Abstract
Massive, irreparable rotator cuff tears are a source of pain and disability. Although most rotator cuff tears can be completely repaired, a significant number are considered massive and irreparable. Numerous operative techniques have been described for the treatment of these kinds of tears including arthroscopic debridement, biceps tenotomy, tendon transfer, grafting, and reverse arthroplasty. We describe a surgical technique using a biodegradable subacromial balloon spacer (InSpace; OrthoSpace, Kfar Saba, Israel) implanted between the humeral head and acromion that permits smooth, frictionless gliding, restoring the shoulder biomechanics. The technique is easy to perform and is less invasive than the conventional surgical techniques available, and it may potentially serve as a bridging option in patients with massive, irreparable tears who are normally candidates for reverse arthroplasty.
The massive, irreparable rotator cuff tear is a challenging problem to manage. A range of surgical options are available for this condition, including subacromial decompression or debridement, various techniques for partial tendon repair, tendon transfer, muscle-tendon slide procedures, the use of rotator cuff allograft and synthetic graft materials, arthrodesis, hemiarthroplasty, and reverse total shoulder arthroplasty.1, 2, 3, 4, 5 There is no consensus or definitive guidelines concerning the preferred surgical option designed to treat massive, irreparable rotator cuff tears when nonoperative modalities have failed, and it is understood that primary repairs are associated with comparatively poor functional outcomes due to definable muscle atrophy and fatty infiltration6 and that failed initial surgery is dependent on the size of the tear.7 A novel surgical technique uses a biodegradable subacromial spacer, the InSpace balloon (OrthoSpace, Kfar Saba, Israel), implanted between the acromion and the humeral head that permits smooth, frictionless gliding, restoring shoulder biomechanics.
The InSpace system contains an introducer and a preshaped spacer made of poly(L-lactide-co-ε-caprolactone), which is a copolymer of poly-lactide and ε-caprolactone, a biodegradable and widely used material that biodegrades over a period of 12 months.8, 9 To enable insertion, the balloon is folded into a cylinder-shaped insertion tube, which is removed once the spacer is inserted into the subacromial space.
The device is contraindicated for patients with known allergy to device material or patients having active or latent infection or signs of tissue necrosis in the subacromial area.
As with any other implantable material, the expected risks after implantation may include foreign body response, local irritation at the wound site, local infection, inflammation, and tissue necrosis. Moreover, device displacement may occur and increase pain. All mentioned adverse effects are relatively rare and can be treated by routine medications such as antibiotics and/or injectable steroids. Alternatively, the implant can be punctured or removed by an arthroscopic procedure. Additional highlights and tips are described in Table 1, and the treatment algorithm is further described in Fig 1.
Table 1.
Highlights and Tips for InSpace Use
| Indications |
| Torn and irreparable supraspinatus |
| Torn and irreparable supraspinatus and torn and reparable infraspinatus |
| Torn and irreparable supraspinatus and infraspinatus |
| Torn and irreparable supraspinatus, torn and reparable or irreparable infraspinatus, and torn and reparable subscapularis |
| Contraindications |
| Glenohumeral arthropathy |
| Active or latent infection or tissue necrosis |
| Allergy to device material (poly-lactide and poly-ε-caprolactone) |
| Axillary palsy |
| Risks⁎ |
| Spacer displacement |
| Tissue responses to implant, which could include local irritation, infection, and inflammation |
| Pitfalls and tips |
| The lateral port should be made parallel to the supraglenoid tubercle to enable easy insertion and correct orientation of the device. |
| Debridement and bursectomy should be performed to allow proper selection of spacer size and to enable correct positioning to minimize implant displacement risk. |
| In cases where the measurement of the distance between the glenoid rim and the lateral border of the acromion falls between 2 spacer sizes (e.g., 49 mm, which is borderline between the small and medium spacer sizes), the larger spacer size should be used (to minimize implant displacement). |
| Using a probe through the anterior port could assist in achieving stabilization and proper placement of the device. |
| It is highly recommended to follow the manufacturer's instructions regarding inflation volumes and avoid overinflation. To avoid over-tensioning of the deltoid muscle. |
| In case of a torn subscapularis, reconstruction is recommended. |
| In case of a partially torn long head of the biceps, biceps tenotomy or tenodesis is recommended. |
| It is recommended to repair the subscapularis to create anterior/posterior coupling before spacer insertion. If the subscapularis is irreparable, spacer implantation should be considered with caution. |
The risks mentioned are relatively rare and may occur with every implantable device. If adverse effects occur, they can be treated by routine medications or by arthroscopic puncture and/or removal of the device.
Figure 1.

InSpace treatment algorithm. (ISP, infraspinatus; SSP, supraspinatus; SUBSCAP, subscapularis.)
The InSpace insertion technique is easy to perform, less invasive than many other procedures, reproducible, and safe, with initial results showing efficacy that is maintained beyond the 12-month period of natural biodegradation of the implant.
Technique
The patient is placed either on a beach chair or in the lateral decubitus position (Video 1). The standard arrangement for arthroscopic rotator cuff repair requires a 50-mL Luer-lock syringe filled with 0.9% saline solution prewarmed to 40°C and an extension tube connected to a 3-way valve.
Step 1
A standard posterior arthroscopic port is inserted to explore the joint and to allow glenohumeral visualization and treatment of shoulder pathology as indicated.
Step 2
Subacromial visualization is conducted through the posterior port while the surgeon inserts a needle into the subacromial space using a secondary lateral arthroscopic port. The lateral 1.5-cm port is made parallel to the supraglenoid tubercle, where placement of this port is critical to permit easy insertion and correct orientation of the balloon system.
Step 3
Debridement and/or bursectomy is performed with a shaver to allow better visualization of the subacromial space and to define the type and extent of rotator cuff tear. The soft tissue over the glenoid rim and the rotator cuff muscle stump is cleaned to a level that permits insertion and accurate positioning of the spacer.
Step 4
The InSpace subacromial spacer is available in 3 different sizes: small (40 × 50 mm), medium (50 × 60 mm), and large (60 × 70 mm) (Table 2). To select the appropriate spacer size, measurements are made with an arthroscopic probe with marked lines, where the medial point is defined 1 cm medial to the superior glenoid rim and the lateral point is the lateral border of the acromion (Figure 2, Figure 3). In those cases where the measurement lies between 2 main sizes, the larger spacer size is used to ensure proper positioning and to minimize the likelihood of implant displacement.
Table 2.
InSpace Spacer Size and Recommended Inflation Volumes
| Balloon Size | Width (mm) | Length (mm) | Maximal Inflation Volume (mL) | Recommended Final Volume (mL) |
|---|---|---|---|---|
| Small | 40 | 50 | 15-17 | 9-11 |
| Medium | 50 | 60 | 22-24 | 14-16 |
| Large | 60 | 70 | 40 | 23-25 |
Figure 2.

Definition of medial point in left shoulder with patient in beach-chair position, arthroscope in posterior portal, and arthroscopic probe in lateral portal.
Figure 3.

Definition of lateral point in left shoulder with patient in beach-chair position, arthroscope in posterior portal, and arthroscopic probe in lateral portal.
Step 5
The biodegradable spacer is introduced through the lateral port. The system should be placed approximately 1 cm over the glenoid rim and the rotator cuff tendon stump, inside the medial point. There is a lateral (black) marker on the system deployer that should be in line with the lateral border of the acromion (Fig 4). In some patients this step can be challenging and may require stabilization and placement assistance by advancing a probe through the anterior port (Fig 5).
Figure 4.

Lateral marker of spacer sheath (arrow) in left shoulder with patient in beach-chair position, arthroscope in posterior portal, and spacer sheath in lateral portal.
Figure 5.
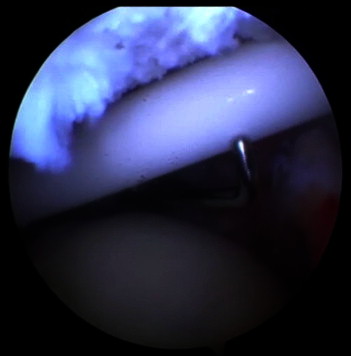
Assistance in spacer (balloon) deployment using probe in left shoulder with patient in beach-chair position, arthroscope in posterior portal, and arthroscopic probe in anterior portal.
Step 6
Once accurate positioning of the spacer is achieved, the protective sheath is withdrawn to reveal the spacer (Fig 6). The extension tubing is connected to the distal side of the Luer-lock connector, and the spacer is inflated to its maximal volume depending on the spacer size. The valve should remain open to permit backflow of saline solution into the syringe until the recommended volume is achieved that allows a full range of motion of the shoulder. It is important to maintain the recommended volume because spacer overinflation may result in excessive tension in the deltoid muscle with pain, as well as an increased likelihood of device displacement over time. It is preferable that, if the correct volume cannot be maintained, the balloon should remain slightly underinflated to avoid these complications.
Figure 6.

(A) Partially exposed spacer and (B) fully exposed spacer in left shoulder with patient in beach-chair position, arthroscope in posterior portal, and spacer in lateral portal.
Step 7
The spacer is sealed and secured in situ by firmly grasping the deployer and withdrawing the connecting syringe (Figure 7, Figure 8). As a final step, the delivery system is removed, and the shoulder is passively moved through a full range of movement to verify that the spacer is accurately placed, is stable in position, and does not interfere with shoulder mobility.
Figure 7.

Final step of deployment and sealed spacer in left shoulder with patient in beach-chair position, arthroscope in posterior portal, and spacer in lateral portal.
Figure 8.

Inflated spacer over humeral head in left shoulder with patient in beach-chair position, arthroscope in posterior portal, and spacer in lateral portal. The sealing mechanism is marked with an arrow.
Discussion
Massive, irreparable rotator cuff tears are a challenging problem, and tears of the posterior cuff result in humeral head migration and narrowing of the subacromial space. Although, on occasion, a shoulder with this pathology can retain acceptable stability, it is frequently very painful and effectively nonfunctional.
In recent publications, numerous management strategies have been used, and studies have shown varied results with regard to shoulder pain, range of motion, strength, and overall improvement of shoulder function, with no consensus regarding the optimal treatment solution for this patient population.
Longo et al.10 summarized 22 retrospective nonrandomized studies describing 493 shoulders, in which latissimus dorsi tendon transfer by an arthroscopic, open, or combined approach as a single procedure or in combination with other muscle-tendon transfer procedures, replacement, or both has been proposed as a possible solution to restore pain-free function, strength, and range of motion in patients with massive, irreparable cuff tears. Lee et al.11 presented satisfactory results with good preservation of the preoperative and postoperative acromiohumeral interval and continuity in the inferior scapulohumeral line, diminishing pain, and improvement of active forward elevation in 36 patients with irreparable, massive rotator cuff tears who were followed up for at least 3 years after arthroscopic tuberoplasty with concomitant acromioplasty, as well as treatment of the biceps tendon when indicated. Gupta et al.12 described a prospective observational study of 24 patients who underwent interposition repair of massive rotator cuff tears with human dermal allograft; all patients showed a significant improvement in pain, range of motion, and strength, with ultrasonography-confirmed “fully intact” repairs in 76% of patients who were followed up for a 3-year period on average. Glanzmann et al.13 performed retrospective assessment of 31 deltoid flap transfers for massive posterosuperior rotator cuff tears at midterm and long-term follow-up examinations (mean, 53 and 175 months, respectively). They showed that functional gains were minor but improvement in pain and patient satisfaction was high. Survival rates for the deltoid flap, confirmed by ultrasound, were 16.5% at midterm follow-up and 12.5% at long-term follow-up. Cranial migration of the humeral head progressed in all cases in their study and could not be prevented by the interposition of a deltoid flap. On the basis of these results, Glanzmann et al. no longer use or recommend this technique. Mulieri et al.7 presented reverse shoulder arthroplasty as a treatment option for patients who had persistent shoulder pain and dysfunction despite a minimum of 6 months of nonoperative treatment with the presence of at least a 2-tendon tear without glenohumeral arthritis. Significant pain relief, along with an improvement in range of motion, was achieved in the 58 patients (60 shoulders) who were evaluated for a minimum of 2 years of follow-up. Nevertheless, this treatment option is a salvage procedure involving major surgery and is associated with a relatively high complication rate (prevalence, 20%).
A balloon implantation technique was previously described by Sartoretti et al.14 for the ankle joint. Kilinc et al.15 used a Foley catheter that was cut just proximal to the balloon end and inserted into the subacromial space to allow better visualization, triangulation of the arthroscopic instruments, and anatomic repair of the rotator cuff tendon.
To our knowledge, this is the first report of the use of the innovative device and technique described for the treatment of patients with massive, irreparable full-thickness rotator cuff tears due to trauma or degradation. The rotator cuff normally provides stability by compression of the humeral head into the glenoid, whereas rotator cuff disruption compromises concavity compression and alters glenohumeral load structure and direction. The deployment of a balloon spacer should reduce subacromial friction during shoulder abduction by lowering the head of the humerus and facilitating humeral gliding against the acromion during movement.
The spacer degrades within 12 months, which is a period that conforms well to the rehabilitation time frame after any arthroscopic procedure performed on the rotator cuff. It is unclear, however, how long the spacer remains inflated, and it is not understood why pain and functional scores continue to improve beyond the period of spacer disintegration.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
This is a U.S. government work. There are no restrictions on its use.
Supplementary data
Technique in left shoulder with arthroscope in posterior portal. Debridement is performed with a shaver from the lateral portal, and the reparability of the rotator cuff is determined. A massive, irreparable tear is present. Measurements are made with an arthroscopic probe with marker lines, where the medial point is defined 1 cm medial to the superior glenoid and the lateral point is the lateral border of the acromion. The InSpace system is introduced from the lateral portal and should be placed over the glenoid rim; a probe to the anterior portal can help with system placement. There is a lateral black marker on the system deployer that should be in line with the lateral border of the acromion. The protecting sheath is withdrawn to reveal the spacer, and the spacer is inflated to its maximal volume depending on the spacer size. The spacer is sealed and secured in situ by firmly grasping the deployer and withdrawing the connecting syringe. The shoulder is passively moved through a full range of movement to verify spacer stability.
References
- 1.Moser M., Jablonski M.V., Horodyski M., Wright T.W. Functional outcome of surgically treated massive rotator cuff tears: A comparison of complete repair, partial repair, and debridement. Orthopedics. 2007;30:479–482. doi: 10.3928/01477447-20070601-05. [DOI] [PubMed] [Google Scholar]
- 2.Cole B.J., ElAttrache N.S., Anbari A. Arthroscopic rotator cuff repairs: An anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23:662–669. doi: 10.1016/j.arthro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Cuff D., Pupello D., Virani N., Levy J., Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald P.B., Altamimi S. Principles of arthroscopic repair of large and massive rotator cuff tears. Instr Course Lect. 2010;59:269–280. [PubMed] [Google Scholar]
- 5.Goutallier D., Postel J.M., Bemageau J. Fatty muscle degeneration in cuff ruptures: Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 2007:78–83. [PubMed] [Google Scholar]
- 6.Matthews T.J., Hand G.C., Rees J.L., Athanasou N.A., Carr A.J. Pathology of the torn rotator cuff tendon: Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 7.Mulieri P., Dunning P., Klein S., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 8.Burks C.A., Bundy K., Fotuhi P., Alt E. Characterization of 75:25 poly(l-lactide-co-epsilon-caprolactone) thin films for the endoluminal delivery of adipose-derived stem cells to abdominal aortic aneurysms. Tissue Eng. 2006;12:2591–2600. doi: 10.1089/ten.2006.12.2591. [DOI] [PubMed] [Google Scholar]
- 9.Levy Y., Paz A., Ben Yosef R. Biodegradable inflatable balloon for reducing radiation adverse effects in prostate cancer. J Biomed Mater Res B Appl Biomater. 2009;91:855–867. doi: 10.1002/jbm.b.31467. [DOI] [PubMed] [Google Scholar]
- 10.Longo U.G., Franceschetti E., Petrillo S., Maffulli N., Denaro V. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: A systematic review. Sports Med Arthrosc. 2011;19:428–437. doi: 10.1097/JSA.0b013e3182390639. [DOI] [PubMed] [Google Scholar]
- 11.Lee B.G., Cho N.S., Rhee Y.G. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27:1341–1350. doi: 10.1016/j.arthro.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A.K., Hug K., Berkoff D.J. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40:141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 13.Glanzmann M.C., Goldhahn J., Flury M., Schwyzer H.K., Simmen B.R. Deltoid flap reconstruction for massive rotator cuff tears: Mid- and long-term functional and structural results. J Shoulder Elbow Surg. 2010;19:439–445. doi: 10.1016/j.jse.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Sartoretti C., Sartoretti-Schefer S., Duff C., Buchmann P. Angioplasty balloon catheters used for distraction of the ankle joint. Arthroscopy. 1996;12:82–86. doi: 10.1016/s0749-8063(96)90224-8. [DOI] [PubMed] [Google Scholar]
- 15.Kilinc A.S., Ebrahimzadeh M.H., Lafosse L. Subacromial internal spacer for rotator cuff tendon repair: “The balloon technique.”. Arthroscopy. 2009;25:921–924. doi: 10.1016/j.arthro.2009.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technique in left shoulder with arthroscope in posterior portal. Debridement is performed with a shaver from the lateral portal, and the reparability of the rotator cuff is determined. A massive, irreparable tear is present. Measurements are made with an arthroscopic probe with marker lines, where the medial point is defined 1 cm medial to the superior glenoid and the lateral point is the lateral border of the acromion. The InSpace system is introduced from the lateral portal and should be placed over the glenoid rim; a probe to the anterior portal can help with system placement. There is a lateral black marker on the system deployer that should be in line with the lateral border of the acromion. The protecting sheath is withdrawn to reveal the spacer, and the spacer is inflated to its maximal volume depending on the spacer size. The spacer is sealed and secured in situ by firmly grasping the deployer and withdrawing the connecting syringe. The shoulder is passively moved through a full range of movement to verify spacer stability.


