Abstract
Iron deficiency anemia is the most common form of anemia worldwide, caused by poor iron intake, chronic blood loss, or impaired absorption. Patients with inflammatory bowel disease (IBD) are increasingly likely to have iron deficiency anemia, with an estimated prevalence of 36%–76%. Detection of iron deficiency is problematic as outward signs and symptoms are not always present. Iron deficiency can have a significant impact on a patient’s quality of life, necessitating prompt management and treatment. Effective treatment includes identifying and treating the underlying cause and initiating iron replacement therapy with either oral or intravenous iron. Numerous formulations for oral iron are available, with ferrous fumarate, sulfate, and gluconate being the most commonly prescribed. Available intravenous formulations include iron dextran, iron sucrose, ferric gluconate, and ferumoxytol. Low-molecular weight iron dextran and iron sucrose have been shown to be safe, efficacious, and effective in a host of gastrointestinal disorders. Ferumoxytol is the newest US Food and Drug Administration-approved intravenous iron therapy, indicated for iron deficiency anemia in adults with chronic kidney disease. Ferumoxytol is also being investigated in Phase 3 studies for the treatment of iron deficiency anemia in patients without chronic kidney disease, including subgroups with IBD. A review of the efficacy and safety of iron replacement in IBD, therapeutic considerations, and recommendations for the practicing gastroenterologist are presented.
Keywords: anemia, inflammatory bowel disease, intravenous iron, iron deficiency, oral iron, therapy
Introduction
Iron deficiency anemia is the most common form of anemia worldwide, representing up to 50% of all cases.1 In the United States, approximately 2% of men and 5% of women are believed to be affected.2 Causes of iron deficiency include poor iron intake, chronic blood loss, impaired absorption, or any combination of the three (Table 1).3–5 Blood loss from the gastrointestinal (GI) tract is the most common cause in men and postmenopausal women and is a common reason for referral to a gastroenterologist.6
Table 1.
| Common causes of iron deficiency anemia | |
|---|---|
| Increased iron loss |
|
| Decreased iron absorption (malabsorption) |
|
| Increased demand for iron |
|
| Decreased iron intake |
|
Abbreviation: GI, gastrointestinal.
In a variety of populations with inflammatory bowel disease (IBD), the prevalence of iron deficiency anemia ranges from 36%–76%.7 Iron is an essential mineral for the function of all body cells and is absorbed at the apical surface of enterocytes to be transported by ferroportin, the only known iron exporter, across the basolateral surface of the enterocyte into circulation.7 Inflammation from IBD interferes with iron absorption by causing an increase in hepcidin, a peptide hormone synthesized in the liver that inhibits ferroportin activity.8 A deficiency of iron can have a significant impact on a patient’s quality of life,9,10 and is associated with diminished exercise tolerance in patients with chronic heart failure.11 Appropriate diagnosis and treatment of iron deficiency anemia are important for the practicing gastroenterologist to improve or maintain the quality of life of patients.4 The purpose of this review is to increase awareness of iron deficiency anemia as a complex condition that requires specific diagnostic and therapeutic approaches and to review the diagnosis and management of iron deficiency anemia in patients with IBD.
Diagnosis of iron deficiency anemia
Iron deficiency anemia may present with symptoms of fatigue and pallor, although these clinical findings are insufficient to establish a diagnosis.12 Rockey and Cello,13 investigating 100 sequential patients with iron deficiency anemia, noted that 62 patients had at least one GI tract lesion with the potential for blood loss. Triester et al,14 utilizing capsule endoscopy, found a 63% yield in obscure GI bleeding while investigating iron deficient patients who had negative esophagogastroduodenos-copies and colonoscopies. The predominant findings were angiodysplasia and Crohn’s disease. Since a large proportion of adult men and postmenopausal women presenting with iron deficiency are not found to have a source of bleeding, malabsorption of iron should be considered as a possible cause in addition to frequent blood donation (Table 1).3–5
Considering the decreased likelihood of outward signs and symptoms, greater vigilance on the part of clinicians is warranted. Diagnostic laboratory tests include a complete blood count and serum iron indices (Table 2).12,15,16 The World Health Organization defines anemia as a hemoglobin level <13 g/dL in men and <12 g/dL in nonpregnant women.17 However, serum iron values alone are not helpful to diagnose iron deficiency, as iron levels are known to vary with the time of day,18 as well as due to systemic causes.3 Low serum ferritin, low transferrin saturation, low serum iron, and increased iron-binding capacity indicate a state of iron deficiency.12 In the absence of inflammation, serum ferritin is the most powerful test for iron deficiency, with values of <12–15 μg/L considered to be diagnostic.19,20 Serum ferritin can be artificially elevated in any chronic inflammatory process, including IBD, infections, malignancies, liver disease, or chronic renal failure.19,21 Higher levels of serum ferritin do not exclude the possibility of iron deficiency, and a serum ferritin level of ≤100 μg/L may still be consistent with iron deficiency in patients with IBD.15 A transferrin saturation of <16% is indicative of iron deficiency, either absolute or functional.15 Other findings on a complete blood count panel that are suggestive of iron deficiency anemia, but are not considered diagnostic, include microcytosis, hypochromia, and elevation of red cell distribution width.12,20 If laboratory values do not prove to be conclusive and suspicion of iron deficiency anemia remains, then a bone marrow aspirate and stain with low iron is considered by some to be evidence of a definitive diagnosis.12 Laboratory findings that distinguish iron deficiency anemia, anemia of chronic disease (also known as iron-restricted erythropoiesis and functional iron deficiency), and anemia of mixed origin are presented in Table 3. Hepcidin-25 assays that can better distinguish between iron deficiency anemia and anemia of chronic disease are currently being investigated.22,23
Table 2.
| Test | Applicability |
|---|---|
| Complete blood count |
|
| Serum ferritin |
|
| Other | |
|
|
Note:
Serum ferritin levels of 50–100 μg/L may be indicative of iron deficiency in IBD.
Abbreviation: IBD, inflammatory bowel disease.
Table 3.
Differential diagnosis of iron deficiency anemia, anemia of chronic disease, and anemia of mixed origin
| Iron deficiency anemia | Anemia of chronic disease | Anemia of mixed origin | |
|---|---|---|---|
| Serum ferritina | Decreased | Normal or increased | Normal |
| Serum iron | Decreased | Decreased | Decreased |
| Transferrin | Increased | Decreased or normal | Decreased |
| Transferrin saturation | Decreased | Decreased | Decreased |
| Mean corpuscular volume | Decreased | Decreased or normal | Decreased or normal |
| Hemoglobin | Decreased | Decreased | Decreased |
| Iron-binding capacity | Increased | Decreased | Decreased to low normal |
| Serum transferrin receptor | Increased | Normal | Increased or normal |
| Serum transferrin receptor index | High (>2) | Low (<1) | High (>2) |
| C-reactive protein | Normal | Increased | Increased |
| Erythropoietin | Increased | Normal or slightly increased | Increased or normal |
| Cytokine levels | Normal | Increased | Increased |
Notes:
The normal range of ferritin values is between 30–100 μg/L. Copyright © 2009, World Journal of Gastroenterology. Adapted with permission from [Bermejo F, García- López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15(37):4638–4643].4 Adapted by permission from Macmillan Publishers Ltd: [Nature Reviews Gastroenterology and Hepatology]. Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7(11):599–610. Copyright 2010.7
Goals of iron deficiency anemia therapy
Anemia is one of the most frequent comorbid conditions associated with mortality in patients with IBD,24 and it is a common cause of hospitalization and delay of discharge when hospitalized,15 which underscores the need for prompt diagnosis and management. The goals of treatment are to treat the underlying cause, limit further blood loss or malabsorption, avoid blood transfusions in hemodynamically stable patients, relieve symptoms, and improve quality of life.15,25 More specifically, therapeutic goals of treatment include normalizing hemoglobin levels within 4 weeks (or achieving an increase of >2 g/dL) and replenishing iron stores (transferrin saturation >30%).7
Management of iron deficiency anemia
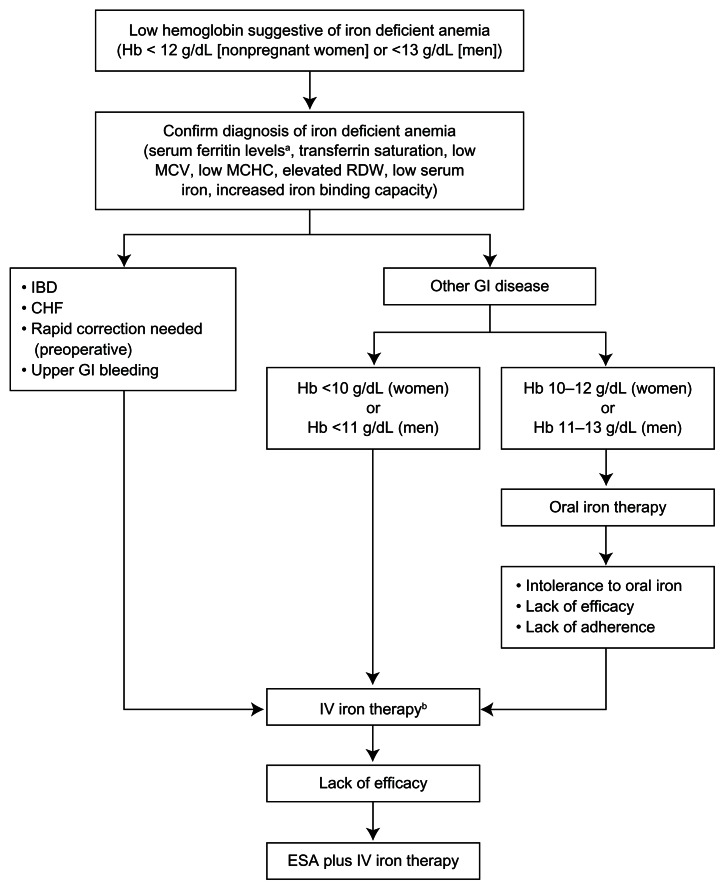
In addition to treating underlying causes, iron supplementation should be administered to all patients with iron deficiency anemia.15,26 An algorithm for the management of iron deficiency anemia in patients with IBD and other GI diseases is shown in Figure 1.
Figure 1.
Algorithm for the management of iron deficiency anemia in patients with IBD and other GI diseases.
Notes:aHigher levels of serum ferritin do not exclude the possibility of iron deficiency. Serum ferritin <100 μg/L may still be consistent with iron deficiency in patients with IBD;15bonly iron dextrans have a broad indication for the treatment of iron deficiency anemia.
Adapted by permission from Macmillan Publishers Ltd: [Nature Reviews Gastroenterology and Hepatology]. Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7(11):599-610, Copyright 2010.7
Abbreviations: Hb, hemoglobin; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; IBD, irritable bowel disease; CHF, congestive heart failure; GI, gastrointestinal; IV, intravenous; ESA, erythropoiesis-stimulating agent.
Oral iron therapy
Oral iron supplementation has been considered standard because of an established safety profile, lower cost, and ease of administration. It has been shown to be effective in correcting anemia and repleting iron stores.27,28 The most widely used oral iron supplements – ferrous fumarate, ferrous sulfate, and ferrous gluconate – contain the ferrous form of iron (Table 4). These agents differ by the amount of elemental iron available for absorption. Ferrous fumarate, ferrous sulfate, and ferrous gluconate contain 33%, 20%, and 12% of elemental iron, respectively.29 For example, a 325 mg tablet of ferrous sulfate contains 65 mg of elemental iron.
Table 4.
Comparison of oral iron preparations available in the United States
| Oral iron preparation | Brand name(s) | Recommended dose | Elemental iron (mg) |
|---|---|---|---|
| Ferrous sulfate | Feosol®, Fer-Gen-Sol®, Fer-in-Sol®, Fer-Iron | 325 mg three times/day | 65 |
| Ferrous gluconate | Fergon® | 325 mg three times/day | 38 |
| Ferrous fumarate | Femiron, Ferretts, Ferro-Sequels®, Nephro-Fer® | 324 mg three times/day | 106 |
Note: Adapted by permission from Macmillan Publishers Ltd: [American Journal of Gastroenterology]. Rizvi S, Schoen RE. Supplementation with oral vs intravenous iron for anemia with IBD or gastrointestinal bleeding: is oral iron getting a bad rap? Am J Gastroenterol. 2011;106(11):1872–1879. Copyright 2011.61
The Centers for Disease Control and Prevention recommended that the dose of elemental iron for the prevention of iron deficiency anemia be 30 mg/day, with 60–120 mg/day recommended for treatment.30 However, the optimal dose of oral iron has not been established in IBD.15 Oral iron therapy for all patients with iron deficiency anemia is recommended to be continued for up to 5 months,7 or for at least 3 months, after the replenishment of iron stores.26
One concern with higher doses of daily oral iron is intolerance due to GI side effects. Symptoms include nausea, vomiting, diarrhea, abdominal pain, constipation, and melena-like stools.28 In a study of 57 patients, intolerance of oral iron resulted in discontinuation for approximately 20% of patients with and without IBD.31 The dark green or black stools seen with oral iron therapy can mimic melena. Because of a higher likelihood of GI events with oral iron and evidence from studies demonstrating efficacy at lower doses, supplementation with no more than 100 mg/day has been recommended in patients with IBD.7,15 Sustained-release formulations developed to help reduce GI intolerances are not recommended due to reduced absorption of oral iron.12
When considering oral iron therapy in patients with IBD, one must take into account the impact of worsening of existing inflammation. Results of a literature review of anemia in Crohn’s disease illustrated that oral iron exacerbates intestinal inflammation and colon carcinogenesis in animals with colitis.32 Proposed mechanisms of increased inflammation include oxidative stress and alteration of the bacterial milieu of the gut.33 Clinicians should consider the impact of increased hepcidin levels on the efficacy of oral iron therapy in patients with IBD. In patients with inflammatory conditions and presumed increases in hepcidin, intravenous (IV) iron may be the preferred choice of treatment compared with oral iron therapy. Other considerations with oral iron therapy include concomitant medications, foods, and drinks that interact with iron. Medications that raise gastric pH (ie, antacids, H2-blockers, and proton pump inhibitors) can limit the intestinal absorption of iron.34 In addition, phytates from bran and tannates from tea also limit iron absorption.35,36 In patients with IBD, the reduced absorption and intolerance of oral iron therapy support an earlier use of IV iron in the treatment paradigm of iron deficiency anemia in IBD.
Intravenous iron therapy
IV iron is more efficacious than oral iron in IBD because of low iron absorption from the GI tract and lower adherence to oral therapy.3 Parenteral iron products consist of nanoparticles of iron oxyhydroxide gel in colloidal suspension held within a stabilizing carbohydrate shell.37 Available preparations in the United States include high molecular weight iron dextran (HMWD; DexFerrum®, American Regent, Inc, Shirley, NY, USA),38 low molecular weight iron dextran (LMWD; INFeD®, Watson Pharma, Inc, Morristown, NJ, USA),39 iron sucrose (Venofer®; American Regent, Inc),40 ferric gluconate (Ferrlecit®; Sanofi-Aventis, Bridgewater, NJ, USA),41 and ferumoxytol (Feraheme®; AMAG Pharmaceuticals, Inc., Lexington, MA, USA).42 A comparison of IV iron preparations and their dosing, administration, and safety profiles is provided in Table 5.
Table 5.
| LMWD | HMWD | Iron sucrose | Ferric gluconate | Ferumoxytol | |
|---|---|---|---|---|---|
| Brand name | INFed® | DexFerrum® | Venofer® | Ferrlecit® | Feraheme® |
| Molecular weight (kD) | 73 | 165 | 43.3 | 37.5 | 731 |
| Labeled indication | Iron deficiency anemiaa | Iron deficiency anemiaa | Iron deficiency anemia in patients with CKD | Iron deficiency anemia in adults and children aged ≥6 years with CKD and receiving dialysis and supplemental epoetin | Iron deficiency anemia in adults with CKD |
| Dosing and administration | |||||
| Test dose required | Yes Initial test dose of 0.5 mL required |
Yes Initial test dose of 0.5 mL required |
No | No | No |
| Adult recommended dose | According to LBW and observed Hb concentration: dose (mL) = 0.0442 (desired Hb − observed Hb) × LBW + (0.26 × LBW) eg, 70 kg adult with Hb range of 3–10 g/dL will require 55–33 mLb | According to LBW and observed Hb concentration: dose (mL) = 0.0442 (desired Hb − observed Hb) × LBW + (0.26 × LBW) eg, 70 kg adult with Hb range of 3–10 g/dL will require 55–33 mL | HDD-CKD: 100 mg slow IV injection (2–5 min) or infusion of 100 mg in max 100 mL normal saline over 15 min at each dialysis session for a total cumulative dose of 1000 mg NDD-CKD: 200 mg slow IV injection (2–5 min) on five separate occasions over 14 days for a total cumulative dose of 1000 mg PDD-CKD: total cumulative dose of 1000 mg by slow infusion in three divided doses over 28 days |
125 mg in 10 mL diluted in 100 mL normal saline for IV infusion over 1 hour. Most pts will require a cumulative dose of 1000 mg delivered over eight sessions at sequential dialysis treatments | 510 mg followed by a second 510 mg dose 3–8 days later administered undiluted as IV injection up to 1 mL/sec |
| Dosage adjustments | None | None | None | Lower doses may be required in elderly patients | None |
Note:
Only LMWD and HMWD have a broad indication for the treatment of iron deficiency anemia;
anecdotally, total doses of 1 to 1.5 g LMWD are commonly used in clinical practice.
Abbreviations: LMWD, low molecular weight dextran; HMWD, high molecular weight dextran; CKD, chronic kidney disease; LBW, lean body weight; Hb, hemoglobin; HDD-CKD, hemodialysis dependent-chronic kidney disease; IV, intravenous; NDD-CKD, nondialysis dependent-chronic kidney disease; PDD-CKD, peritoneal dialysis-chronic kidney disease.
A common concern regarding the choice between IV and oral iron is the risk of serious and potentially life-threatening hypersensitivity reactions with IV iron. The incidence of severe adverse effects is lower with LMWD and newer IV iron formulations compared with HMWD.3 LMWD and HMWD require the administration of a test dose of 0.5 mL over at least 30 seconds for LMWD and at least 5 minutes for HMWD to assess for anaphylactic reactions.38,39 It is recommended that patients be observed for at least 1 hour following the test dose.38,39 Serious adverse reactions requiring intervention are rare and usually occur after the test dose but can occur during the infusion. The signs of anaphylaxis include sudden onset of difficult breathing and cardiovascular collapse.38,39 Patients with a history of multiple drug allergies are believed to be at increased risk for anaphylaxis with IV iron.38,39 It is recommended that IV iron be administered only where therapies and personnel trained in resuscitation and the management of anaphylaxis or hypersensitivity reactions are readily available.38–42
The administration of any of the available iron preparations can be associated with acute chest and back tightness, without accompanying hypotension, tachypnea, tachycardia, wheezing, stridor, and periorbital edema.43 These minor adverse events abate without therapy, rarely recur with rechallenge, and are more common in patients with an allergic diathesis.44 Tryptase levels, a marker for mast cell degranulation, are routinely found to be elevated in anaphylaxis,45 and can serve as a simple test to distinguish between anaphylaxis and a nonspecific reaction.
A minority of patients will experience self-limited arthralgias and myalgias the day after iron infusions. These reactions typically abate without therapy and administration of nonsteroidal anti-inflammatory drugs may shorten their duration. Delayed reactions may also occur with LMWD and HMWD after larger infusions up to 24–48 hours after administration.38,39 Delayed reactions include arthralgia, myalgia, chills, fever, dizziness, nausea, and vomiting.38,39 Physicians frequently premedicate patients with diphenhydramine prior to the administration of LMWD. Premedication with diphenhydramine and acetaminophen prior to iron dextran infusions has been shown to reduce adverse events.46 In a single institution study, the adverse event rate per iron infusion was 4.4% (four events in 91 infusions) with premedication using diphenhydramine and acetaminophen compared with 12.3% (ten events in 81 infusions) without premedication (P = 0.054).46 However, in a study by Auerbach et al47 in 396 iron-deficient patients receiving 570 infusions of 1 g of LMWD in 1 hour without premedication (patients with allergic diathesis received pretreatment steroids), no serious adverse events were reported and only 2.3% of patients had an adverse event requiring treatment.
Ferric gluconate and iron sucrose are two iron salts indicated for the treatment of iron deficiency anemia in adult patients with chronic kidney disease (CKD). Ferric gluconate is indicated for patients with CKD receiving hemodialysis and supplemental epoetin therapy and can be given to pediatric patients aged ≥6 years old. Ferric gluconate and iron sucrose have the advantage of not needing the administration of a test dose; it is recommended that patients be observed for 30 minutes after administration.40,41
Ferumoxytol is the newest approved IV product in the United States and was recently approved in the European Union and Canada. It is currently indicated for the treatment of iron deficiency anemia in adults with CKD to be administered as a 510 mg bolus in >17 seconds. However, administration in around 1 minute has been recommended. 48 Ferumoxytol does not require a test dose and it does not require dilution for slow IV use, in contrast to iron sucrose and sodium ferric gluconate. Full iron repletion with ferumoxytol can be achieved with two sessions, compared with as many as three to ten sessions with iron sucrose.40,49,50 Ferumoxytol, like all IV iron products, can cause severe hypersensitivity reactions and patients should be observed for 30 minutes after administration.42 Other adverse events of ferumoxytol include nausea, dizziness, hypotension, and peripheral edema. IV preparations currently undergoing clinical investigation in the United States include ferric carboxymaltose (Injectafer®/Ferinject®; Luitpold Pharmaceuticals, Inc, Shirley, NY, USA) and iron isomaltoside 1000 (Monofer®; Pharmacosmos A/S, Holbaek, Denmark).
Intravenous iron therapy in patients with IBD
Several smaller trials have investigated the use of LMWD for the treatment of iron deficiency anemia in patients with IBD. In a single-arm study of 50 adult patients, LMWD was associated with an increase in hemoglobin of 1.7 g/dL from baseline after 4 weeks of therapy. Four patients experienced an adverse reaction to the test infusion dose, and one patient experienced an allergic reaction after the total dose was infused.51 None of these reactions left any residual effects. In a case-matched study comparing the efficacy and safety of LMWD with oral iron, patients treated with LMWD were found to have significantly greater increments in hemoglobin from baseline after 8 weeks of therapy compared with oral iron (2.0 g/dL versus 0.6 g/dL; P < 0.0001).52 A total of 15% (5/33) of patients treated with oral iron experienced GI side effects, while 5.7% (2/35) of patients experienced an anaphylactic reaction to a test dose of LMWD.52
Two randomized controlled studies have compared the efficacy and safety of IV iron sucrose to oral iron therapy in patients with IBD.53,54 In one 20-week study of 91 patients with IBD, significantly more patients randomized to receive IV iron sucrose completed treatment compared with oral iron therapy (96% versus 76%; P = 0.0009), and more patients had increased hemoglobin ≥2.0 g/dL, although the difference was not statistically significant (66% versus 47%; P = 0.07).53 A second smaller study of 6 weeks’ duration compared a single dose of iron sucrose 7 mg/kg followed by five infusions of 200 mg each week (n = 22) versus oral iron sulfate at 100–200 mg daily for 6 weeks (n = 24). A comparable median increase in hemoglobin levels was observed in both treatment groups.54 Fewer patients randomized to receive iron sucrose discontinued the study due to side effects compared with oral iron therapy (4.5% versus 20.8%).54 No clinical trials to date have investigated the use of ferric gluconate for the treatment of iron deficiency anemia in patients with IBD.
Ferric carboxymaltose is approved for use in Europe only and has been shown to be effective and safe in IBD-associated iron deficiency anemia in two randomized studies.55,56 In a study by Kulnigg et al,56 patients were randomized 2:1 to receive IV ferric carboxymaltose up to a weekly maximum of 1000 mg (or 15 mg/kg if body weight was <65 kg) until the calculated iron deficit by the Ganzoni formula7,57
was reached, or until they received oral iron sulfate 100 mg twice daily for 12 weeks.56 At 12 weeks, ferric carboxymaltose was found to be noninferior to iron sulfate for the primary endpoint of the change from baseline in hemoglobin (3.6 g/dL versus 3.0 g/dL; P = 0.6967).56 More patients discontinued iron sulfate treatment than ferric carboxymaltose by the end of the study (7.9% versus 1.5%; P = 0.057), and there was no statistically significant difference in the adverse event profile detected between groups (P = 0.0693).56 A second study compared three infusions of 1000 or 500 mg of ferric carboxymaltose to 200 mg infusions of iron sucrose based on Ganzoni-calculated iron deficit up to a maximum of 11 infusions.55 More patients treated with ferric carboxymaltose achieved the primary endpoint of a hemoglobin response of ≥2 g/dL compared to those treated with iron sucrose (65.8% versus 53.6%; P = 0.004). Overall, there was no statistically significant difference in the incidence of any adverse event for ferric carboxymaltose or iron sucrose (P = 0.413), although significantly more hypophosphatemia occurred in the ferric carboxymaltose group (2.5% versus 0.0%; P = 0.030).55
Phase 3 clinical trials are currently ongoing to evaluate the use of ferumoxytol in patients with iron deficiency anemia, including subgroups of patients with IBD (ClinicalTrials.gov identifier: NCT01114139, NCT01114217, and NCT01114204. A Phase 3 clinical trial of iron isomaltoside (NCT01017614) and a Phase 4 study of iron sucrose (NCT01067547) are currently recruiting patients with iron deficiency anemia and IBD.
Indications of IV iron therapy
Guidelines on the Diagnosis and Management of Iron Deficiency and Anemia in Inflammatory Bowel Diseases recommend IV iron therapy over oral iron supplementation in the treatment of iron deficiency anemia in patients with IBD, citing faster and prolonged response to treatment, decreased irritation of existing GI inflammation, improved patient tolerance, and improved quality of life.15 Patients with severe anemia (hemoglobin level of <10 g/dL), failure to respond or intolerance to oral iron therapy, severe intestinal disease, or patients receiving concomitant erythropoietin are recommended indications for IV iron therapy (Table 6).15 Other conditions where patients should be considered for first-line IV therapy over oral therapy include congestive heart failure, upper GI bleeding, and in situations where rapid correction of anemia may be required.3,7
Table 6.
Indications for IV iron therapy in patients with GI disease
|
When to consider using IV iron as first-line therapy
|
Abbreviations: IV, intravenous; GI, gastrointestinal.
Data for the management and follow-up of IBD patients with iron deficiency anemia who have been successfully treated with IV iron is lacking. It is recommended to measure hemoglobin levels within 4 weeks in patients who have symptomatic anemia in order to gauge the therapeutic response.15 An increase in hemoglobin of 2 g/dL or normalization of hemoglobin levels is considered to be a positive response to therapy.15 Monitoring of serum ferritin levels every 3 months for at least 4–6 weeks after therapy with a target of >400 μg/L has also been proposed.7,58
Conclusion
The major goal in treating patients with IBD and iron deficiency anemia is to correct the underlying cause of anemia, increase hemoglobin levels to normal values, and replenish iron stores. Iron deficiency anemia may significantly impact patients’ quality of life and morbidity. Although some patients with IBD may respond to oral iron supplementation, recent evidence from clinical studies and clinical experience supports that IV iron repletion is preferable. Although few head-to- head clinical trials have been conducted, newer IV iron preparations are more convenient with rapid infusion times and no requirement for test dosing. Patients with IBD should be checked for iron deficiency whenever they are anemic and have serum ferritin levels of <100 μg/L, which is consistent with iron deficiency in IBD.
Acknowledgments
Maria McGill, RPh, CMPP, and Robert Schupp, PharmD, of inScience Communications, Springer Healthcare, provided medical writing support funded by AMAG. Dr Goldberg would like to thank Michael Auerbach, MD, for his review and commentary during the preparation of this manuscript. The development of this manuscript was supported by AMAG.
Footnotes
Disclosure
Dr Goldberg has served on the Speaker’s Bureau for Salix Pharmaceuticals previously. The author reports no other conflicts of interest in this work.
References
- 1.World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277(12):973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 3.Bayraktar UD, Bayraktar S. Treatment of iron deficiency anemia associated with gastrointestinal tract diseases. World J Gastroenterol. 2010;16(22):2720–2725. doi: 10.3748/wjg.v16.i22.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermejo F, García-López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15(37):4638–4643. doi: 10.3748/wjg.15.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidelines and Protocols Advisory Committee on behalf of the Medical Services Commission, British Columbia Medical Association. Iron Deficiency – Investigation and Management. 2010. [Accessed April 26, 2012]. [updated June 15, 2010]. Available from: http://www.bcguidelines.ca/pdf/iron_deficiency.pdf.
- 6.McIntyre AS, Long RG. Prospective survey of investigations in outpatients referred with iron deficiency anaemia. Gut. 1993;34(8):1102–1107. doi: 10.1136/gut.34.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7(11):599–610. doi: 10.1038/nrgastro.2010.151. [DOI] [PubMed] [Google Scholar]
- 8.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzi LT, Weston CM, Goldfarb NI, et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(1):47–52. doi: 10.1097/01.mib.0000191670.04605.e7. [DOI] [PubMed] [Google Scholar]
- 10.Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12(2):123–130. doi: 10.1097/01.MIB.0000196646.64615.db. [DOI] [PubMed] [Google Scholar]
- 11.Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58(12):1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 12.Killip S, Bennett JM, Chambers MD. Iron deficiency anemia. Am Fam Physician. 2007;75(5):671–678. [PubMed] [Google Scholar]
- 13.Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329(23):1691–1695. doi: 10.1056/NEJM199312023292303. [DOI] [PubMed] [Google Scholar]
- 14.Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101(5):954–964. doi: 10.1111/j.1572-0241.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 15.Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(12):1545–1553. doi: 10.1002/ibd.20285. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4(3):177–184. doi: 10.1177/1756283X11398736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers. Geneva: World Health Organization; 2001. [Google Scholar]
- 18.Favier A, Ruffieux D. Physiological variations of serum levels of copper, zinc, iron and manganese. Biomed Pharmacother. 1983;37(9–10):462–466. [PubMed] [Google Scholar]
- 19.Cook JD, Baynes RD, Skikne BS. Iron deficiency and the measurement of iron status. Nutr Res Rev. 1992;5(1):198–202. doi: 10.1079/NRR19920014. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7(2):145–153. doi: 10.1007/BF02598003. [DOI] [PubMed] [Google Scholar]
- 21.Bartels U, Pedersen NS, Jarnum S. Iron absorption and serum ferritin in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;13(6):649–656. doi: 10.3109/00365527809181777. [DOI] [PubMed] [Google Scholar]
- 22.Geerts I, Vermeersch P, Joosten E. Evaluation of the first commercial hepcidin ELISA for the differential diagnosis of anemia of chronic disease and iron deficiency anemia in hospitalized geriatric patients. ISRN Hematol. 2012;2012:567491. doi: 10.5402/2012/567491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeulen E, Vermeersch P. Hepcidin as a biomarker for the diagnosis of iron metabolism disorders: a review. Acta Clin Belg. 2012;67(3):190–197. doi: 10.2143/ACB.67.3.2062654. [DOI] [PubMed] [Google Scholar]
- 24.Cucino C, Sonnenberg A. Cause of death in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2001;7(3):250–255. doi: 10.1097/00054725-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24(2):109–116. doi: 10.1097/MEG.0b013e32834f3140. [DOI] [PubMed] [Google Scholar]
- 26.Goddard AF, James MW, McIntyre AS, Scott BB for British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309–1316. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 27.Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low-dose iron supplements during pregnancy: a randomized controlled trial. Am J Clin Nutr. 2003;78(1):145–153. doi: 10.1093/ajcn/78.1.145. [DOI] [PubMed] [Google Scholar]
- 28.Rimon E, Kagansky N, Kagansky M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118(10):1142–1147. doi: 10.1016/j.amjmed.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health. Dietary Supplement Fact Sheet: Iron. Bethesda, MD: National Institutes of Health; 2007. [Accessed May 3, 2012]. Office of Dietary Supplements. Available from: http://ods.od.nih.gov/pdf/factsheets/Iron-HealthProfessional.pdf. [Google Scholar]
- 30.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(RR-3):1–29. [PubMed] [Google Scholar]
- 31.de Silva AD, Tsironi E, Feakins RM, Rampton DS. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther. 2005;22(11–12):1097–1105. doi: 10.1111/j.1365-2036.2005.02700.x. [DOI] [PubMed] [Google Scholar]
- 32.Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24(11–12):1507–1523. doi: 10.1111/j.1365-2036.2006.03146.x. [DOI] [PubMed] [Google Scholar]
- 33.Dostal A, Chassard C, Hilty FM, et al. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J Nutr. 2012;142(2):271–277. doi: 10.3945/jn.111.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AstraZeneca Pharmaceuticals LP. Prilosec (Omeprazole) Delayed- Release Capsules and Prilosec (Omeprazole Magnesium) for Delayed- Release Oral Suspension. Wilmington, DE: AstraZenca Pharmaceuticals LP; 2012. [Accessed April 26, 2012]. Available from: http://www1.astrazeneca-us.com/pi/Prilosec.pdf. [Google Scholar]
- 35.Disler PB, Lynch SR, Charlton RW, et al. The effect of tea on iron absorption. Gut. 1975;16(3):193–200. doi: 10.1136/gut.16.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallberg L, Rossander L, Skånberg AB. Phytates and the inhibitory effect of bran on iron absorption in man. Am J Clin Nutr. 1987;45(5):988–996. doi: 10.1093/ajcn/45.5.988. [DOI] [PubMed] [Google Scholar]
- 37.Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J Am Soc Nephrol. 2004;15(Suppl 2):S93–S98. doi: 10.1097/01.ASN.0000143814.49713.C5. [DOI] [PubMed] [Google Scholar]
- 38.American Regent, Inc. DEXFERRUM (iron dextran) injection, solution [webpage on the Internet] Bethesda, MD: US National Library of Medicine; 2011. [Accessed April 26, 2012]. Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=51879. [Google Scholar]
- 39.Watson Pharma, Inc. INFeD®(iron dextran injection USP) [ webpage on the Internet] Morristown, NJ: Watson Pharma, Inc; 2009. [Accessed April 26, 2012]. Available from: http://pi.watson.com/data_stream.asp?product_group=1251&p=pi&language=E. [Google Scholar]
- 40.American Regent, Inc. VENOFER (Iron Sucrose Injection, USP) Shirley, NY: American Regent, Inc; 2011. [Accessed April 26, 2012]. Available from: http://www.americanregent.com/documents/41.pdf. [Google Scholar]
- 41.Sanofi-Aventis US LLC. FERRLECIT sodium ferric gluconate complex in sucrose injection [webpage on the Internet] Bridgewater, NJ: 2011. [Accessed April 26, 2012]. Available from: http://products.sanofi.us/ferrlecit/ferrlecit.html. [Google Scholar]
- 42.AMAG Pharmaceuticals, Inc. FERAHEME Ferumoxytol Injection. Lexington, MA: AMAG Pharmaceuticals, Inc; 2009. [Accessed April 26, 2012]. Available from: http://www.amagpharma.com/documents/feraheme-productinsert.pdf. [Google Scholar]
- 43.Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet. 2007;369(9572):1502–1504. doi: 10.1016/S0140-6736(07)60689-8. [DOI] [PubMed] [Google Scholar]
- 44.Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis. 1996;28(4):529–534. doi: 10.1016/s0272-6386(96)90463-1. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26(3):451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Laman CA, Silverstein SB, Rodgers GM. Parenteral iron therapy: a single institution’s experience over a 5-year period. J Natl Compr Canc Netw. 2005;3(6):791–795. doi: 10.6004/jnccn.2005.0047. [DOI] [PubMed] [Google Scholar]
- 47.Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. 2011;86(10):860–862. doi: 10.1002/ajh.22153. [DOI] [PubMed] [Google Scholar]
- 48.Rosner MH, Auerbach M. Ferumoxytol for the treatment of iron deficiency. Expert Rev Hematol. 2011;4(4):399–406. doi: 10.1586/ehm.11.31. [DOI] [PubMed] [Google Scholar]
- 49.Provenzano R, Schiller B, Rao M, Coyne D, Brenner L, Pereira BJ. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(2):386–393. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh H, Reed J, Noble S, Cangiano JL, Van Wyck DB United States Iron Sucrose (Venofer) Clinical Trials Group. Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anemia: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1(3):475–482. doi: 10.2215/CJN.01541005. [DOI] [PubMed] [Google Scholar]
- 51.Koutroubakis IE, Oustamanolakis P, Karakoidas C, Mantzaris GJ, Kouroumalis EA. Safety and efficacy of total-dose infusion of low molecular weight iron dextran for iron deficiency anemia in patients with inflammatory bowel disease. Dig Dis Sci. 2010;55(8):2327–2331. doi: 10.1007/s10620-009-1022-y. [DOI] [PubMed] [Google Scholar]
- 52.Khalil A, Goodhand JR, Wahed M, Yeung J, Ali FR, Rampton DS. Efficacy and tolerability of intravenous iron dextran and oral iron in inflammatory bowel disease: a case-matched study in clinical practice. Eur J Gastroenterol Hepatol. 2011;23(11):1029–1035. doi: 10.1097/MEG.0b013e32834a58d1. [DOI] [PubMed] [Google Scholar]
- 53.Lindgren S, Wikman O, Befrits R, et al. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: A randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol. 2009;44(7):838–845. doi: 10.1080/00365520902839667. [DOI] [PubMed] [Google Scholar]
- 54.Schröder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease – a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100(11):2503–2509. doi: 10.1111/j.1572-0241.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 55.Evstatiev R, Marteau P, Iqbal T, et al. for FERGI Study Group. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141(3):846–853. e1–e2. doi: 10.1053/j.gastro.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103(5):1182–1192. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 57.Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. 1970;100(7):301–303. German. [PubMed] [Google Scholar]
- 58.Kulnigg S, Teischinger L, Dejaco C, Waldhör T, Gasche C. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol. 2009;104(6):1460–1467. doi: 10.1038/ajg.2009.114. [DOI] [PubMed] [Google Scholar]
- 59.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 60.Patterson C, Guyatt GH, Singer J, Ali M, Turpie I. Iron deficiency anemia in the elderly: the diagnostic process. CMAJ. 1991;144(4):435–440. [PMC free article] [PubMed] [Google Scholar]
- 61.Rizvi S, Schoen RE. Supplementation with oral vs intravenous iron for anemia with IBD or gastrointestinal bleeding: is oral iron getting a bad rap? Am J Gastroenterol. 2011;106(11):1872–1879. doi: 10.1038/ajg.2011.232. [DOI] [PubMed] [Google Scholar]