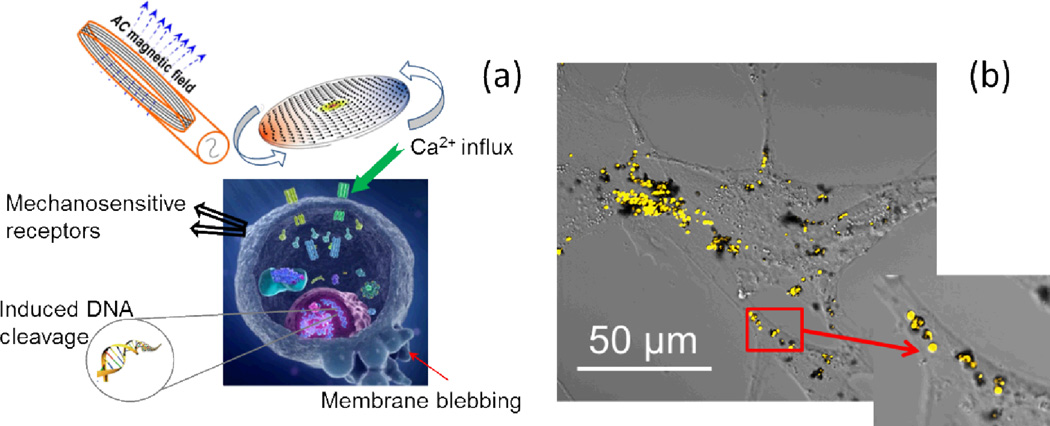
Targeted delivery of the disks to cancer cells was achieved by coating the disks with IL-13α2R antibody which is specific to the IL13α2 receptor expressed on human glioma cells. The schematic diagram of the experimental approach can be seen in Figure 2 (a). Figure 2(b) demonstrates the confocal image of A172 glioblastoma (human brain cancer) cells with functionalized microdisks on their surface. The inset shows the close up view of the disks. Following the incubation with the biofunctionalized disks and treatment with a weak low frequency a.c. field, the cells showed the signs of apoptotic degeneration 4 hours after treatment.
Figure 2.
(a) Schematic diagram of the principle of targeted stimulation of cell membrane with ferromagnetic disks. (b) The image showing magnetic disks on the cell surface. The excessive amount of disks was used for illustrative purposes. Close up view of single microdisks is shown in the inset image.
Magnetomechanical stimulation resulted in the increase of intracellular calcium which is likely to be the result of the mechanosensitive channel activation [26]. Membrane-bound ferromagnetic disks are likely to stretch the cell membrane thus actuating the mechanosensitive channels which respond to the change in membrane tension [29]. Stretch-activated mechanosensitive channels trigger the intracellular calcium response [30–31]. The inability of a cell to chemically alter calcium forces the cell to regulate its calcium concentration through compartmentalization, chelation, or extrusion [32–33]. The prolonged elevated intracellular calcium level triggers apoptosis since the cell is not able to sustain the impact of calcium disbalance [34]. Actin filaments of the cytoskeleton have recently been identified as the key responders to mechanical actuation of cell membrane [35–36].
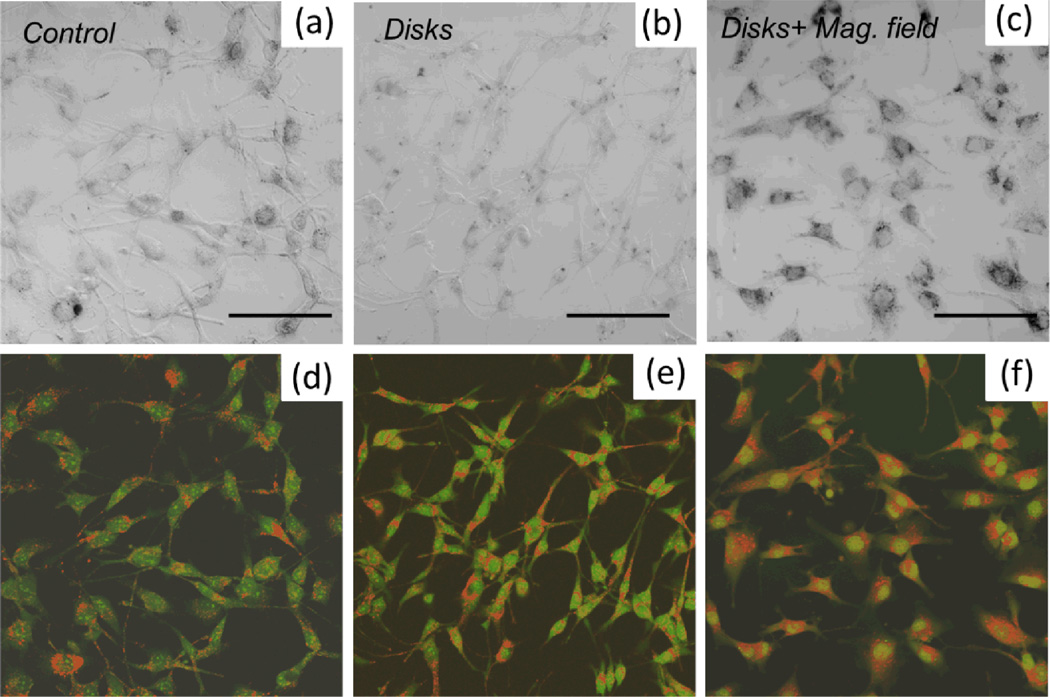
Similar effect was observed on human glioblastoma cells A172. The cells were stained with acridine orange (AO) and ethidium bromide (EB) to evaluate the apoptotic changes. The final concentration of each dye was 14µg/ml. AO intercalates into DNA making it appear green, whereas in cytoplasm it binds to RNA staining it red [37]. EB enters only non-viable cells with damaged membrane and it stains nucleus red. The cells were cultured in a standard DMEM medium supplemented with 10% fetal bovine serum in a humidified atmosphere at 37°C, 5% CO2. Three samples were prepared - 1) control without magnetic disks, 2) cells incubated with magnetic disks, 3) cells incubated with magnetic disks and subjected to a.c. magnetic field (10 Hz, 100 Oe) for 15 min. After the a.c. field treatment of the sample 3) all samples were incubated for 24 hours prior to the AO/EB staining. Figure 3 shows the differential interference contrast (DIC) and the corresponding confocal fluorescence images of the A172 glioblastoma cells. Only the cells which were exposed to a.c. magnetic field (panel (f)) exhibit the signs of early apoptosis as indicated by the enhanced nuclear staining. At the same time, by comparing the distribution of AO/EB staining in panels (d) and (e), one can conclude that culturing glioblastoma cells with magnetic disks present of their membrane does not compromise the cell viability. The absence of orange nuclear staining suggests that for the given cell line, the magnetomechanically induced apoptosis does not necessarily involve the rupture of the cell membrane since EB does not enter the nucleus, at least in the early stages.
Figure 3.
DIC image of (a) control human glioblastoma cells A172, (b) cells cultured with ferromagnetic disks, (c) cells cultured with magnetic disks and subjected to a.c. magnetic field. Scale bar 100 µm, 20x magnification objective. Each sample was stained with acridine orange/ethidium bromide for assessing the apoptotic changes. Panels (d–f) show the corresponding confocal fluorescence images. Only the cells which were exposed to a.c. magnetic field (panel (f)) exhibit the signs of early apoptosis as indicated by enhanced nuclear staining. At the same time, by comparing the distribution of AO/EB staining in panels (d) and (e), one can conclude that culturing glioblastoma cells with magnetic disks present of their membrane does not compromise the cell viability.
III. Dual magnetic field approach for expanding the range of frequency response
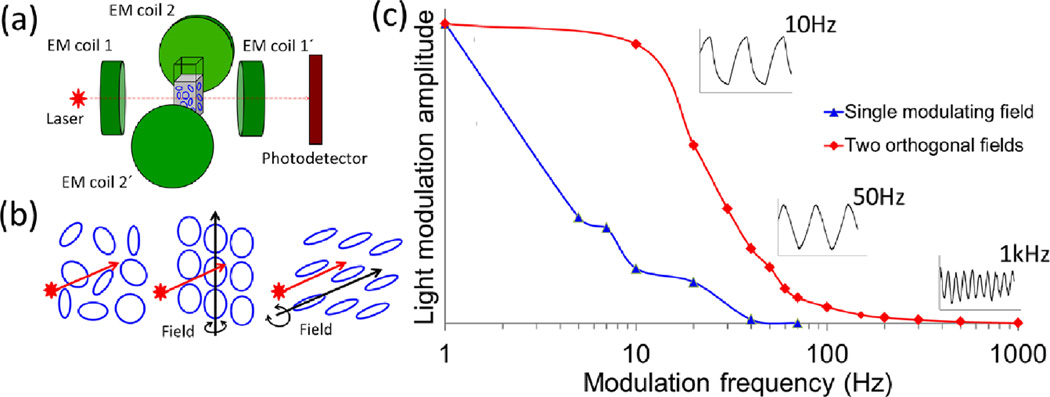
Magnetic methods allow for precise control over the forces applied to the cell membrane. These forces depend on the size of magnetic particles, the strength of the external field and the mechanical properties of the cell membrane. By controlling the dynamic behavior of the disks, one can potentially increase the efficiency of magnetic treatment for induction of cell apoptosis. In our original work, the disks’ rotation was controlled by a single magnetic field. Their dynamic behavior was investigated by monitoring the modulation of light intensity transmitted through the aqueous suspension containing ~107 disks/ml. The maximum modulation frequency achieved in this setup was approximately 60Hz. To expand the range of accessible frequencies, we employed a dual magnetic field setup by using two a.c. magnetic fields orthogonal to each other [28]. Figure 4(a) shows the schematic diagram of experimental setup and panel (b) illustrates the principle of the disks behavior depending on the direction of the applied magnetic field. Employing the second field leads to significant broadening of the modulation frequency range up to 1 kHz, as seen in Figure 4(c) The inset graphs demonstrate that the optical signal modulation is well resolvable even at higher frequencies for the dual-field setup. In this case, the relaxation dynamics of the disks is fully controlled by the magnetic field, thus eliminating the constraints of Brownian motion which otherwise poses a limitation on their frequency response. We are currently in the process of testing this method for in vitro cell studies.
Figure 4.
(a) Schematic diagram of dual-field setup. (b) Schematic diagram of the magnetic disks before (L) and after (M, R) the external field is switched on. (Middle) The field which is perpendicular to the illumination beam results in the decrease of the total transmitted light. The opposite effect is observed when the incident beam and the magnetic field are parallel (Right). (c) Normalized frequency dependent light modulation amplitude for single modulating magnetic field and two orthogonal magnetic fields. Addition of the second magnetic field results in significant broadening of the frequency modulation range. The inset graphs demonstrate the shape of the light intensity signal at different modulation frequencies for the dual field configuration. Adapted from [28].
IV. Magneto-mechanically-induced controlled drug release
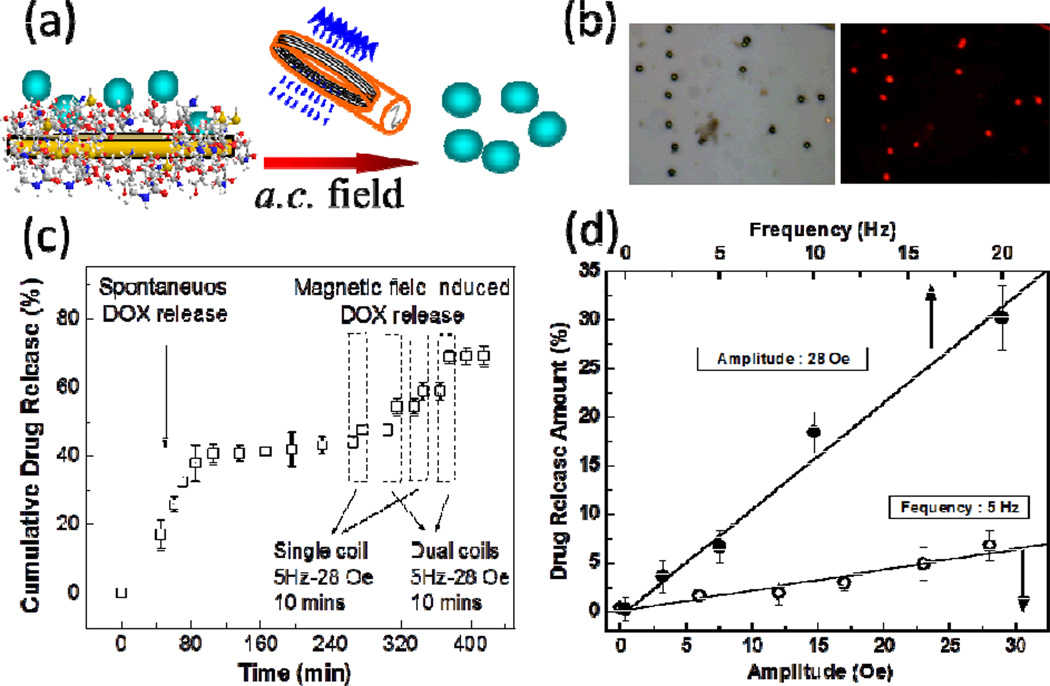
The dual field approach described in the previous section has also been proven to be advantageous for magnetically controlled drug release recently demonstrated by our group [38]. The microdisks were functionalized with chitosan polymer brushes and then loaded with anti-cancer drug doxorubicin (DOX) via entrapping the drug in between chitosan polymer chains on a surface of the disks, Figure 5(a–b). Chitosan brush in this case serves as the DOX binding agent and also facilitates the dispersion of the disks in solution. The cumulative drug release as a function of time for both single and dual field setups is shown in Figure 5(c). A certain amount of drug is released spontaneously without external stimulus application. However, once magneto-mechanical stimulus is applied, a drug is “shaken off” the disks carriers.. Moreover, an application of two orthogonal a.c. fields allows further increasing of the efficiency of the magneto-mechanically-induced DOX release which is likely to be due to larger disk rotation amplitude compared to that for the single field. In addition, the dose of magnetically delivered drug can be controlled by changing the amplitude and the frequency of magnetic field, as seen in Figure 5(d).
Figure 5.
(a) Schematic diagram of the a.c. field-induced drug release by magnetic disks–chitosan–DOX composites. (b) DOX-loaded chitosan–magnetic disks: (L) bright field and (R) fluorescent (Ex 543/Em
V. Ferromagnetic disks in hyperthermia and Magnetic Resonance Imaging
Hyperthermia is one of the widely explored methods for nanoparticle-assisted cancer treatment [39]. Laser-induced hyperthermia with plasmonic particles allows for fast elevation of temperature, however this method is complicated by the necessity of delivering laser light to the desired location in human body [40]. Near-infrared absorbing particles offer a partial solution to this problem, however their applications are still restricted by the tissue penetration depth limiting the access to tumor located in internal organs. Magnetically driven hyperthermia, on the other hand, can be performed non-invasively since the heating of magnetic particles can be remotely controlled [41]. Superparamagnetic particles have been traditionally employed for this application [22, 39, 42]. In our work, we demonstrate that ferromagnetic metal microdisks are also instrumental for hyperthermia when subjected to 100s kHz a.c. fields.
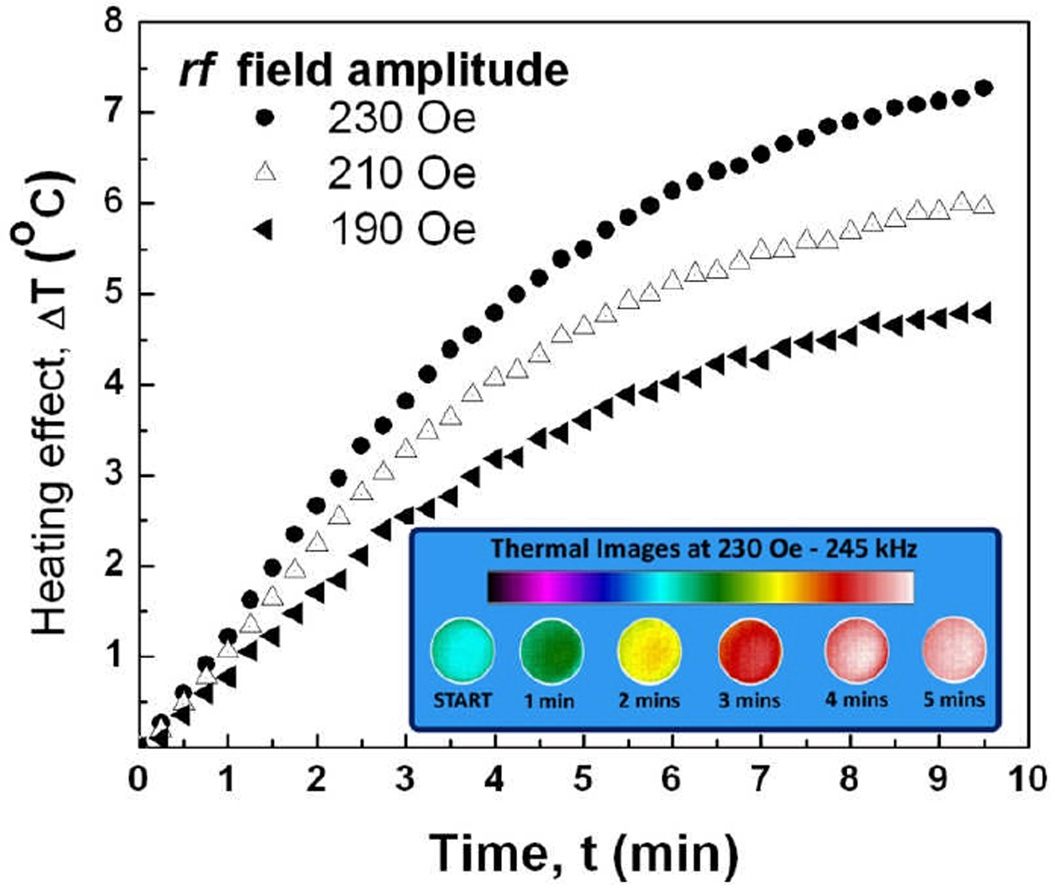
To demonstrate the proof-of-principle, we imaged 1ml of aqueous suspension of 1 µm diameter disks using IR camera. High frequency (240 kHz) a.c. magnetic field was applied for 10 min. The results shown in Figure 6 demonstrate that the disks are very efficient mediators of inductive heating and the temperature response can be further improved by using stronger magnetic fields. The specific absorption rate of magnetic disks was measured to be 121.6 W/g for 332 kHz, 141 Oe magnetic field which is comparable to that of Fe3O4 10 nm nanoparticles reported in the literature (120 W/g at 300 kHz at ~192Oe) [42].
Figure 6.
Heating temperature of ferromagnetic microdisks as a function of time at different magnitudes of the applied a.c. magnetic field. The inset shows calorimetric images of the sample acquired for 230 Oe, 245 kHz field.
Another facet of multifunctional ferromagnetic disks is their applicability in magnetic resonance imaging (MRI). Metal anisotropically-shaped particles have demonstrated advanced properties as MRI enhancement agents [43–44]. We have carried out preliminary MRI studies for comparing the effectiveness of ferromagnetic microdisks as MRI contrast agents with conventional Fe3O4 nanoparticles (11 nm diameter). The particles were visualized in polymer matrix (1% agarose gel serving as a tissue phantom). The concentrations of stock solutions of the magnetic disks and Fe3O4 were determined with Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES). Different concentrations of the magnetic materials were dispersed in the Phantom. MRI measurements of the samples were performed on a Bruker Biospec Avance 9.4 Tesla system.
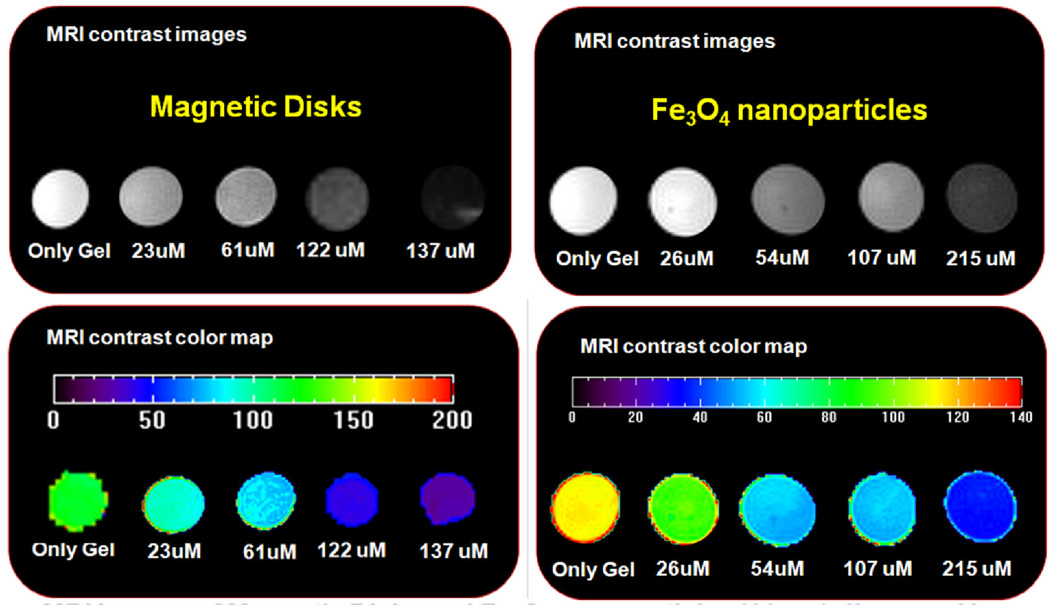
In MRI, the application of a transverse RF pulse results in proton perturbation [45]. The subsequent process through which these protons return to their original state is known as relaxation a phenomenon that is characterized by two independent processes – longitudinal relaxation (T1-recovery) and transverse relaxation (T2-decay). Local variation in relaxation due to the presence of magnetic nanoparticles results in contrast enhancement. Spin-echo pulse sequences with inversion recovery and multiple spin echoes of various time delays were utilized to obtain pixel-by-pixel T1 and T2 maps of each sample. T1 and T2 relaxation times were measured from large regions of interest and results were inverted to obtain the R1 and R2 relaxation rates. Figure 7 shows the resulting T2 images for both sample types. The relaxation rate for the magnetic disks was measured to be 140.2 mM−1S−1, whereas the Fe3O4 nanoparticles exhibited much lower rate of 83.1 mM−1S−1. The results demonstrate that due to significantly higher value of the magnetization of saturation, the ferromagnetic disks provide much stronger MRI enhancement than typically used iron oxide nanoparticles of the same volume concentration.
Figure 7.
MRI T2 images of magnetic disks and Fe3O4 nanoparticles (11 nm) dispersed in Phantom (1% agarose gel) with increasing concentrations.
VI. Conclusions
Magnetic materials hold a great promise for noninvasive control over cell function, including targeted drug delivery, hyperthermia, and imaging using magnetic resonance. Superparamagnetic particles were traditionally employed as primary magnetic carriers. However, their intrinsically low magnetization imposes a severe limitation on their applications. Recent application of the ferromagnetic particles with spin-vortex ground state as mediators of cellular mechanotransduction may represent the new development for the “spin-doctoring”. Superior properties, such as zero remanence, high saturation magnetization and strong magnetomechanical coupling along with developed technologies of their fabrication at controllable size, shape and composition make these particles promising multifunctional tools in the field of cell biology and medicine. Moreover, the state-of-the-art microfabrications techniques allow for scaling the dimensions of the disks down to less than 100 nm, while still preserving the vortex structure [46–47]. This opens a vast realm of opportunities available for employing the microdisk-based methods for in vivo applications.
ACKNOWLEDGMENT
We thank our collaborators Drs. S. D. Bader, T. Rajh, V. G. Yefremenko, R. Divan and Mr J. Pearson from Argonne National Laboratory and Prof. M. Lesniak from the University of Chicago Brain Tumor Center, for continued interest to this project. We also thank Dr. D.-H. Kim from the Northwestern University for assistance with RF heating and MRI experiments. The work at Argonne, including use of the Center for Nanoscale Materials was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357, and by in part by the by Grant Number R01NS077388 from the National Institute of Neurological Disorders And Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
References
- 1.Martinac B. Mechanosensitive ion channels: Molecules of mechanotransduction. J. Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 2.Heilbronn A. Eine neue methode zur bestimmung der viskositat lebender protoplasten (A new method for the estimation of viscosity in living protoplasts) Jahrb. Wiss. Bot. 1922;61:284–38. [Google Scholar]
- 3.Seifriz W. An elastic value of protoplasm, with further observations on the viscosity of protoplasm. J. Exp. Biol. 1924;2:1–11. [Google Scholar]
- 4.Crick FHC, Hughes AFW. The physical properties of cytoplasm—a study by means of the magnetic particle method. Exp. Cell Res. 1950;1:37–80. [Google Scholar]
- 5.Hiramoto Y. Mechanical properties of the protoplasm of the sea urchin egg. Exp. Cell Res. 1969;56:201–208. doi: 10.1016/0014-4827(69)90003-2. [DOI] [PubMed] [Google Scholar]
- 6.King M, Macklem PT. Rheological properties of microliter quantities of mucus. J. Appl. Physiol.: respire. Environ. Exercise Physiol. 1977;42:797–802. doi: 10.1152/jappl.1977.42.6.797. [DOI] [PubMed] [Google Scholar]
- 7.Gehr P, Brain JD, Bloom SB, Valberg PA. Magnetic particles in the liver: a probe for intracellular movement. Nature. 1983;302:336–338. doi: 10.1038/302336a0. [DOI] [PubMed] [Google Scholar]
- 8.Valberg PA, Albertini DF. Cytoplasmic motions, rheology and structure probed by a novel magnetic particle method. J. Cell Biol. 1985;101:130–40. doi: 10.1083/jcb.101.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adair RK. Constraints of biological effects of weak extremely-low-frequency electromagnetic fields. Phys. Rev. A. 43(2):1039–1048. doi: 10.1103/physreva.43.1039. [DOI] [PubMed] [Google Scholar]
- 10.Kirschvink JL. Constraints on biological effects of weak extremely-low-frequency electromagnetic fields—comment. Phys. Rev. A. 1992;46:2178–2184. doi: 10.1103/physreva.46.2178. [DOI] [PubMed] [Google Scholar]
- 11.Gould JL. Honey bee recruitment – dance-language controversy. Science. 1975;189(4204):685–693. doi: 10.1126/science.1154023. [DOI] [PubMed] [Google Scholar]
- 12.Walker MW, Bitterman ME. Conditioned responding to magnetic fields by honeybees. J. Comp. Physiol. A. 1985;157:67–71. [Google Scholar]
- 13.De Jong D. Orientation of comb building by honeybees. J. Comp. Physiol. 1982;147:495–501. [Google Scholar]
- 14.Kirschvink JL, Defeyes KS. Bees have magnetic remanence. Science. 1992;201:1026–1028. doi: 10.1126/science.201.4360.1026. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 16.Pommerenke H, Schreiber E, Durr F, Nebe B, Hahnel C, Moller W, Rychly J. Stimulation of integring receptors using a magnetic drag force device induces an intracellular free calcium response, Europ. J. Cell. Biology. 1996;70(2):157–64. [PubMed] [Google Scholar]
- 17.Chambers R, Fell HB. Micro-operations on cells in tissue cultures. Proc. R. Soc. Lond. B. 1931;109:380–403. [Google Scholar]
- 18.Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem. 1999;274(3):1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 19.Brohawn SG, delMármol J, MacKinnon R. Crystal Structure of the Human K2P TRAAK, a Lipid- and Mechano-Sensitive K+ Ion Channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobson J. Remote control of cellular behavior with magnetic nanoparticles. Nature Nanotech. 2008;3:139–143. doi: 10.1038/nnano.2008.39. [DOI] [PubMed] [Google Scholar]
- 21.Hughes S, McBain S, Dobson J, El Haj AJ. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface. 2008;5:855–863. doi: 10.1098/rsif.2007.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan KM. Biomedical nanomagnetics: a spin through new possibilities in imaging, diagnostics and therapy. IEEE Trans. Mag. Adv. in Mag. 2010;46:2523–2558. doi: 10.1109/TMAG.2010.2046907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankhurst QA, Thanh NK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys D-Appl Phys. 2009;42:224001. [Google Scholar]
- 24.C Berry C. Progress in functionalization of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl. Phys. 2009;42:224003. [Google Scholar]
- 25.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Kim D-H, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, Novosad V. Nature Materials. 2010;9:165–171. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozhkova EA, Novosad V, Kim D-H, Pearson J, Divan R, Rajh T, Bader SD. J. Appl. Phys. 2009;105:07B306. [Google Scholar]
- 28.Vitol EA, Yefremenko VG, Jain S, Pearson J, Rozhkova EA, Bader SD, Novosad V. Optical transmission modulation by disk-shaped ferromagnetic particles. J. Appl. Phys. 2012 [Google Scholar]
- 29.Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol. Rev. 1998;78(3):763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 30.Sigurdson W, Ruknudin A, Sachs F. Calcium imaging of mechanically induced fluxes in tissue-cultured chick heart: role of strerch-activated ion channels. Am. J. Physiol. Heart and Circulatory Physiology. 1992;262(4):H1110–H1115. doi: 10.1152/ajpheart.1992.262.4.H1110. [DOI] [PubMed] [Google Scholar]
- 31.Yang XC, Sachs F. Block of stretch-activated ion channels in oocytes by gadolinium and calcium ions. Science. 1989;243(4894):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 32.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 33.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 34.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nature Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J. Cell. Science. 2008;121:496–503. doi: 10.1242/jcs.022053. [DOI] [PubMed] [Google Scholar]
- 36.Orynbayeva Z, Singhal R, Vitol EA, Schrlau MG, Papazoglou E, Friedman G, Gogotsi Y. Physiological validation of cell health upon probing with carbon nanotube endoscope and its benefit for single-cell interrogation. Nanomedicine: Nanotechnology, Biology and Medicine. 2011 doi: 10.1016/j.nano.2011.08.008. In press. [DOI] [PubMed] [Google Scholar]
- 37.Mercille S, Massie B. Induction of apoptosis in nutrient-deprived cultures of hybridoma and myeloma cells. Biotech. Bioeng. 1994;44:1140–1154. doi: 10.1002/bit.260440916. [DOI] [PubMed] [Google Scholar]
- 38.Kim D-H, Karavayev P, Rozhkova EA, Pearson J, Yefremenko V, Bader SD, Novosad V. Mechanoresponsive system based on sub-micron chitosan-functionalized ferromagnetic disks. J. Mater. Chemistry. 2011;21:8422. [Google Scholar]
- 39.Rozhkova EA. Nanoscale materials for tackling brain cancer: Recent Progress and Outlook. Adv. Mater. 2011;23:H136–H150. doi: 10.1002/adma.201004714. [DOI] [PubMed] [Google Scholar]
- 40.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near-infrared absorbing nanoparticles. Cancer Letters. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Jordan A, Scholz R, Wust P, Fahling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Mag. Mag. Mater. 1999;201(1–3):413–419. [Google Scholar]
- 42.Pradhan P, Giri J, Samanta G, Sarma HD, Mishra KP, Bellare J, Bannerjee R, Bahadur D. Comparative evaluation of heating ability and biocompatibility of difference ferrite-based magnetic fluids for hyperthermia application. J. Biomed. Mat. Res. Pt B: Appl. Biomat. 2006;27:12–22. doi: 10.1002/jbm.b.30630. [DOI] [PubMed] [Google Scholar]
- 43.Zabow G, Dodd S, Moreland J, Koretsky A. Micro-engineered local field control for high-sensitivity multispectral MRI. Nature. 2008;453:1058. doi: 10.1038/nature07048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zabow G, Dodd SJ, Moreland J, Koretsky AP. The fabrication of uniform cylindrical nanoshells and their use as spectrally tunable MRI contrast agents. Nanotechnology B. 2009:385301. doi: 10.1088/0957-4484/20/38/385301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merbach AE, Toth E, editors. The chemistry of contrast agents in medical magnetic resonance imaging. Wiley: 2001. p. 300. [Google Scholar]
- 46.Scholz W, Yu Guslienko K, Novosad V, Suess D, Schrefl T, Chantrell RW, Fidler J. Transition from single-domain to vortex state in soft magnetic cylindrical nanodots. J. Magn. Magn. Mater. 2003;266:155–163. [Google Scholar]
- 47.Koh AL, Hu W, Wilson RJ, Earhart CM, Wang S, Sinclair R. Structural and magnetic characterizations of high moment synthetic antiferromagnetic nanoparticles fabricated using self-assembled stamps. J. Appl. Phys. 2010;107:09B522. doi: 10.1063/1.3358067. [DOI] [PMC free article] [PubMed] [Google Scholar]