Abstract
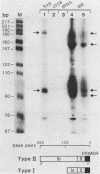
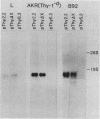
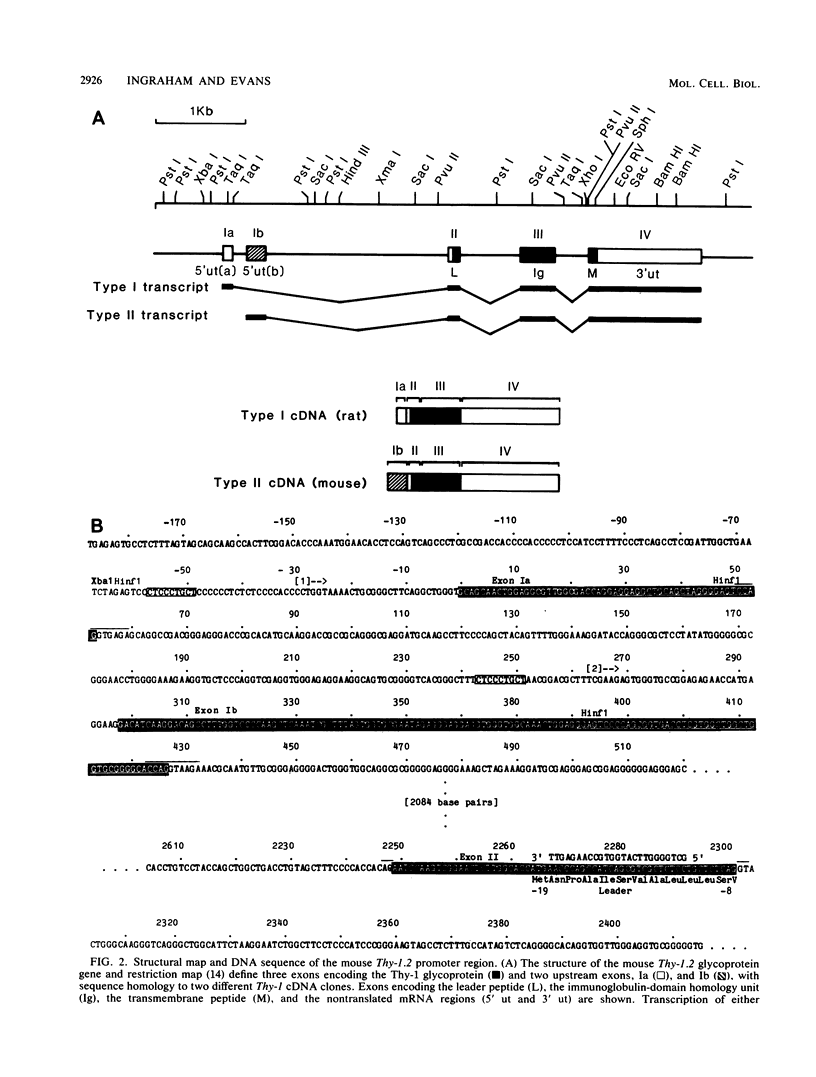
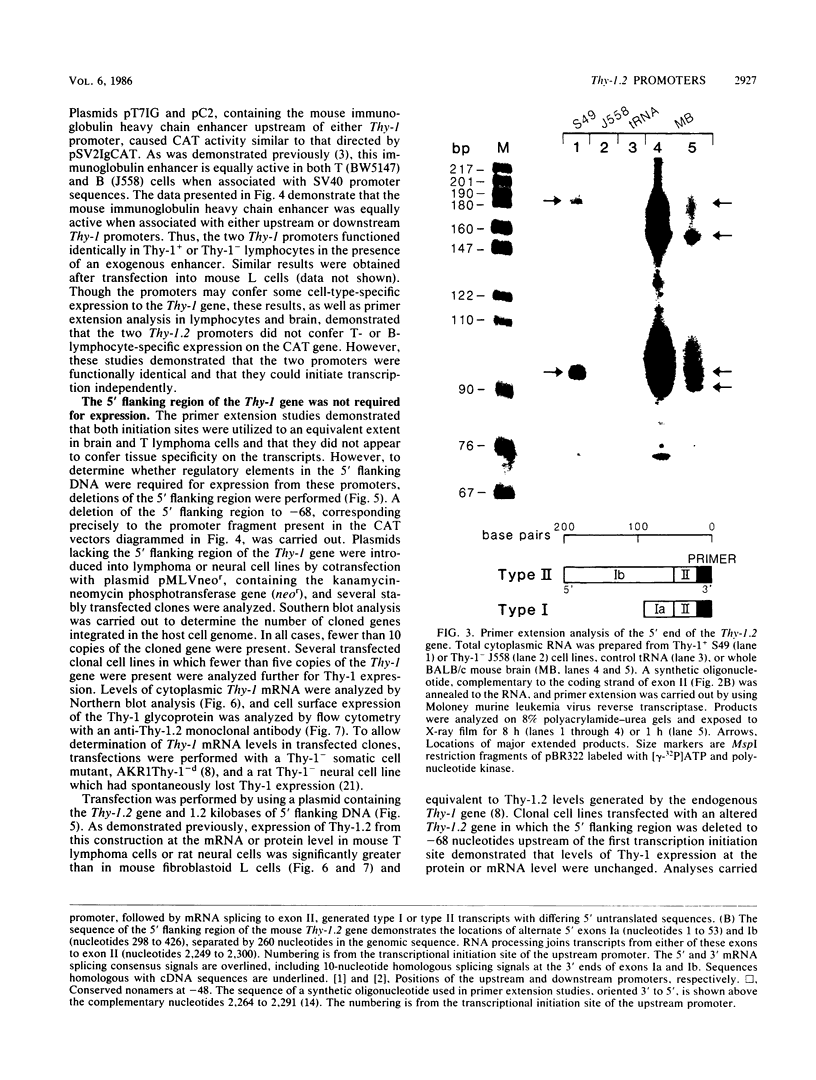
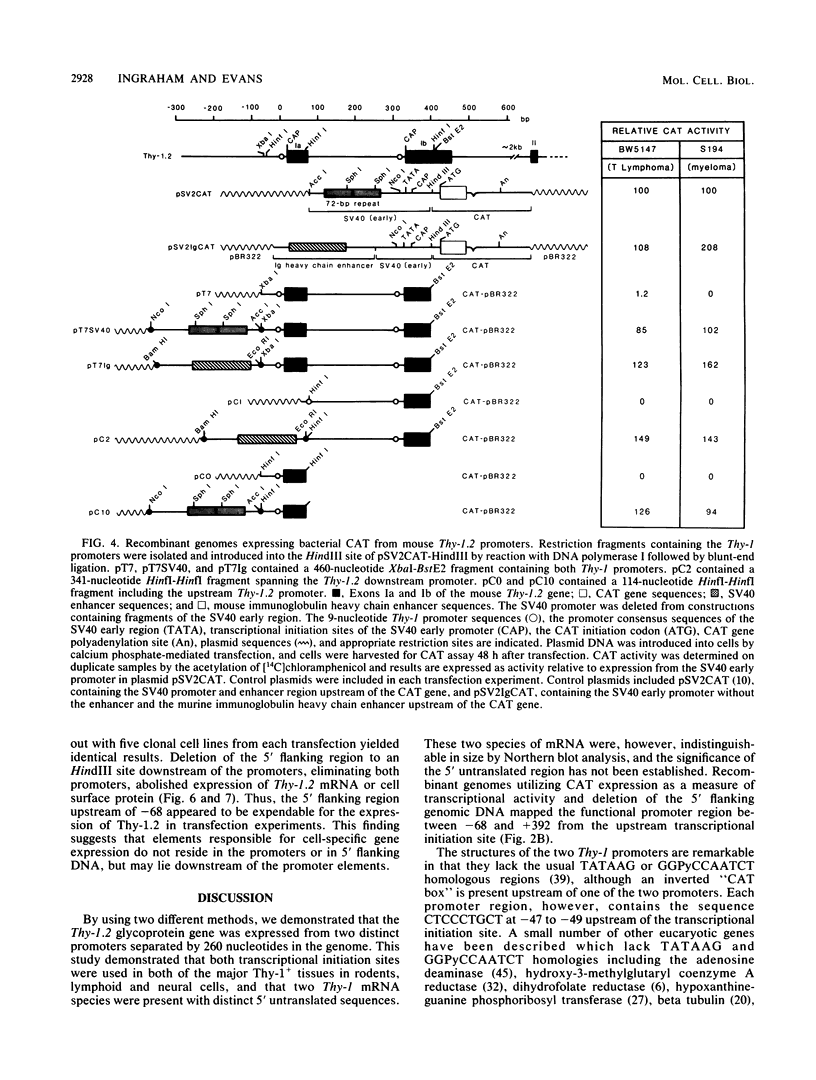
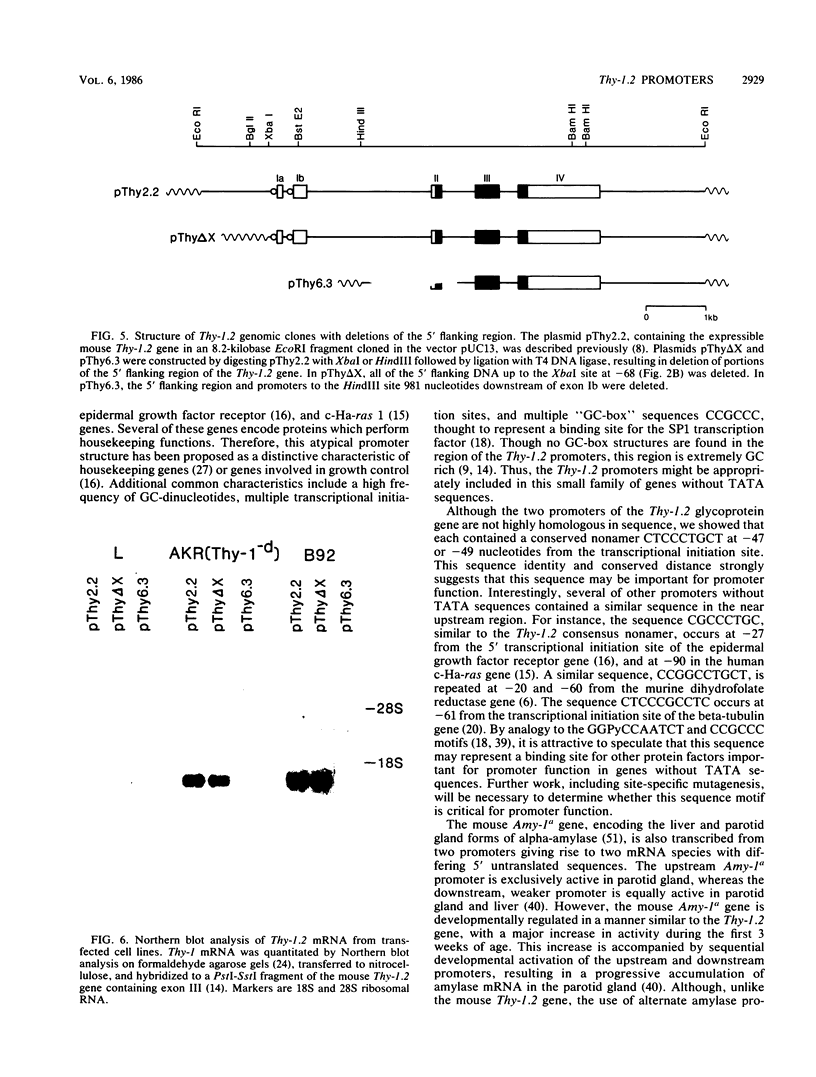
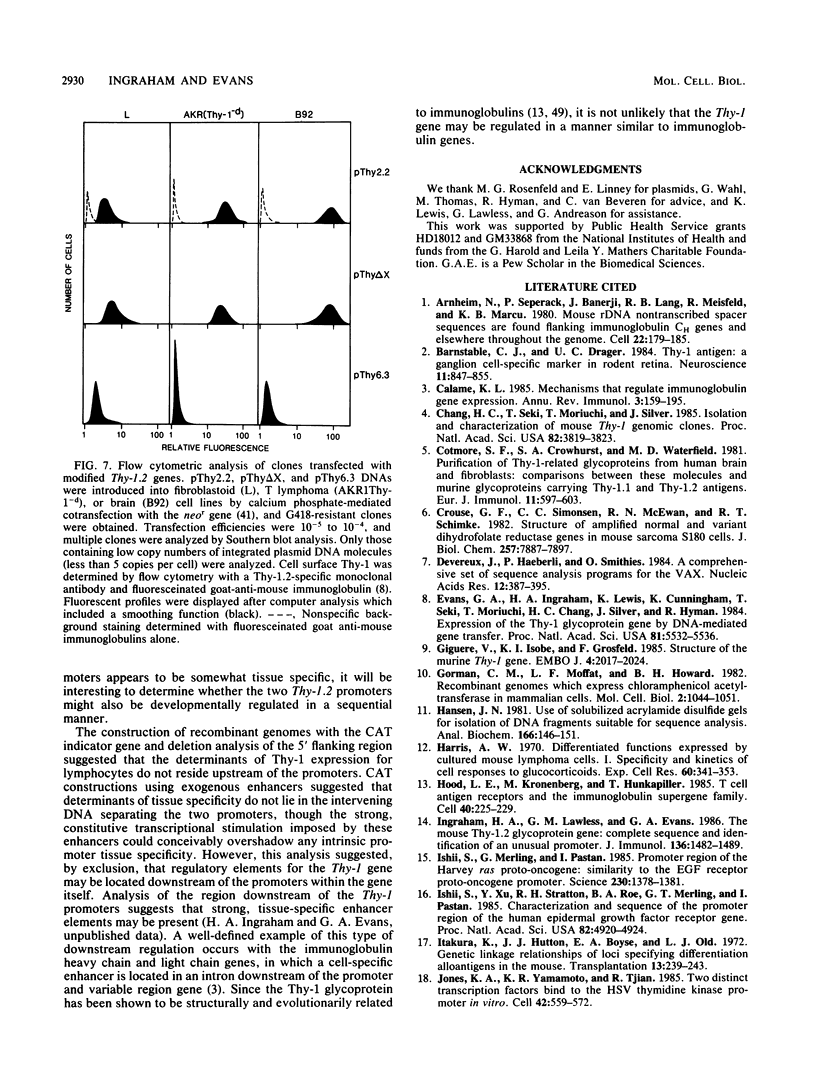
The promoter and 5' flanking region of the mouse Thy-1.2 glycoprotein gene were characterized by DNA sequencing, primer extension analysis, and deletion analysis. Transcriptional initiation sites were identified which corresponded to two separate exons upstream of the portion of the gene encoding the Thy-1.2 glycoprotein. We demonstrated that the mouse Thy-1.2 gene was transcribed from two atypical promoters separated by 260 base pairs in the genomic sequence. These promoters contained neither TATAAG nor GGPyCCAATCT homologous sequences but defined a conserved nonamer CTCCCTGCT at -48 from each initiation site. Two Thy-1.2 mRNA species of 1,835 and 1,939 nucleotides, differing in the 5' untranslated region of the mRNA, were thus transcribed from the single Thy-1.2 gene by mRNA splicing to the same downstream exon. Recombinant genomes in which the bacterial chloramphenicol acetyltransferase gene was expressed from either of the two Thy-1.2 promoters demonstrated that each promoter functioned independently and did not direct cell-specific expression in lymphoid cells. The 5' flanking region of the Thy-1.2 gene upstream of -68 could be eliminated without altering cell-type-specific expression. This suggests that regulatory elements responsible for tissue and developmental stage-specific expression of the Thy-1.2 gene are not present in the 5' flanking DNA but may reside downstream of the promoters.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Seperack P., Banerji J., Lang R. B., Miesfeld R., Marcu K. B. Mouse rDNA nontranscribed spacer sequences are found flanking immunoglobulin CH genes and elsewhere throughout the genome. Cell. 1980 Nov;22(1 Pt 1):179–185. doi: 10.1016/0092-8674(80)90166-x. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Dräger U. C. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984 Apr;11(4):847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Calame K. L. Mechanisms that regulate immunoglobulin gene expression. Annu Rev Immunol. 1985;3:159–195. doi: 10.1146/annurev.iy.03.040185.001111. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Seki T., Moriuchi T., Silver J. Isolation and characterization of mouse Thy-1 genomic clones. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3819–3823. doi: 10.1073/pnas.82.11.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Crowhurst S. A., Waterfield M. D. Purification of Thy-1-related glycoproteins from human brain and fibroblasts: comparisons between these molecules and murine glycoproteins carrying Thy-1.1 and Thy-1.2 antigens. Eur J Immunol. 1981 Aug;11(8):597–603. doi: 10.1002/eji.1830110802. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Simonsen C. C., McEwan R. N., Schimke R. T. Structure of amplified normal and variant dihydrofolate reductase genes in mouse sarcoma S180 cells. J Biol Chem. 1982 Jul 10;257(13):7887–7897. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Ingraham H. A., Lewis K., Cunningham K., Seki T., Moriuchi T., Chang H. C., Silver J., Hyman R. Expression of the Thy-1 glycoprotein gene by DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5532–5536. doi: 10.1073/pnas.81.17.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguére V., Isobe K., Grosveld F. Structure of the murine Thy-1 gene. EMBO J. 1985 Aug;4(8):2017–2024. doi: 10.1002/j.1460-2075.1985.tb03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. N. Use of solubilizable acrylamide disulfide gels for isolation of DNA fragments suitable for sequence analysis. Anal Biochem. 1981 Sep 1;116(1):146–151. doi: 10.1016/0003-2697(81)90337-7. [DOI] [PubMed] [Google Scholar]
- Harris A. W. Differentiated functions expressed by cultured mouse lymphoma cells. I. Specificity and kinetics of cell responses to corticosteroids. Exp Cell Res. 1970 Jun;60(3):341–353. doi: 10.1016/0014-4827(70)90527-6. [DOI] [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Lawless G. M., Evans G. A. The mouse Thy-1.2 glycoprotein gene: complete sequence and identification of an unusual promoter. J Immunol. 1986 Feb 15;136(4):1482–1489. [PubMed] [Google Scholar]
- Ishii S., Merlino G. T., Pastan I. Promoter region of the human Harvey ras proto-oncogene: similarity to the EGF receptor proto-oncogene promoter. Science. 1985 Dec 20;230(4732):1378–1381. doi: 10.1126/science.2999983. [DOI] [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Hutton J. J., Boyse E. A., Old L. J. Genetic linkage relationships of loci specifying differentiation alloantigens in the mouse. Transplantation. 1972 Mar;13(3):239–243. doi: 10.1097/00007890-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Lathe R., Kieny M. P., Skory S., Lecocq J. P. Linker tailing: unphosphorylated linker oligonucleotides for joining DNA termini. DNA. 1984;3(2):173–182. doi: 10.1089/dna.1984.3.173. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lesley J. F., Lennon V. A. Thy-L antigen expression on rat brain cell lines. Brain Res. 1978 Sep 15;153(1):109–120. doi: 10.1016/0006-8993(78)91132-0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Lundblad A., Steller R., Kabat E. A., Hirst J. W., Weigert M. G., Cohn M. Immunochemical studies on mouse myeloma proteins with specificity for dextran or for levan. Immunochemistry. 1972 May;9(5):535–544. doi: 10.1016/0019-2791(72)90063-8. [DOI] [PubMed] [Google Scholar]
- Mansour M. H., Cooper E. L. Purification and characterization of Rana pipiens brain Thy-1 glycoprotein. J Immunol. 1984 May;132(5):2515–2523. [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- McKenzie J. L., Fabre J. W. Studies with a monoclonal antibody on the distribution of Thy-1 in the lymphoid and extracellular connective tissues of the dog. Transplantation. 1981 Apr;31(4):275–282. doi: 10.1097/00007890-198104000-00009. [DOI] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. J., Sutcliffe J. G. Gene expression in rat brain. Nucleic Acids Res. 1983 Aug 25;11(16):5497–5520. doi: 10.1093/nar/11.16.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriuchi T., Chang H. C., Denome R., Silver J. Thy-1 cDNA sequence suggests a novel regulatory mechanism. Nature. 1983 Jan 6;301(5895):80–82. doi: 10.1038/301080a0. [DOI] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse nervous tissue iso-antigens. Nature. 1966 Jan 29;209(5022):523–523. doi: 10.1038/209523a0. [DOI] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jobe A., Cohn M. Mouse myelomas producing precipitating antibody to nucleic acid bases and-or nitrophenyl derivatives. Nature. 1968 Nov 30;220(5170):882–885. doi: 10.1038/220882a0. [DOI] [PubMed] [Google Scholar]
- Seki T., Chang H. C., Moriuchi T., Denome R., Ploegh H., Silver J. A hydrophobic transmembrane segment at the carboxyl terminus of thy-1. Science. 1985 Feb 8;227(4687):649–651. doi: 10.1126/science.2857501. [DOI] [PubMed] [Google Scholar]
- Seki T., Moriuchi T., Chang H. C., Denome R., Silver J. Structural organization of the rat thy-1 gene. Nature. 1985 Feb 7;313(6002):485–487. doi: 10.1038/313485a0. [DOI] [PubMed] [Google Scholar]
- Seki T., Spurr N., Obata F., Goyert S., Goodfellow P., Silver J. The human Thy-1 gene: structure and chromosomal location. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6657–6661. doi: 10.1073/pnas.82.19.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Sordat B., Schibler U. The two promoters of the mouse alpha-amylase gene Amy-1a are differentially activated during parotid gland differentiation. Cell. 1985 Apr;40(4):907–912. doi: 10.1016/0092-8674(85)90350-2. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R., Mazauskas C. The synthesis and properties of T25 blycoprotein in Thy-1-negative mutant lymphoma cells. Cell. 1978 May;14(1):21–32. doi: 10.1016/0092-8674(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Many cells in rat bone marrow have cell-surface Thy-1 antigen. Eur J Immunol. 1976 Jul;6(7):526–528. doi: 10.1002/eji.1830060716. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]
- van Rijs J., Giguère V., Hurst J., van Agthoven T., Geurts van Kessel A., Goyert S., Grosveld F. Chromosomal localization of the human Thy-1 gene. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5832–5835. doi: 10.1073/pnas.82.17.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]