Abstract
Objective:
Neem plant (Azadirachta indica) has been extensively used in Ayurvedic system of medicine for female fertility regulation for a long time, but its mechanism of action remains poorly understood. Hence, the present study was aimed to determine whether an increase of granulosa cell apoptosis is associated with aqueous neem leaf extract (NLE)-induced oocyte apoptosis.
Materials and Methods:
Sexually immature female rats of 20 days old were fed NLE (50 mg/day) for 10 days and then subjected to superovulation induction protocol. The morphological changes in cumulus oocyte complexes (COCs), rate of oocyte apoptosis, hydrogen peroxide (H2O2), total nitrite, and cytochrome c concentrations, inducible nitric oxide synthase (iNOS), cytochrome c, p53, Bcl2 and Bax expressions, deoxyribonucleic acid (DNA) fragmentation, and estradiol 17β level in granulosa cells collected from preovulatory COCs were analyzed.
Results:
Aqueous NLE increased H2O2 concentration and decreased catalase activity, increased iNOS expression and total nitrite concentration, increased p53, Bax, and p53 expressions but decreased Bcl2 expression, increased cytochrome c concentration and induced DNA fragmentation in granulosa cells. An increased granulosa cell apoptosis resulted in reduced estradiol 17β concentration and induced apoptosis in ovulated oocytes.
Conclusion:
We conclude that aqueous NLE-induced granulosa cell apoptosis through the mitochondria-mediated pathway, reduced estradiol 17β concentration and induced apoptosis in ovulated oocytes. Thus, granulosa cell apoptosis mediates NLE-induced oocyte apoptosis during female fertility regulation in rat.
Keywords: Aqueous neem leaf extract, Bax/Bcl2, deoxyribonucleic acid fragmentation, granulosa cell apoptosis, rat oocytes, reactive oxygen species
INTRODUCTION
Inside the follicular microenvironment, oocyte is surrounded by several layers of granulosa cells that are differentiated into mural and cumulus granulosa cells during final stages of folliculogenesis. The cumulus granulosa cells are closer to the oocyte and communication between cumulus granulosa cells and oocyte is ensured via cytoplasmic extensions of corona radiata cells that pass through zona pellucida.[1,2] Both mural as well as cumulus granulosa cells provide nutrients and maturation-enabling factors to ensure successful maturation and developmental competency of oocytes.[2–5] Further, granulosa cells protect oocyte from oxidative stress damage[6,7] because granulosa cells have their own enzymatic antioxidant system that regulate reactive oxygen species (ROS) levels during maturation of oocytes.[8] The morphology and number of encircling granulosa cells have been used as biomarkers for developmental competency, embryo and pregnancy outcome.[4,9,10]
The premature rupture of communication between granulosa cells and oocyte or removal of granulosa cells from oocyte or granulosa cell apoptosis reduces developmental competence of oocyte[9,11–17] and induces degenerative changes in oocytes.[17] Although apoptosis has been reported in cumulus granulosa cells lying in the peripheral region of newly ovulated cumulus oocyte complexes (COCs),[18] incidence of cumulus granulosa cell apoptosis must be kept below the threshold level to ensure the acquisition of oocyte developmental competency of follicular COCs.[19] A higher incidence of apoptotic granulosa cells has been associated with an increased number of empty follicles, fewer oocyte retrieval and poor quality of oocytes.[20]
Neem (Azadirachta indica) leaves are frequently used to control fertility in the traditional system of medicine world-wide.[21] However, the possible mechanism of neem leaf extract (NLE) action during fertility control remains to be elucidated. Previous studies suggest that aqueous NLE-induced oocyte apoptosis in vitro,[22] as well as in vivo through the generation of ROS.[23] Aqueous NLE reduces the number of granulosa cells encircling oocyte and induces dispersion of granulosa cells.[23] These findings led us to hypothesize that granulosa cell apoptosis could be one of the causes for NLE-induced oocyte apoptosis. Granulosa cell apoptosis in preovulatory follicles not only deprive nutrients, maturation enabling factors and survival factors that are required for growth and development of preovulatory oocytes but also reduce estradiol 17β production that may induce susceptibility of oocyte towards apoptosis. However, there is no evidence to support this hypothesis. Therefore, the present study was designed to find out whether an increase of granulosa cell apoptosis mediates NLE-induced oocyte apoptosis in rat.
MATERIALS AND METHODS
Chemicals and preparation of culture medium
All chemicals used in the present study were purchased from Sigma Chemical Co., St. Louis, MO, USA unless stated otherwise. The culture medium (M-199; HiMedia, Mumbai, India) was freshly prepared as per company manual protocol. The penicillin (100 IU/ml) and streptomycin (100 ug/ml) were added to the culture medium.
Experimental animal
Sexually immature female rats (Charles-Foster strain) of 20 days old (40 ± 5 g body weight) along with their mother were separated from existing colony of departmental animal facility and maintained in normal husbandry conditions with food and water ad libitum. All procedures confirmed to the stipulations of the Departmental Animal Ethical Committee of Banaras Hindu University, Varanasi - 221 005, India and followed the guidelines for the care and use of laboratory animals (NIH Publication).
Preparation of aqueous NLE and treatment
The aqueous NLE was prepared as described earlier,[24] extracted and lyophilized following our published protocol.[22] The working concentration of NLE (250 mg/ml) was prepared in normal saline (0.9% NaCl) and kept at 4°C until use. Experimental animals were divided into two groups of three animals each. Immature non-cyclic female rats of 20 days old were fed 50 mg/day (25 mg/100 μl of normal saline twice a day) for 10 days through gavage and control animals were given the same amount of normal saline as vehicle control. This dose and route of administration was selected based on our recent published protocol.[24] After 10 days of NLE feeding, experimental rats were subjected to super ovulation induction protocol as previously described.[24] The 40-45 COCs was collected from one animal, hence three animals per group were sufficient in the present study.
Collection of COCs and analysis of their morphological changes
The ovary along with oviduct was collected in 35 mm Petri dish containing 5 ml of pre-worm culture medium from control as well as NLE-treated animals. Both ovary and oviduct were punctured using 26 gauge needle attached to tuberculin syringe under the dissecting microscope (Nikon Dissecting Microscope, Model C-DS; Tokyo, Japan) and COCs from ovary and oviduct were collected separately in 35 mm Petri dish containing 5 ml of pre-worm culture medium. Preovulatory COCs were used for the collection of granulosa cells and deoxyribonucleic acid (DNA) fragmentation analysis. Ovulated COCs (10-12 COCs from each group) were transferred to a grooved slide containing 100 μl of culture medium and immediately analyzed for their morphological changes using a phase-contrast microscope (Nikon, Eclipse; E600, Tokyo, Japan).
Collection of granulosa cells from preovulatory COCs
The granulosa cells (mural as well as cumulus cells) were separated from preovulatory COCs using 0.01% hyaluronidase in culture medium at 37°C followed by repeated pipetting. Granulosa cells were washed 3 times with fresh culture medium to remove enzyme activity. Washed granulosa cells were divided into two groups. The first group of granulosa cells was lysed in 1 ml of lysis buffer (5 mM Tris, 20 mM ethylenediaminetetra acetic acid (EDTA), 0.5% Triton X-100, pH 8) for 1 h at 4°C. Lysates were used for the quantitative analysis of hydrogen peroxide (H2O2) and total nitrite concentrations, catalase activity, cytochrome c and estradiol 17β concentrations. The second group of granulosa cells were washed 3 times with phosphate buffered saline (PBS) and fixed in 3.7% buffered formaldehyde for 15 min and then loaded on the slide and air-dried. These slides were used for in situ analysis of inducible nitric oxide synthase (iNOS), p53, Bax, Bcl2, cytochrome c expression, and DNA fragmentation by immunocytochemistry or fluorescence microscopy.
Quantitative analysis of H2O2 and total nitrite concentrations
The intracellular H2O2 concentration in granulosa cell lysate was analyzed using H2O2 assay kit purchased from Northwest Life Science Specialties, LLC, WA, USA and total nitrite concentration was analyzed using NO assay kit purchased from R & D Systems, MN, USA. The granulosa cells collected from preovulatory COCs were transferred to a microcentrifuge tube containing 300 μl of hypotonic lysis buffer (5 mM Tris, 20 mM EDTA, 0.5% Triton X-100, pH 8) for 1 h on ice for lysis. Lysates were centrifuged at 10,000 g at 4°C for 15 min and clear supernatant was immediately diluted by 5-fold with sample diluent and then used for the quantitative estimation of H2O2 and total nitrite concentrations by colorimetric assay as per company manual protocols. The optical density was determined using a microplate reader (Micro Scan MS5608A, ECIL, Hyderabad, India) set at 560 nm for H2O2 and 540 nm for total nitrite. The samples were run in triplicate and all samples were run in one assay to avoid inter-assay variation. The intra-assay variation for H2O2 and total nitrite concentrations were 2.8% and 2.2%, respectively.
Catalase activity assay
The catalase activity in granulosa cells lysate was analyzed following our previous published protocol[23] using catalase activity assay kit purchased from BioVision, Inc., CA, USA. The granulosa cell lysates were prepared as described above for the measurement of H2O2 concentration. Lysates were immediately used for the estimation of catalase activity as per company manual protocol and enzyme activity was calculated as the amount of H2O2 decomposed/min/ml and represented as μU/mg protein of cell lysate.
Detection of iNOS expression
Immunostaining for iNOS was carried out using anti-iNOS antibody purchased from Santa Cruz Biotechnology Inc., CA, USA. In brief, 50 μl of granulosa cell suspension from each group were fixed in 3.7% formaldehyde solution in PBS (0.01 M, pH 7.4) for 15 min and then air-dried. Slides were washed with PBS 2 times and then treated with 0.3% H2O2 in absolute methanol for 15 min to quench endogenous peroxidase activity. After two washed with PBS, slides were exposed to PBS containing 0.1% Triton X-100 to for permeabilization. Slides were exposed to 100 μl of blocking buffer (0.5% bovine serum albumin (BSA), 0.1% tween-20 in 100 ml PBS) at room temperature for 1 h and then incubated with 100 μl of diluted (1:100 in PBS) iNOS polyclonal rabbit antibody (Santa Cruz Biotechnology Inc., CA, USA) tagged with horseradish peroxidase (HRP) at room temperature for 1 h in a humidified chamber. At the end of the incubation period, slides were washed 3 times with PBS and then exposed to 100 μl of freshly prepared diaminobenzidine (DAB) solution (1 μl of 30% H2O2 and 5 μl of DAB in 1 ml of PBS; R & D Systems Inc., MN, USA) for 15 min. Thereafter, slides were washed 4 times in PBS and mounted in distyrene plasticizer xylene. The mounted slides were analyzed for DAB-positive staining using a phase-contrast microscope (Nikon, Eclipse; E600; Tokyo, Japan) at × 400 magnification. The experiment was repeated 3 times and a representative photograph is shown in the result section.
Detection of p53, Bax, and Bcl2 expressions
Granulosa cells were harvested from preovulatory COCs and immediately fixed with 3.7% buffered formaldehyde for 10 min at room temperature. The fixed cells (50 μl of cell suspension) were loaded on each slide and then air-dried. Slides were washed 3 times with pre-warmed PBS and exposed to Triton X-100 (0.01% in PBS) for 10 min at 37°C for permeabilization. Slides were washed 3 times with pre-warmed PBS and then treated with sodium citrate solution (0.01 M) at 37°C for 10 min. Slides were again washed 3 times with pre-warmed PBS and then incubated with blocking buffer (5% PBS-BSA solution) at 37°C for 30 min. Thereafter, slides were exposed to 100 μl of their respective primary antibodies (1:500 dilutions in blocking buffer) at 37°C for 2 h. After five washes with pre-warmed PBS, slides were exposed to 100 μl of fluorescein isothiocyanate (FITC) labeled secondary antibody (1:1000 dilution in blocking buffer) for 1 h at 37°C in a humidified chamber. After 1 h of incubation, slides were washed 5 times with pre-warmed PBS, mounted with fluorescence mounting medium and then observed under a fluorescence microscope (Olympus, Japan) at 520 nm at × 400 magnification.
Detection of cytochrome c expression
Immunostaining for cytochrome c was carried out using anti-cytochrome c antibody purchased from Santa Cruz Biotechnology Inc., CA, USA. In brief, granulosa cells from preovulatory COCs were fixed in 3.7% formaldehyde solution in PBS (0.01 M, pH 7.4). After two washes with PBS for 5 min each, cells (50 μl of cell suspension) were loaded on the slide and air-dried. The slides were processed as described above for the detection of iNOS expression. Slides were exposed to 100 μl of blocking buffer and then incubated with 100 μl of diluted (1:500 in PBS) cytochrome c polyclonal rabbit antibody (Santa Cruz Biotechnology Inc., CA, USA) tagged with HRP at room temperature for 1 h in a humidified chamber. At the end of the incubation period, slides were washed 3 times with PBS and then used for the analysis of cytochrome c. The experiment was repeated 3 times and a representative photograph is shown in the result section.
Cytochrome c enzyme-linked immunosorbent assay
The cytochrome c concentration in granulosa cells lysates was analyzed using cytochrome c assay kit purchased from R & D Systems, MN, USA. The granulosa cell lysates were prepared as described above for the measurement of H2O2 and total nitrite concentrations. The cytochrome c concentration was analyzed as per company manual protocol. The plate was read at 450 nm using a micro-plate reader (Micro Scan, ECIL, India). All samples were run in triplicate to avoid inter-assay variation and intra-assay variation was 1.12%.
DNA fragmentation analysis
The DNA fragmentation was assessed using acridine orange/ethidium bromide (AO/EB) staining following previous published protocol[25] with some modifications. Briefly, granulosa cells (50 μl of cell suspension), preovulatory and ovulated COCs (10-12 in each group) were transferred on a separate slide and fixed using 3.7% buffered formaldehyde for 15 min and then air-dried. Slides were washed 3 times with PBS (pH 7.5) and then labeled using the nucleic acid-binding dye mix (100 μl of 1:1 mixture of AO/EB solutions (4 μg/ml) for 1 min. Slides were then washed 3 times with PBS and photographed using fluorescence light microscope (TE-Eclipse 800, Nikon; Tokyo, Japan). The viable cells, apoptotic cells and necrotic cells were identified depending on their color change due to binding of ethidium bromide to fragmented DNA as described earlier.[26] Normal cells with intact DNA had green fluorescence of AO. The extent of EB binding to fragmented DNA changes the color from green to yellow (for early apoptotic cells), orange color (late apoptotic cells), and bright red color (necrotic cell). Experiment was repeated 3 times and representative photographs are shown in the result section.
Estradiol 17β ELISA
The granulosa cells from preovulatory COCs from each group were lysed in 1 ml of lysis buffer (5 mM Tris, 20 mM EDTA, 0.5% Triton X-100, pH 8) for 1 h at 4°C. The supernatant was collected after centrifugation at 10,000 g for 15 min at 4°C and used for the determination of estradiol 17β concentration using ELISA kit purchased from DiaMetra, Milano, Italy. In brief, 25 μl of standards and samples were added to microwells plate in duplicate. Thereafter, 200 μl of 17β estradiol-HRP conjugate was added to each well, mixed well and then incubated for 2 h at 37°C. At the end of the incubation period, microplate was rinsed and flicked 5 times with wash buffer. After washing, 100 μl of 3,3’,5,5’-tetramethylbenzidine (TMB) substrate was added to each well and incubated for 30 min at room temperature in the dark. Thereafter, 100 μl of stop solution was added to each well, mixed for 30 s and absorbance was read at 450 nm using micro plate reader (Micro Scan, ECIL, India) within 15 min. All samples were run in one assay to avoid inter-assay variation and intra-assay variation was 1.4%. The estradiol 17β concentration is presented in ng/mg protein of granulosa cell lysate.
Statistical analysis
Data are expressed as mean ± standard error of mean of triplicate samples. Data were analyzed by either Student's t-test using Statistical Package for the Social Sciences (SPSS) software, version 17.0 (SPSS, Inc., Chicago, IL, USA). A probability of P < 0.05 was considered significant.
RESULTS
NLE reduced number of granulosa cells and induced oocyte apoptosis
As shown in Figure 1a, aqueous NLE treatment (50 mg/day) for 10 days reduced number of granulosa cells encircling oocyte and induced granulosa cell dispersion [Figure 1a; NLE-treated-A] as compare to control ovulated COCs where a large number of granulosa cells are tightly attached to the oocyte [Figure 1a; control]. The NLE treatment induced apoptosis [Figure 1a; NLE-treated-A] in the majority of oocytes [76.41 ± 3.65%; Figure 1b], remaining oocytes were looking morphologically normal with a reduced number of granulosa cells encircling with oocyte [Figure 1a; NLE-treated-N].
Figure 1.
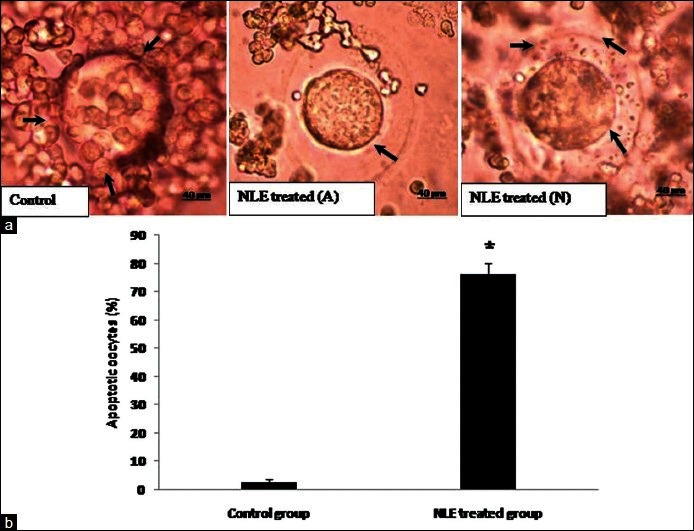
Representative photographs showing neem leaf extract (NLE) induced morphological changes in ovulated cumulus oocyte complexes (COCs). (a) Control, cumulus oocytes complex showing greater number and tightly intact cumulus granulosa cells with oocyte (arrows). NLE-treated-A, NLE (50 mg/day for 10 days) reduced number of granulosa cells and induced shrinkage in the majority of COCs (shrinkage; arrow). NLE-treated-N, Remaining COCs showing normal morphology with a reduced number of granulosa cells (arrows). (b) Effect of NLE treatment on the rate of oocytes apoptosis. Data are mean ± SE of three replicates. *Denotes significantly (P < 0.05) higher as compare to control (n = 3/group; Student's t-test). Bar = 40 μm
NLE increased H2O2 concentration and reduced catalase activity in granulosa cells
The quantitative analysis of H2O2 suggests that NLE treatment significantly (P < 0.05) increased H2O2 concentration in granulosa cells (38.58 ± 8.56 μM/mg protein) as compare to control granulosa cells lysate [13.13 ± 0.94 μM/mg protein; Figure 2a]. However, NLE treatment significantly (P < 0.05) reduced catalase activity (15.22 ± 0.72 μU/mg protein) as compare to control granulosa cells lysate [21.24 ± 1.91 μU/mg protein; Figure 2b].
Figure 2.
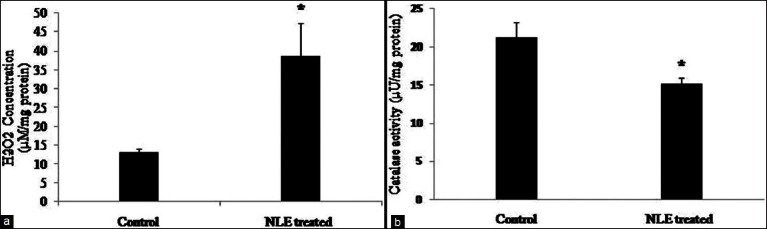
Neem leaf extract (50 mg/day for 10 days) treatment increased H2O2 concentration (a) and reduced catalase activity (b) in granulosa cells of preovulatory cumulus oocyte complexes. Data are mean ± SE of three replicates. *Denotes significantly (P < 0.05) different as compare to control (n = 3/group; Student's t-test)
NLE increased iNOS expression and total nitrite concentration in granulosa cells
As shown in Figure 3a, NLE increased iNOS expression in most of the treated granulosa cells as compare to control granulosa cells. The quantitative analysis of total nitrite concentration further strengthens our data and suggest that NLE significantly (P < 0.05) increased total nitrite concentration in treated granulosa cells lysate (57.45 ± 4.68 μM/mg protein) as compare to control granulosa cells lysate [44.43 ± 0.90 μM/mg protein; Figure 3b].
Figure 3.
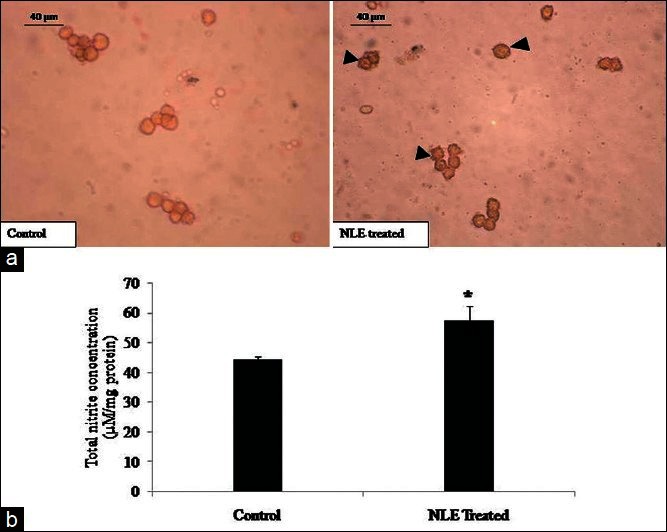
Representative photograph showing the effect of neem leaf extract (NLE) (50 mg/day for 10 days) on immunocytochemical localization of inducible nitric oxide synthase (iNOS) expression and total nitrite concentration in granulosa cells of preovulatory COCs. Most of the granulosa cells collected from preovulatory COCs of NLE-treated animal show higher iNOS expression as compared to control (a; arrows). NLE treatment increased total nitrite concentration in granulosa cells of preovulatory COCs (b). Data are mean ± SE of three replicates. *Denotes significantly (P < 0.05) higher as compare to control (n = 3/group; Student's t-test). Bar = 40 μm
NLE increased p53/Bax expressions and reduced Bcl2 expression in granulosa cells
As shown in Figure 4a, NLE treatment significantly increased number of granulosa cells showing high expression of p53 [Figure 4a] and pro-apoptotic protein such as Bax expression [Figure 4b] as compare to their respective controls. However, NLE significantly reduced anti-apoptotic protein such as Bcl2 expression in treated granulosa cells as compare to control granulosa cells [Figure 4c].
Figure 4.
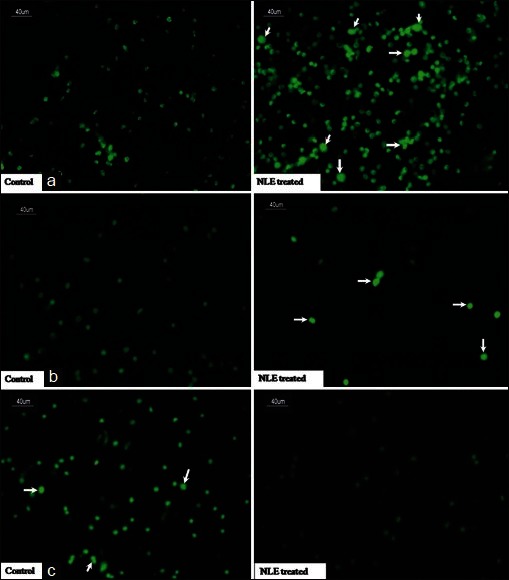
Representative immunofluorescence photograph showing the effects of neem leaf extract (NLE) (50 mg/day for 10 days) treatment on p53, Bax, and Bcl2 expression in granulosa cells of preovulatory cumulus oocyte complexes. NLE treatment increased p53 (a; arrows), Bax (b; arrows), and decreased Bcl2 (c; arrows) expression in granulosa cells. Bar = 40 μm
NLE increased cytochrome c level in granulosa cells
As shown in Figure 5a, NLE treatment significantly increased number of granulosa cells showing higher expression of cytochrome c as compared to control granulosa cells. The quantitative analysis of cytochrome c further strengthened our results and suggests that NLE treatment significantly (P < 0.05) increased cytochrome c concentration (72.30 ± 5.01 ng/mg protein) as compared to control granulosa cells [47.33 ± 4.91 ng/mg protein; Figure 5b].
Figure 5.
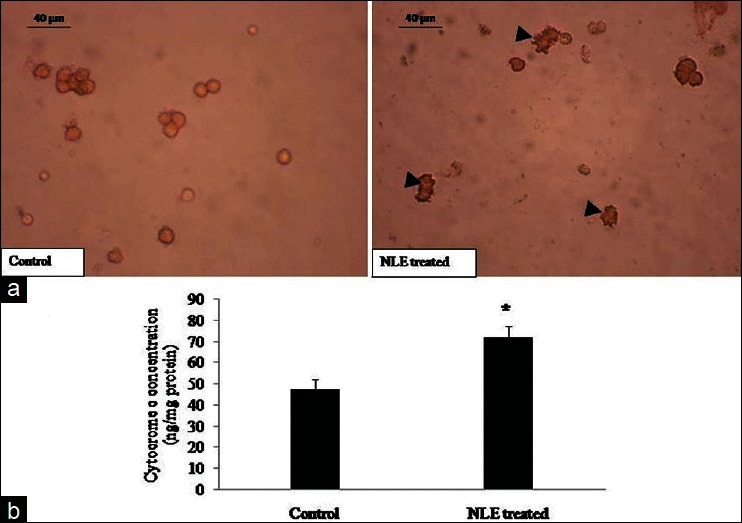
Representative photograph showing effects of neem leaf extract (NLE) (50 mg/day for 10 days) treatment on immunocytochemical localization of cytochrome c expression and cytochrome c contration in granulosa cells isolated from cumulus oocyte complexes (COCs) collected from ovary. Most of the granulosa cells collected from preovulatory COCs of NLE-treated animal show higher cytochrome c expression as compared to control (a; arrows) and increased cytochrome c concentration (b) in granulosa cells collected from ovary. Data are mean ± SE of three replicates. *Denotes significantly (P < 0.05) higher as compare to control (n = 3/group; Student's t-test). Bar = 40 μm
NLE-induced DNA fragmentation in granulosa cells
To confirm the occurrence of DNA fragmentation, granulosa cells collected from preovulatory COCs were exposed to nucleic acid binding dye (AO/EB). As shown in Figure 6a, granulosa cells collected from control preovulatory COCs did not show DNA fragmentation as evidenced by green fluorescence of AO as a background color of fixed cells. However, NLE-induced initiation of apoptosis since most of the treated granulosa cells showed yellow color fluorescence [Figure 6b]. Although granulosa cells collected from ovulated COCs also showed initiation of apoptosis as evidenced by yellow color fluorescence [Figure 6c], NLE treatment induced cell death in almost all granulosa cells [Figure 6d]. The preovulatory as well as ovulated COCs of NLE-treated animals showing normal oocytes morphology were labeled with nucleic acid binding dye (AO/EB). Granulosa cells encircling control oocyte did not show any DNS fragmentation as evidenced by greenish background color of AO [Figure 7a], while NLE treatment, induced apoptosis in most of the granulosa cells as evidenced by yellowish orange color [Figure 7b]. Although initiation of apoptosis was observed in granulosa cells encircling control ovulated oocyte [Figure 7c], NLE treatment induced granulosa cell death, reduced granulosa cell number and induced dispersion [Figure 7d].
Figure 6.
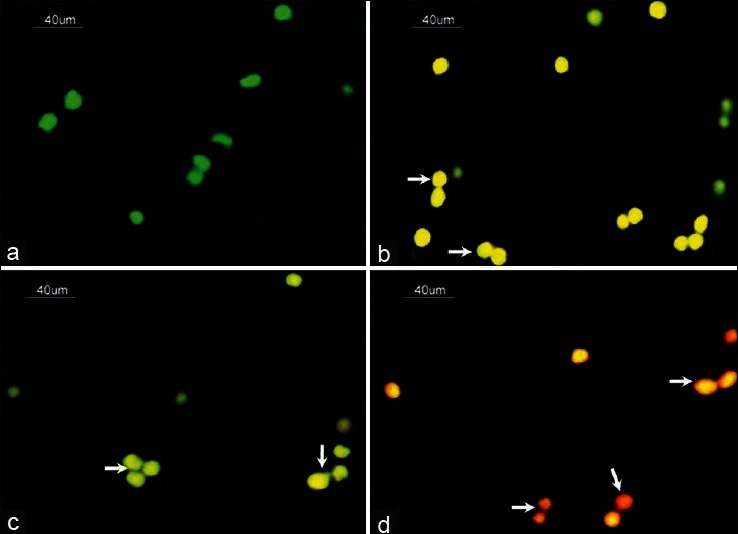
Representative photograph showing effects of neem leaf extract (NLE) (50 mg/day for 10 days) treatment on deoxyribonucleic acid (DNA) fragmentation in granulosa cells isolated from preovulatory as well as ovulated cumulus oocyte complexes (COCs). (a) Granulosa cells collected from preovulatory COCs did not show any DNA fragmentation as evidenced by green fluorescence of acridine orange (AO). (b) NLE-induced apoptosis in most of the granulosa cells of preovulatory COCs as evidenced by yellowish orange fluorescence of ethidum bromide (EB) (arrows). (c) The initiation of apoptosis as evidenced by yellow fluorescence of EB was observed in granulosa cells of ovulated COCs (arrows). (d) NLE treatment induced cell death as evidenced by reddish fluorescence of EB and reduced number of granulosa cells of ovulated COCs (arrows) (n = 3/group). Bar = 40 μm
Figure 7.
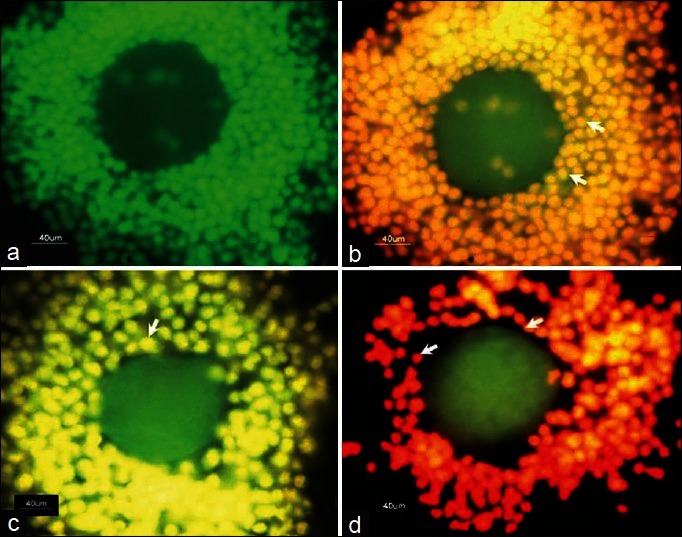
Representative photograph showing neem leaf extract (NLE) (50 mg/day for 10 days) induced deoxyribonucleic acid (DNA) fragmentation in granulosa cells encircling oocytes that showed normal morphology at the time of collection. (a) Granulosa cells encircling preovulatory oocytes did not show DNA fragmentation as evidenced by green fluorescence of acridine orange (AO). (b) NLE-induced apoptosis in most of the granulosa cells encircling preovulatory oocyte as evidenced by yellowish orange fluorescence of ethidium bromide (EB) (arrows). (c) The initiation of apoptosis was also observed in granulosa cells encircling ovulated oocyte as evidenced by yellow fluorescence of EB (arrow). (d) NLE treatment induced cell death thereby reduced number of granulosa cells encircling ovulated oocyte as evidenced by reddish fluorescence of EB (arrows) (n = 3/group). Bar = 40 μm
NLE reduced estradiol 17β concentration in granulosa cells
Inside the follicular microenvironment, active granulosa cells of preovulatory COCs synthesize estradiol 17β required for growth and development of oocyte. However, granulosa cell apoptosis may result in reduction of estradiol 17β. Therefore, we analyzed estradiol 17β concentration in granulosa cell lysate collected from preovulatory COCs. As shown in Figure 8, NLE treatment significantly (P < 0.05) reduced estradiol 17β level in granulosa cells (0.62 ± 0.06 ng/mg protein) as compare to control (1.37 ± 0.14 ng/mg protein).
Figure 8.
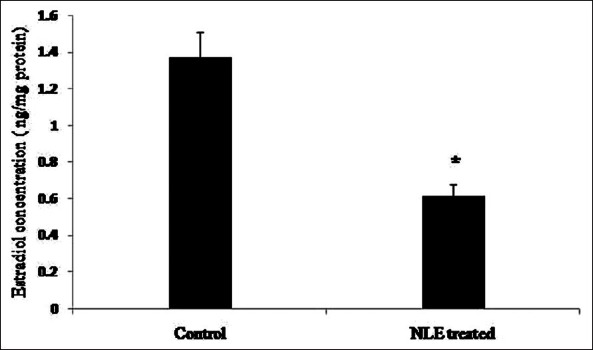
Neem leaf extract (50 mg/day for 10 days) treatment reduced estradiol 17β concentration in granulosa cells isolated from preovulatory cumulus oocyte complexes. Data are mean ± SE of three replicates. *Denotes significant (P < 0.05) decrease as compare to control granulosa cell lysate (n = 3/group; Student's t-test)
DISCUSSION
Preovulatory as well as ovulated oocytes are encircled with several layers of cumulus and mural granulosa cells. These granulosa cells are generally discarded at the time of in vitro fertilization (IVF) but these cells can be used for the assessment of oocyte quality.[9] Through gap junctions, granulosa cells provide nutrients and maturation-enabling factors to ensure developmental competency of preovulatory oocytes.[2–5,10] However, premature rupture of communication between granulosa cells and oocyte or removal of granulosa cells from oocyte or cumulus cell apoptosis may reduce developmental competence of oocyte[9,11–13,15,17] and increase susceptibility of oocyte towards apoptotic cell death.[17] Data of the present study suggests that NLE (50 mg/day) treatment for 10 days reduced number of granulosa cells encircling oocytes, induced granulosa cell dispersion and induced oocyte apoptosis in the majority of ovulated COCs. The reduced number of granulosa cells and disruption in the cell-cell communication might have deprived oocyte of nutrients and survival factors inside the preovulatory follicle and induced apoptosis in ovulated oocytes. These results further strengthen our recent observations that NLE reduced number, induced granulosa cells dispersion in ovulated COCs and induced oocytes apoptosis through the mitochondria-mediated pathway.[24] Based on these studies, we propose that granulosa cell apoptosis in preovulatory COCs could be one of the causes associated with NLE-induced oocytes apoptosis in vivo.
The aqueous NLE contains bioactive flavonoids including quercetin,[21,27] which induces generation of ROS and thereby apoptosis in various somatic cell types.[28–31] A possibility exist that NLE may induce generation of ROS and thereby apoptosis in granulosa cells of preovulatory COCs. To strengthen this possibility, we isolated granulosa cells from preovulatory COCs and analyzed whether NLE induces apoptosis. Our results suggest that NLE treatment significantly increased H2O2 concentration and decreased catalase activity in granulosa cell lysate. Further, iNOS expression and total nitrite concentrations were also increased in granulosa cells suggesting that NLE increases ROS levels in granulosa cells encircling oocyte of preovulatory follicle. Similarly, NLE-induced generation of ROS has recently been reported in apoptotic oocytes.[24] The increased levels of ROS act as upstream signal to trigger activation of p53, which play a central role in induction of apoptosis.[32,33] Data of the present study suggests that NLE treatment induced p53 expression in most of the granulosa cells encircling preovulatory oocyte. The expression was mainly in the cell cytoplasm. When p53 accumulates in the cell cytoplasm, it induces proapoptotic protein such as Bax to induce apoptosis.[34,35] Our data revealed that NLE-induced underexpression of Bcl2 and overexpression of Bax protein in treated granulosa cells. The NLE-induced overexpression of Bax has been reported in apoptotic oocyte cultured in vitro.[22] These results together with our previous findings suggest that the activation of p53 in the cytoplasm induced overexpression of Bax protein and setting NLE-treated granulosa cell toward apoptosis.
Changes in the ratio of apoptotic promoters to suppressors (Bax/Bcl-2) within a cell disrupts mitochondria membrane potential and induces the release of cytochrome c which initiate apoptotic signals in a wide variety of somatic cells.[30,35–44] Similarly, a possibility exist that NLE-induced overexpression of Bax may disrupt mitochondria membrane potential and increase cytochrome c release in granulosa cells. Data of the present study suggests that NLE increased cytochrome c expression in granulosa cells. The quantitative analysis further revealed that NLE increased cytochrome c concentration in granulosa cell lysate. These data corroborates with recent observations that NLE increased cytochrome c concentration in apoptotic oocytes.[24] Taken together, these data with previous observations suggest that NLE-induced overexpression of Bax might have disrupted mitochondria membrane potential to release cytochrome c and thereby apoptosis in granulosa cells.
The release of cytochrome c from mitochondria activates upstream and downstream caspases to induce apoptotic cell death. Although we did not analyze caspases activities in the present study, our previous observations suggest that NLE induce caspase-9 and caspase-3 activities and thereby apoptosis in rat oocytes.[24] The activated procaspase-3 cleaves key structural and regulatory proteins that result in the biochemical such as DNA fragmentation in 180-200 base-pair, a hallmark feature of apoptosis.[45] The fragmented DNA can be detected on the basis of color changes in a cell using nucleic acid-binding dye mix of AO/EB[26] in a fixed cell.[25] Data of the present study suggests that NLE-induced DNA fragmentation in granulosa cells encircling preovulatory oocyte. Although initiation of apoptosis in peripheral granulosa cells of ovulated oocyte have also been observed, NLE treatment induced DNA fragmentation in almost all granulosa cells encircling oocyte after ovulation. Although apoptosis has also been observed in ovulated control COCs, NLE treatment induced cell death in most of the granulosa cells encircling ovulated COCs. These data suggest that NLE-induced apoptosis in granulosa cells of preovulatory COCs that could be one of the causes for a reduced number of granulosa cells encircling oocytes after ovulation. Similarly, it has been reported that granulosa cells encircling preovulatory oocytes did not show DNA fragmentation while peripheral granulosa cells encircling oocyte shortly after ovulation showed DNA fragmentation.[18]
Granulosa cells in preovulatory follicles are the major source for estradiol 17β biosynthesis. Granulosa cell apoptosis reduces estradiol 17β biosynthesis and generates hypoestrogenic condition in the ovary.[22,46] The reduced estradiol 17β level reduces oocyte quality by inducing apoptosis.[46] We hypothesize that NLE-induced granulosa cell apoptosis may reduce estradiol 17β biosynthesis and thereby oocyte quality by inducing susceptibility towards apoptosis. The quantitative analysis of estradiol 17β using ELISA in the present study suggest that NLE reduced estradiol 17β concentration in granulosa cell lysate. These data support our previous observations that granulosa cell apoptosis generates hypoestrogenic condition and affects oocyte quality by inducing apoptosis.[22,46]
CONCLUSIONS
On the basis of results obtained in this study, it can be concluded that NLE-induced generation of ROS, increased p53 and thereby Bax protein expression in granulosa cells of preovulatory COCs. The overexpression of Bax modulated mitochondria membrane potential and increased cytochrome c release. A rise in cytochrome c concentration was associated with induced DNA fragmentation in granulosa cells of preovulatory COCs. The induced granulosa cell apoptosis was associated with the reduced estradiol 17β concentration. Reduced estradiol 17β concentration might have affected the oocytes quality by inducing oocytes apoptosis in rat. These data suggest that aqueous NLE generates ROS and induces granulosa cell apoptosis through a mitochondria-mediated pathway. The reduced number of granulosa cell and estradiol 17β production may induce oocyte apoptosis during female fertility regulation. Further studies are required to standardize aqueous NLE dose for fertility regulation in human.
ACKNOWLEDGMENT
This study was supported financially by Department of Science and Technology, New Delhi, India.
Footnotes
Source of Support: This study was supported financially by Department of Science and Technology, New Delhi, India.
Conflict of Interest: None declared.
REFERENCES
- 1.Allworth AE, Albertini DF. Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Dev Biol. 1993;158:101–12. doi: 10.1006/dbio.1993.1171. [DOI] [PubMed] [Google Scholar]
- 2.Barrett SL, Albertini DF. Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J Assist Reprod Genet. 2010;27:29–3. doi: 10.1007/s10815-009-9376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–77. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 4.Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: Potential early markers of oocyte competence. Reproduction. 2010;140:835–52. doi: 10.1530/REP-10-0248. [DOI] [PubMed] [Google Scholar]
- 5.Albertini DF. A cell for every season: The ovarian granulosa cell. J Assist Reprod Genet. 2011;28:877–8. doi: 10.1007/s10815-011-9648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: Role of cumulus cells. Biol Reprod. 2000;63:805–10. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol Reprod. 2009;80:493–502. doi: 10.1095/biolreprod.108.072017. [DOI] [PubMed] [Google Scholar]
- 8.Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51:57–64. doi: 10.1080/15216540119253. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, McKenzie LJ, Matzuk MM. Revisiting oocyte-somatic cell interactions: In search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod. 2008;14:673–8. doi: 10.1093/molehr/gan064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assou S, Haouzi D, De Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod. 2010;16:531–8. doi: 10.1093/molehr/gaq032. [DOI] [PubMed] [Google Scholar]
- 11.Chian RC, Niwa K, Sirard MA. Effects of cumulus cells on male pronuclear formation and subsequent early development of bovine oocytes in vitro. Theriogenology. 1994;41:1499–508. doi: 10.1016/0093-691x(94)90201-s. [DOI] [PubMed] [Google Scholar]
- 12.Perez GI, Tilly JL. Cumulus cells are required for the increased apoptotic potential in oocytes of aged mice. Hum Reprod. 1997;12:2781–3. doi: 10.1093/humrep/12.12.2781. [DOI] [PubMed] [Google Scholar]
- 13.Modina S, Luciano AM, Vassena R, Baraldi-Scesi L, Lauria A, Gandolfi F. Oocyte developmental competence after in vitro maturation depends on the persistence of cumulus-oocyte comunications which are linked to the intracellular concentration of cAMP. Ital J Anat Embryol. 2001;106:241–8. [PubMed] [Google Scholar]
- 14.Jancar N, Kopitar AN, Ihan A, Virant Klun I, Bokal EV. Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. J Assist Reprod Genet. 2007;24:91–7. doi: 10.1007/s10815-006-9103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan Y, Hao ZD, Liu J, Wu Y, Yang L, Liu GS, et al. Heat shock at the germinal vesicle breakdown stage induces apoptosis in surrounding cumulus cells and reduces maturation rates of porcine oocytes in vitro. Theriogenology. 2008;70:168–78. doi: 10.1016/j.theriogenology.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Kim YS, Kim EY, Moon J, Yoon TK, Lee WS, Lee KA. Expression of interferon regulatory factor-1 in the mouse cumulus-oocyte complex is negatively related with oocyte maturation. Clin Exp Reprod Med. 2011;38:193–202. doi: 10.5653/cerm.2011.38.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Wang XL, Liu JH, Bao ZJ, Tang DW, Wu Y, et al. BIM EL-mediated apoptosis in cumulus cells contributes to degenerative changes in aged porcine oocytes via a paracrine action. Theriogenology. 2011;76:1487–95. doi: 10.1016/j.theriogenology.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Szołtys M, Tabarowski Z, Pawlik A. Apoptosis of postovulatory cumulus granulosa cells of the rat. Anat Embryol (Berl) 2000;202:523–9. doi: 10.1007/s004290000122. [DOI] [PubMed] [Google Scholar]
- 19.Grupen CG, Armstrong DT. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: Temporal effects of follicular fluid during IVM. Reprod Fertil Dev. 2010;22:1100–9. doi: 10.1071/RD09307. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko T, Saito H, Takahashi T, Ohta N, Saito T, Hiroi M. Effects of controlled ovarian hyperstimulation on oocyte quality in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet. 2000;17:580–5. doi: 10.1023/A:1026439409584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subapriya R, Nagini S. Medicinal properties of neem leaves: A review. Curr Med Chem Anticancer Agents. 2005;5:149–6. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 22.Chaube SK, Prasad PV, Khillare B, Shrivastav TG. Extract of Azadirachta indica (neem) leaf induces apoptosis in rat oocytes cultured in vitro. Fertil Steril. 2006;85(Suppl 1):1223–31. doi: 10.1016/j.fertnstert.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi A, PremKumar KV, Pandey AN, Khatun S, Mishra SK, Shrivastav TG, et al. Melatonin protects against clomiphene citrate-induced generation of hydrogen peroxide and morphological apoptotic changes in rat eggs. Eur J Pharmacol. 2011;667:419–24. doi: 10.1016/j.ejphar.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Tripathi A, Shrivastav TG, Chaube SK. Aqueous extract of Azadirachta indica (neem) leaf induces generation of reactive oxygen species and mitochondria-mediated apoptosis in rat oocytes. J Assist Reprod Genet. 2012;29:15–23. doi: 10.1007/s10815-011-9671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natesan S, Kataria JM, Dhama K, Bhardwaj N, Sylvester A. Anti-neoplastic effect of chicken anemia virus VP3 protein (apoptin) in Rous sarcoma virus-induced tumours in chicken. J Gen Virol. 2006;87:2933–40. doi: 10.1099/vir.0.82085-0. [DOI] [PubMed] [Google Scholar]
- 26.Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–47. doi: 10.1210/endo.140.11.7135. [DOI] [PubMed] [Google Scholar]
- 27.Sithisarn P, Gritsanapan W. Variability of antioxidative quercetin content in Siamese neem tree leaves in Thailand by TLC-densitometry. Acta Hort (ISHS) 2008;786:161–9. [Google Scholar]
- 28.Lee YK, Park SY, Kim YM, Lee WS, Park OJ. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009;41:201–7. doi: 10.3858/emm.2009.41.3.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YK, Hwang JT, Kwon DY, Surh YJ, Park OJ. Induction of apoptosis by quercetin is mediated through AMPKalpha1/ASK1/p38 pathway. Cancer Lett. 2010;292:228–36. doi: 10.1016/j.canlet.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Priyadarsini Vidya R, Murugan Senthil R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Jung JH, Lee JO, Kim JH, Lee SK, You GY, Park SH, et al. Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J Cell Physiol. 2010;223:408–14. doi: 10.1002/jcp.22049. [DOI] [PubMed] [Google Scholar]
- 32.Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–81. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- 33.Liu B, Chen Y, St Clair DK. ROS and p53: A versatile partnership. Free Radic Biol Med. 2008;44:1529–35. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee S, Chakraborty P, Saha P, Bandyopadhyay SA, Banerjee S, Kabir SN. Ovotoxic effects of galactose involve attenuation of follicle-stimulating hormone bioactivity and up-regulation of granulosa cell p53 expression. PLoS One. 2012;7:e30709. doi: 10.1371/journal.pone.0030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun T, Dar S, Vorobiov D, Lindenboim L, Dascal N, Stein R. Expression of Bcl-x (S) in Xenopus oocytes induces BH3-dependent and caspase-dependent cytochrome c release and apoptosis. Mol Cancer Res. 2003;1:186–94. [PubMed] [Google Scholar]
- 37.Johnson CE, Freel CD, Kornbluth S. Features of programmed cell death in intact Xenopus oocytes and early embryos revealed by near-infrared fluorescence and real-time monitoring. Cell Death Differ. 2010;17:170–9. doi: 10.1038/cdd.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig. 2006;13:451–8. doi: 10.1016/j.jsgi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Kumar Harish G, Mohan Chandra KV, Rao Jagannadha A, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Invest New Drugs. 2009;27:246–52. doi: 10.1007/s10637-008-9170-z. [DOI] [PubMed] [Google Scholar]
- 43.Kumar Harish G, Priyadarsini Vidya R, Vinothini G, Letchoumy Vidjaya P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010;28:392–401. doi: 10.1007/s10637-009-9263-3. [DOI] [PubMed] [Google Scholar]
- 44.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–8. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 45.Jurisicova A, Acton BM. Deadly decisions: The role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction. 2004;128:281–91. doi: 10.1530/rep.1.00241. [DOI] [PubMed] [Google Scholar]
- 46.Chaube SK, Prasad PV, Thakur SC, Shrivastav TG. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis. 2005;10:863–74. doi: 10.1007/s10495-005-0367-8. [DOI] [PubMed] [Google Scholar]


