Abstract
Background:
Alzheimer's disease (AD), a progressive neurodegenerative disorder characterized by multiple cognitive deficits, is often accompanied by behavioral disorders and mood changes. Because of the non-availability of proper curative/preventive therapy for AD, the present study was designed to evaluate the possible role of Azadirachta indica in experimental AD in rats.
Materials and Methods:
Experimental AD in rats was produced by nucleus basalis magnacellularis lesion with ibotenic acid (IB) and intacerebroventricular administration of colchicine (Col). Different behavioral tests and biochemical analysis were performed to explore the role to A. indica in AD.
Results:
A. indica exhibited anxiolytic activity in the open field test in Col lesion animals, which was comparable to that of diazepam. In the Elevated plus maze test, A. indica significantly alleviated IB and Col-induced anxiety. IB and Col-induced depression was mitigated by A. indica, and the results were comparable to that of imipramine. In Morris’ water maze test, A. indica pre-treatment improved reference memory, working memory and spatial learning, which are at par with the effects of donepezil. Both IB and Col-induced deficits in active avoidance learning and retention of learned behavior were significantly reversed by A. indica. IB and Col-induced increased lipid peroxidase activity was significantly reversed by A. indica (reductions in malondialdehyde level). A. indica stabilized rise in superoxide dismutase and a decreasing trend in acetylcholine-esterase (AChE) activity was seen with IB and Col lesions. A. indica had no effect over the AChE activity.
Conclusion:
A. indica might be effective in clinical AD by virtue of its cognition enhancement, antidepressant and antianxiety properties.
Keywords: Alzheimer's disease, Azadirachta indica, flavonoids, phytosterols
INTRODUCTION
In the present era, anxiety, depression, dementia and other cognitive disorders are becoming increasingly prevalent. These behavioral changes are the outcome of either functional or organic aberrations in the central nervous system (CNS). One of the important CNS disorders incapacitating mainly elderly people toward end of their life is Alzheimer's disease (AD). This neurodegenerative dementing disorder accounts for approximately 70% of dementia affecting 17-25 million elderly people worldwide.[1–3] The prevalence of this disease doubles every 5 years after the age of 60-64 years. With advancement in the medical health care system, the life expectancy has increased with a parallel increase in the prevalence of AD.[4]
A number of hypotheses have been envisaged to delineate the pathophysiology of AD. The neurobiological theory of cholinergic system has been targeted for the symptomatic treatment of AD.[5–11] In spite of remarkable increase in the scientific knowledge about the pathobiology of AD, attempts other than modifying the cholinergic neurotransmission have proved futile for the treatment of AD. Scientists are left blindfolded in the information jungle, still trying to hit upon a suitable remedy for AD.
In the Indian indigenous system of medicine, the Ayurveda, a number of agents are in use since ancient time for chronic debilitating disorders. “Rasayanas,” a class of Ayurvedic preparation having adaptogenic properties, have been claimed to increase the longevity on prolonged administration.[12,13] Azadirachta indica, a Juss (Maliaceae), commonly known as “Neem,” is a large evergreen tree found throughout India and its neighboring countries. It is known to posses many beneficial properties and has been described as “Rasayana.”[13,14] It has been in use in the Indian system of medicine for its various medicinal properties from ancient times. Different parts of the A. indica tree have been studied and reported to contain many limonoids, viz. nimbin, azadirachtin and meliantriol, etc., which have a variety of properties ranging from insecticidal to male spermicidal actions.[14] A. indica is known to be beneficial in microbial infections, skin diseases and dental disorders, and is known to possess hypoglycemic, antiulcerogenic, anti-inflammatory and anti-stress properties.[15,16] Fresh leaf extract of A. indica has been found to possess anxiolytic activity in rat models of anxiety.[17] Earlier studies on A. indica leaf juice have demonstrated anxiolytic, bronchial smooth muscle relaxing and hepatoprotective actions.[17–19] It has been reported to possess hypoglycemic, anti-inflammatory, immunomodulatory, antioxidant and cognition-enhancing properties.[20–23] Recently, it has been demonstrated that the A. indica pre-treatment is protective in ischemia-reperfusion injury of brain due to its antioxidant property and that it can reverse cognitive deficits induced by chronic hypoperfusion of brain in rats on bilateral carotid artery ligation.[19] Various active principles present in the plant are responsible for their varying biological and medicinal properties, viz. compounds like azadirachtin and nimbidin have been shown to be responsible for blood sugar lowering and antihypertensive actions, and flavonoids, limonoids and phytosterols play a major role in its anti-inflammatory, antioxidant, immunomodulatory and adaptogenic properties.[22–27]
In AD, the symptomatology mainly comprises of cognitive deficits, apathy, dysphoria/depression and anxiety along with other affective symptoms. Because A. indica have earlier been proved to possess antianxiety and cognition-enhancing attributes in different models of cerebrodegenerative disorders, the present study was undertaken to explore the possible role of this drug of plant origin in an experimental model of AD.
MATERIALS AND METHODS
Animals
After Institutional Animal Ethics Committee approval, the present study was conducted on standard inbred Charles-Foster albino rats of either sex weighing 150-200 g. They were kept in an animal house in colony cages at an ambient temperature of 25 ± 2°C and relative humidity of 45-55% with 12 h: 12 h day and night cycle. Throughout their stay, they had free access to standard rodent pellet food and drinking water. The food was withdrawn 12 h prior to the surgical procedure; however, water was allowed ad libitum. Throughout the experiment, the Laboratory Animal Care Guidelines by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) were followed.[28]
Chemicals and reagents
Ibotenic acid (IB), colchicine (Col), acetylcholine chloride, thiobarbituric acid, nicotinamide adenine dinucleotide-reduced form (NADH), nitroblue tetrazolium (NBT) and phenazine methosulfate (PMS) were obtained from Sigma, USA. 1, 1, 3, 3-Tetraetoxypropane was obtained from Merck, Germany. All other chemicals and reagents were purchased locally and were of highest analytical grade. Donepezil (DOZ) and Imipramine (IMI) were purchased from Sun Pharma and Diazepam (DZP) was purchased from Glaxo.
Plant material and standardization of extract
Fresh green leaves of A. indica were collected from the Ayurvedic garden of our institute in the month of April. The collected leaves were washed and diseased/dried leaves were discarded. The clean leaves weighing 5.3 kg were shade dried for 3 days. They were crushed and macerated in double distilled water in a ratio of 1:4 for 3 days and filtered through a Whatman No. 1 filter paper. The filtrate was heat evaporated to dry and the aqueous extract was stored in a refrigerator until use. The yield was 2.7%. It was filtered and the filtrate was concentrated over a water bath to obtain the solidified extract that weighed 81g. The aqueous extract was subjected to high-performance thin-layer chromatography (HPTLC) and gas chromatographic analysis for quantification of flavonoids (total, quercetin and rutin) and phytosterols (total, β-sitosterol, camposterol and stigmasterol).
For determination of sterols, the extract was quantified as described earlier.[28] The sample (1 g) was dispersed in 20 mL of water, transferred to a separating funnel and extracted with chloroform till color persisted. The chloroform extract was passed through anhydrous sodium sulfate and the solvent was evaporated completely over a water bath. The residue thus obtained was acetylated using the mixture of acetic anhydride and pyridine as per the usual procedure. The solvent was evaporated and the residue was dissolved in 1 mL of chloroform. Five microliters of this test sample along with four different concentrations of standard were injected into a Varian 3800 gas chromatograph. The column used was VA 17, 30 m × 0.25 mm id. The flame ionization and injector temperatures were set at 330°C and 250°C, respectively. The carrier gas was nitrogen at a flow rate of 5 mL/min. The contents of phytosterols were quantified using the linear regression equation obtained from the calibration curve of the standard. The percentage of total phytosterols was 0.394% w/w (β-sitosterol – 0.267%, camposterol – 0.048% and stigmasterol – 0.078%).
The bioflavonoids were quantified using the following method – 100 mg of the test drug was dissolved in 10 mL of aqueous methanol (1:1) and 5 μl and 10 μl of the same were was applied on a pre-coated Thin layer chromatography (TLC) plate (E. Merck, Cat No. 5554). Along with five different concentrations of standard, this was then subjected to HPTLC analysis (Camag TLC system, with CATS v 4.05, S/N 0202A016/Sc3 v1.14, S/N: 041123 evaluation software). The plate was developed in ethyl acetate: Formic acid: Acetic acid: Water = 100:11:11:27 upto 80 mm, which was then air dried and scanned at 360 nm. The contents of bioflavonoids (total, quercetin and rutin) were determined using a linear regression equation plotted between concentration and area of standard. The yield was 1.25% total flavonoids, quercetin 0.083% and rutin 0.41%.
Experimental procedure
Grouping of animals
For the study purpose, the animals (N = 360) were randomly allocated into groups comprising 10 animals in each group, expecting 20-25% post-procedure mortality [Table 1].
Table 1.
Grouping of animals
The groups used for the different tests were IB DZP and Col DZP for the open field test; IB IMI and Col IMI for the Porsolts’ swim test and Active avoidance paradigm; and IB DOZ and Col DOZ for the Elevated plus maze test, Morris’ water maze test, lipid peroxidation and acetylcholinesterase activity.
Dosing schedules
Pre-operatively, the animals received vehicle and the respective drugs for 7 days. On the 7th day, 1 h after the drug dosing, experimental AD was induced in the animals. Day of induction of AD was considered as the 0th day. The neurobehavioral, cognitive and biochemical experiments on the 7th, 14th and 28th days were conducted 1 h after the administration of the respective drug schedule of the day.
Induction of experimental Alzheimer's disease
Intacerebroventricular (i.c.v.) administration of Col: The right lateral ventricle was cannulated in rats under pentobarbitone sodium (40 mg/kg i.p.) anesthesia using stereotaxic coordinates, 0.6 mm posterior to bregma, 1.8 mm right lateral and 2.7 mm below cortical surface. Col (15 μg/rat), dissolved in 5 μL of artificial cerebrospinal fluid (ACSF; in nM: NaCl 147, KCl 2.9, MgCl2 1.6, CaCl2 1.7 and dextrose 2.2), was slowly injected into the cannulated right lateral ventricle using a 10 μL Hamilton syringe, and the needle was held at place for 2 min for proper dispersal of drug from the tip.[29]
IB-induced lesion of nucleus basalis magnacellularis (nbm): Bilateral nbm lesions were induced in rats by injecting IB (10 μg/rat), dissolved in 5 μL of ACSF with the help of a 10 μL Hamilton syringe under pentobarbitone sodium (40 mg/kg i.p.) anesthesia, using stereotaxic coordinates 1.0 mm posterior to bregma, 2.6 mm right/left lateral and 7.9 mm below the cortical surface. The needle was held in position for 2 min so that the drug was properly dispersed.[29]
Neurobehavioral studies
Open field test
Locomotor activity and exploratory behavior were evaluated using the open field test. The open field apparatus consists of a cubicle box made up of plywood and is of the dimension 96 cm × 96 cm. All the walls are painted black except for the floor, which is divided into 16 squares by 6 mm thick white lines. Each of the animals was placed in a corner and in the next 5 min, was observed for ambulation (number of squares crossed), immobility period (in s), grooming, rearing and fecal pellets.[30–32]
Elevated plus maze behavior test
Anxiety parameter was tested by the Elevated plus maze test. This maze consists of two opposite arms (50 cm × 10 cm) crossed with two opposite enclosed arms of the same dimension, with walls 40 cm high. The arms are connected with a central square (10 cm × 10 cm) to give the apparatus a plus sign appearance. The Elevated plus maze was kept Elevated 50 cm above the floor in a dimly lit room. The rats were placed individually on the central square of the plus maze facing one enclosed arm. The time spent and the number of entries made by the rat during the next 5 min on the open and closed arms was recorded. An arm entry was defined when all four limbs of the rat were on the arm.[33,34]
Behavioral despair/Porsolt's swim test
The rats were placed individually in a cylinder (45 cm × 20 cm) containing 38 cm water (25 ± 2°C) so that the rats could not touch the bottom of the cylinder with their hind limbs or tail or climb over the edge of the chamber. Two swim sessions were conducted, an initial 15 min pre-test followed by a 5 min test 24 h later. Drug was administered after the pre-test. The period of immobility (remained floating in water without struggling and making only those movements necessary to keep its head above the water level) during the 5 min test period was noted.[35]
Cognitive studies
Morris’ water maze test
On 7th, 14th and 28th days of surgery, spatial learning and memory were tested in a water maze. The maze consisted of a black circular pool (diameter 214 cm, height 80 cm) filled to a depth of 44 cm with water (25 ± 2°C). Four days of training (1 min each day) was given initially and on the 27th day, the rats received habituation (exposure in water maze for 1 min) in which there was no platform. Then, on the 28th day, a circular platform (9 cm in diameter) was kept hidden 2 cm below the water level in the center of a quadrant. The platform remained in the same position during the training days (reference memory procedure). At the beginning of each session, a random sequence of four starting poles along the perimeter of the pool was generated. All animals followed this sequence for that session. Each rat was placed in the water facing the wall at the start location and was allowed 90 s to find the hidden platform. The animal was allowed a 20 s rest on the platform. The latency to reach the platform was recorded. If the rat was unable to locate the hidden platform, it was lifted out and placed on the platform for 20 s. The procedure was repeated for all the four start locations.
Three escape trials were conducted on the 1st day of testing separated by 4 h. After that, the platform was removed and a probe trial (without platform) was conducted after a gap of 4 h. Each rat was placed in the pool at the same, randomly selected the starting pole, and the swimming path was observed and time spent in the quadrant of the pool that initially contained the platform was measured. On completion of the probe trial, a black platform that extended 1 cm above the water surface was placed in a quadrant other than that chosen for the submerged platform. Each rat was then given four trials of 90 s to locate the same. The latency to reach the platform was recorded (working memory procedure).[36,37]
Conditioned avoidance behavior
Active avoidance learning acquisition and its retention were tested by the method of Bhattacharya et al.[29,38] The apparatus used was conventional shuttle avoidance box (Techno, India), which consisted of two grid floor compartments (28 cm × 29 cm × 25 cm), each separated by a Plexiglas transparent partition with a single opening (14 cm × 17 cm), and had a buzzer. The rats were singly placed in the right compartment of the shuttle box and were allowed to adapt for 15 s, and were then exposed to an acoustic (buzzer, situated in the ceiling of the shuttle box) stimulus (2.8 kHz, 70 dB) (conditioning stimulus, [CS]) for 3 s. This was followed by both the acoustic stimulus and an electric shock (unconditioned stimulus, 1.0 mA, 50 Hz) through the grid floor of the right compartment for 30 s. If the rat crossed to the un-electrified safe compartment during presentation of CS, an avoidance response was recorded; otherwise unconditional stimulus was applied. Each rat was given 20 trials for 5 days, with an inter-trial interval of 30 s, before lesioning, until it reached the criterion of 100% active avoidance response. Rats not reaching this criterion were discarded from the study. Retention of the active avoidance response in the different treatment groups was assessed on days 7, 14 and 28, following lesioning with Col or IB, by noting the number of trials required to criterion of 100% active avoidance response.
Biochemical estimation
At the end of the experiment, animals were sacrificed by decapitation under anesthesia and their forebrains were collected and rinsed with ice cold 0.9% NaCl. Both halves of the brain, except the cerebellum, were transferred to an appropriate homogenizing medium.
Lipid peroxidation
Lipid peroxidation was estimated by measuring malondialdehyde (MDA), a by-product of lipid peroxidation. One gram of wet rat forebrains was transferred to 9 mL ice cold 1.15% KCl (1:9 w/v) and homogenized with the help of a Teflon homogenizer under ice cold conditions. Two hundred microliters of this homogenate was taken for further assay. Results were expressed as n mols of MDA/g of wet tissue. 1, 1’, 3, 3’-tetraethoxypropane was used as the external standard to prepare the standard curve.[39]
Superoxide dismutase activity
For measuring SOD activity, rat forebrains were homogenized with a Teflon homogenizer in ice cold 0.052 M sodium pyrophosphate (pH 8.3) and 200 μL of this homogenate was used for further assay. The inhibition of reduction of NBT to blue-colored tetrazolium, in the presence of PMS and NADH, by SOD was measured at 560 nm. One unit of enzyme activity is defined as the enzyme concentration required to inhibit the optical density of chromogen production at 560 nm by 50% in 1 min under experimental conditions, and was expressed as specific activity in milliunits/mg of protein.[40]
Acetylcholinesterase activity
Rat brains were homogenized with a Teflon homogenizer in M/15 ice cold phosphate buffer (pH 7.2) and 10 μL of this homogenate was used for further assay. The color developed was read at 540 nm and the results are expressed as μmol of acetylcholine (Ach) hydrolyzed/mL/h.[41]
Statistical analysis
Statistical analysis of data (using GraphPad InStat, Version 3.05, 32 bit for Win95/NT, GraphPad Software, San Diego, California, USA) was performed by applying one-way analysis of variance (ANOVA) followed by Tukey test for biochemical parameters, and the Newman–Kueles's test for behavioral observations. A P < 0.05 was considered statistically significant.
RESULTS
In this study, we observed 17% (n = 62) post-procedure mortality. Data were collected for statistical analysis from six animals chosen randomly in each group among the groups where more than six animals survived after the procedure.
Results of neurobehavioral studies
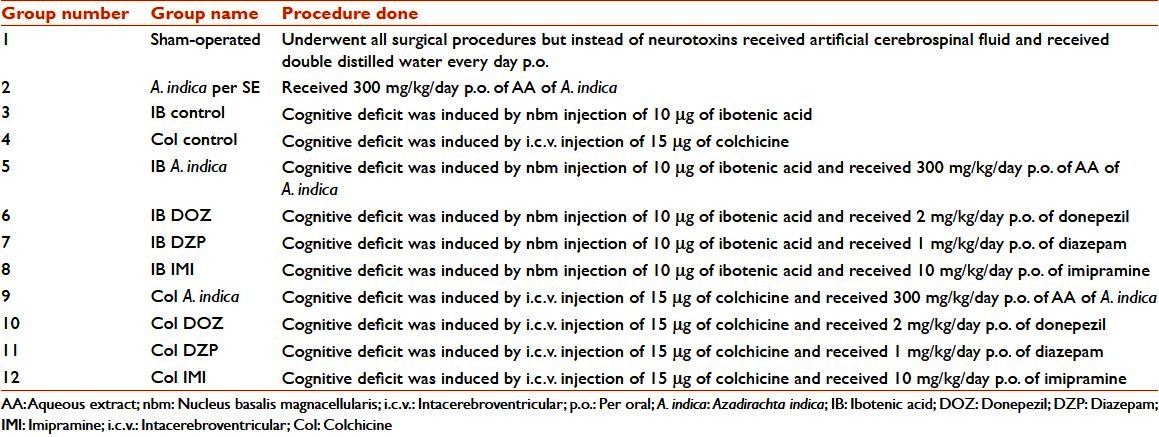
In the open field test, no difference was seen between sham-operated animals and A. indica per SE groups on all days for all parameters. In ambulation, the IB control group had showed significantly (P < 0.05) decreased number of ambulation only on Day 7, but no change in number of ambulation on Day 14 and Day 28 when the values were compared with that of sham-operated animals. The comparison between sham-operated animals and Col control group showed no significance at all. A. indica treatment in IB and Col lesion animals increased the number of ambulation, which were comparable to that of DZP treatment (P < 0.05) throughout the experimental period. In comparison with the sham-operated group animals, the IB control and Col control groups showed increased propensity toward being immobile (P < 0.05), and these were significantly reduced by A. indica treatment in both IB and Col lesion animals, which was comparable with that of the DZP group (P < 0.05). This finding was consistent on all the study days. Only on the 7th day the animals of the IB control and Col control groups showed significantly (P < 0.05) decreased number of rearing when compared with that of sham-operated animals. There was no reversal of this change with A. indica. No statistically observable significance was noted in parameters, grooming and fecal pellets [Table 2].
Table 2.
Effect of Azadirachta indica on ambulations, immobility and rearing in the open field test in ibotenic acid and colchicine induced Alzheimer's disease in rats
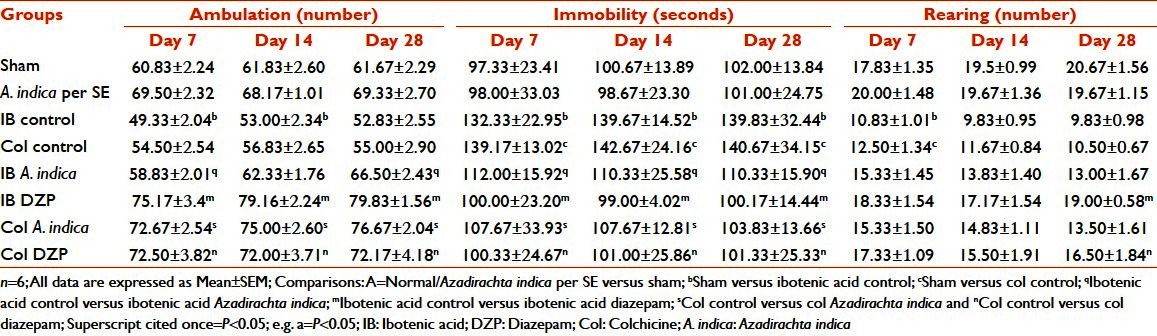
In the Elevated plus maze test, the IB and Col lesion animals spent less time in the open arm of the Elevated plus maze when compared with the sham-operated animals (P < 0.05), while they spent significantly more time in the closed arm (P < 0.05). Reversal of this change was seen only in the Col A. indica group (P < 0.05). Number of entries into the open arm significantly (P < 0.05) reduced and entries into the closed arm correspondingly increased in both IB control and Col control groups, which was consistent only on Day 28 but not on Day 7 and Day 14. There was a reversal of change in number of entries into the open arm in the IB A. indica and Col A. indica groups (P < 0.05). This type of change was not seen with the closed arm entry. The ratio of time spent in the open arm to closed arm was significantly reduced (P < 0.05) in the IB control and Col control groups on all days of the study with respect to the sham-operated animals, and this was reversed only in the Col A. indica group (P < 0.05). The ratio of entries in the open arm to closed arm was significantly (P < 0.05) increased in the IB A. indica group on Days 14 and 28. In the Col A. indica group, this change was seen on all days [Table 3].
Table 3.
Effect of Azadirachta indica on time spent and number of entries on Elevated plus maze test in ibotenic acid and colchicine-induced Alzheimer's disease in rats
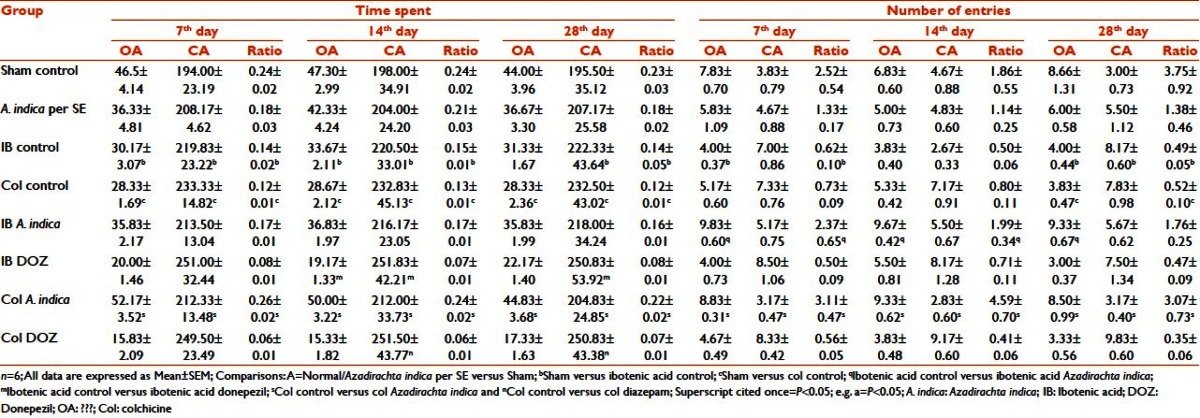
In the Porsolts’ swim test, both IB control and Col control group animals showed propensity toward increasing levels of despair when compared with sham-operated animals, which was significant at P < 0.01. As days progressed, even the behavioral despair increased, which reached a significance level (P < 0.01) on the 28th day in both control groups. A. indica treatment in the IB and Col lesion animals reduced the period of immobility (P < 0.05) on the same days. No significance was observable on comparing the values on different days within the groups. The values obtained were comparable to those observed with IMI on the corresponding days. IMI treatment had significantly (P < 0.05) reduced the despair level in the IB and Col lesion animals [Table 4].
Table 4.
Effect of Azadirachta indica on porsolts’ swim test in ibotenic acid and colchicine
Result of cognitive studies
In case of Morris’ water maze test, in escape latency 1, the IB control animals on all days and Col control animals on the 14th and 28th days took more time to reach the hidden platform (P < 0.05). No difference was seen between sham and other groups (P > 0.05). In escape latency 2, the same tendency as above was found with the IB control and Col control groups, and was significant on all days. IB A. indica animals took significantly shorter time (P < 0.05) to reach the platform on all days, and the same response was evident with the Col A. indica groups at a significance level of P < 0.01 when they were compared with their corresponding control groups. In escape latency 3, when compared with the sham-operated animals, the IB control and Col control animals took more time to find the hidden platform (P < 0.01) on all days. A. indica treatment in the IB lesion animals decreased this time requirement, P < 0.05 on Day 7 and P < 0.01 on Day 14 and Day 28. When a comparison was drawn in between the Col control and Col A. indica group on Day 7, animals of the later group took less time (P < 0.05), which was further decreased on Day 14 and Day 28 (P < 0.01). The animals of the IB control, Col control and A. indica treatment groups in both types of lesions took progressively less time to reach the hidden platform, which was significant, in both control groups and in all the treatment groups in escape latency 1 through escape latency 3. In the probe trial, the animals of both IB and Col control groups swam for lesser time on Day 7 (P < 0.05) in the quadrant where the platform was kept earlier. However, the change in swimming time did not alter in the IB and Col control groups on Day 14 and Day 28. Day 14 and Day 28 comparisons of IB A. indica with IB control and Col A. indica with Col control showed that the A. indica-treated groups swam for a significantly longer time (P < 0.05) compared with the corresponding control groups. In the new platform trial, the animals of the IB control and Col control groups took more time to find the platform kept in a different quadrant than that of the sham-operated animals, which reached a significant level (P < 0.05) on Day 14 and Day 28. A. indica treatment in the IB lesion animals made them reach the new platform much earlier than the IB control group animals, which was significant on Day 14 and Day 28. A. indica treatment showed a decreasing trend in time required, which was significant at the level P < 0.05 on Day 7, and reached P < 0.01 on Day 14 and Day 28 [Table 5].
Table 5.
Effect of Azadirachta indica on Morris’ water maze test in ibotenic acid and colchicine induced Alzheimer's disease in rats
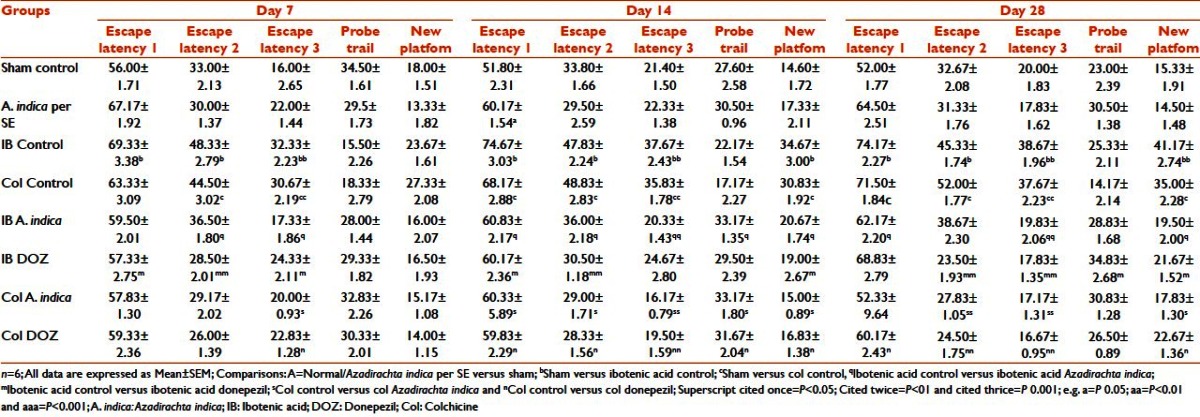
The data of the active avoidance study showed that in normal, sham-operated and A. indica perse animals, there was a decreasing trend in the number of trials required to achieve 100% avoidance from Day 7 to Day 28, which reached a significant level only on the 28th day. No significance was found between these groups when the comparison was performed for the corresponding days. Comparison of sham versus IB control showed that, from Day 7, there was an increase in the number of trials required to achieve 100% learning. On Day 7, it was significant at P < 0.05, but on Day 14 and Day 28, the significance level was P < 0.01. Intra-group comparison of the IB control group showed an increasing trend in the number of trials required, but they were not significant. Comparison between the sham and the Col control groups showed significant increased number of trials required on all days. A. indica treatment in both the IB and the Col lesion groups decreased the number of trials required to reach 100% acquisition when compared with the corresponding control groups (P < 0.05). In both the IB and the Col lesion groups, DOZ significantly reduced the number of trials required (P < 0.01) [Table 6].
Table 6.
Effect of Azadirachta indica on active avoidance paradigm in ibotenic acid and colchicine induced Alzheimer's disease in rats
Result of biochemical studies
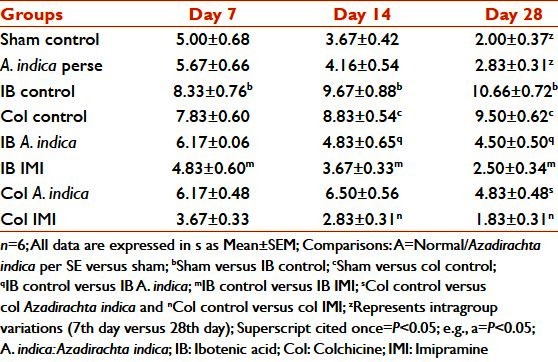
In both the IB and the Col control groups, there was a significant increase in MDA levels with respect to sham-operated animals (P < 0.05). Pre-treatment with A. indica in both the IB and the Col lesion animals decreased the MDA levels on all days compared with their corresponding control groups (P < 0.05) [Table 7].
Table 7.
Effect of Azadirachta indica on lipid peroxidation and acetylcholinesterase activity in ibotenic acid and colchicine induced in rats
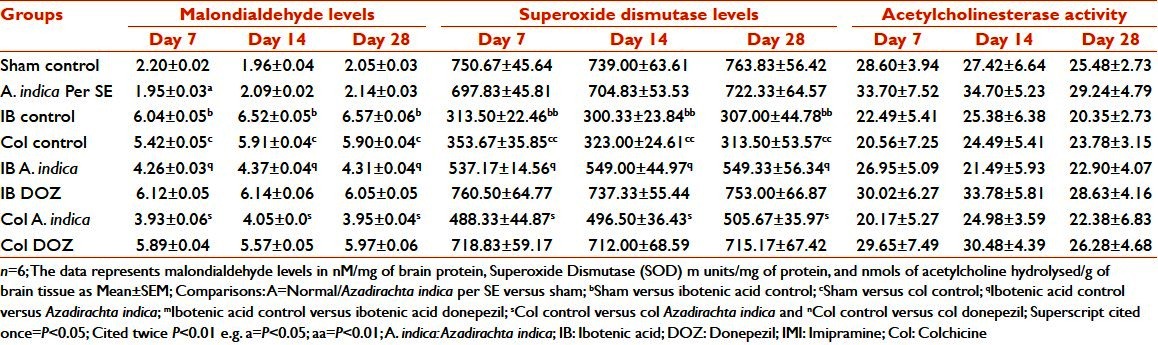
In the IB and the Col control groups, there was a significant decrease (P < 0.01) in SOD activity with respect to the sham-operated group. Pre-treatment with A. indica in both the IB and the Col lesion groups increased the SOD activity on all days when compared with their corresponding control (P < 0.05). These alterations were of significance on the same days (inter-group) but not on different days (intra-group) [Table 7].
Both the IB and the Col control groups showed a decrease in the values of Ach hydrolyzed with respect to the sham-operated animals on corresponding days, but this was not significant. A. indica pre-treatment in the IB and Col lesion groups did not significantly alter the values of Ach hydrolyzed with respect to the corresponding control animals [Table 7].
DISCUSSION
AD is the fourth largest cause of death among people over 65 years of age. Because specific treatment for AD is not available, the consensus is to improve the quality of patients’ life by improving both cognitive and behavioral symptoms. A number of Indian Ayurvedic herbal preparations are claimed to have a beneficial effect in cognitive and behavioral dysfunctions by virtue of their “Medharasayana” activity. In this study, A. indica was studied for its possible effect in experimental AD.
The open field test is conventionally designed to evaluate the state of anxiety. It is well documented by the previous investigators that if the animals are having anxiety, their ambulation will be decreased and inverse to this, the period of immobility will be correspondingly increased. A. indica pre-treatment increased the number of ambulations, which was comparable to that of DZP. There was no significance in the parameters of rearing, grooming and fecal pellets.
The Elevated plus maze test depends on the fact that the anxiety-prone animals will prefer to stay in the dark closed arm of the apparatus than in the open white arms. The open/enclosed arms entries and time spent ratios provide a measure of fear-induced inhibition of exploratory activity. These ratios are increased by anxiolytics and reduced by anxiogenic agents.[29] It is quite evident from the results of the Elevated plus maze test that the IB and Col control animals spent significantly less time in open arms and more time in enclosed arms. This change was reversed by A. indica pre-treatment, which reached a significance level only in the Col lesion animals. However, propensity of A. indica to reverse IB-induced change was not statistically significant.
The forced swim test, originally described by Porsolt et al., is the most widely used pharmacological animal model for assessing depressant/antidepressant activity.[42–44] In the present study, A. indica pre-treatment significantly reduced the immobility period with respect to their corresponding control animals, suggesting the antidepressant activity of this medicinal plant.
It is not out of place to mention that this is the first time the antidepressant activity of A. indica has come to light. A survey of the literature revealed no such earlier claim. These findings confirm that there exists a state of anxiety in AD along with depression that corroborates with the clinical scenario.[45] The anxiolytic effect observed in the present study is in accordance with the earlier reports of the anxiolytic properties of A. indica.[19,46]
Morris’ water maze test incorporates three different parts of memory, viz. reference memory, working memory and spatial learning, and is an excellent model for testing the lesions associated with the hippocampal system of animals. Lesions of nbm cause relatively selective deficits in the reference-memory component. Damage to the septohippocampal system has previously been reported to impair working memory without affecting reference memory.[47] Although the IB destroys cholinergic neurons in the nbm, it also damages non-cholinergic neurons in the vicinity of this group of cells. It remains possible that the reference memory deficit is a consequence of destruction of some of these non-cholinergic neurons. The degeneration of the nbm in AD may contribute directly to some of the learning and memory impairments. Earlier studies have found a significant correlation between cortical choline acetyltransferase depletion after IB lesions and several neurological parameters (like weight loss, limb use) or performance in the Morris’ water maze.[48]
In the present study, the results of Morris’ water maze showed that reference memory improved in all groups (escape latency 1 through 3), but, in the IB and Col lesion animals, the degree of learning was less when compared with the sham-operated animals. However, A. indica pre-treatment in both IB and Col lesions improved the reference memory component in comparison with their respective control animals. The result of the probe trial, an indication of working memory, showed that the IB and Col lesion animals swam in the quadrant in which the platform was kept earlier for a lesser period of time and A. indica pre-treatment had significantly increased the time spent in the same quadrant, suggesting that A. indica pre-treatments prevented the damage caused by IB and Col. The spatial learning, as evidenced by new platform test, was affected by IB and Col lesions and was normalized by A. indica pre-treatments in both IB and Col cholinergic deprivation. Yanpallewar et al. have shown that memory defects in chronically hypoperfused animals was effectively reversed by A. indica treatment.[19,46] The present study confirms the memory-enhancing effect of this herbal drug.
The results of the present study on active avoidance paradigm showed marked deficits in the active avoidance learning task and retention of the memory in rats lesioned with either IB or Col, which is in consonant with earlier reports.[49,50] The pre-treatment with A. indica in both IB and Col lesions preserved learning and retention of learned behavior.
It has been reported that central administration of Col causes impairment in learning and memory by altering the antioxidant enzymes like SOD, catalase and Lipid Hydroperoxide (LPO).[51] IB, an N-Methyl-D-aspartic Acid (NMDA) receptor agonist, brings about neuronal toxicity by overloading calcium into the neurons.[49] Increased intracellular calcium has been implicated as one of the causes behind neuronal oxidative damage following ischemia reperfusion injury and post-stroke syndromes.[19,46] An effective antioxidant agent should be capable of augmenting intracellular concentration of not only SOD but also that of Catalase and/or Glutathione peroxidase, and should also be capable of reducing lipid peroxidation.[52,53] It is evident from the results that both IB and Col increased brain MDA levels by 2.75 and 2.46 folds on the 7th day, 3.33 and 3.02 folds on the 14th day and 3.20 and 2.88 folds on the 28th day. In the IB lesion, A. indica pre-treatment reduced the same by 29.47%, 32.98% and 34.4% on the 7th, 14th and 28th days, respectively. In Col lesion, A. indica pre-treatment reduced the MDA level by 27.49%, 31.47% and 33.05% on the 7th, 14th and 28th days, respectively. DOZ treatment had no effect on MDA levels. A. indica by perse had no effect on MDA levels. Results of SOD activity estimation showed that IB lesion decreased brain SOD levels by 2.39, 2.46 and 2.49 folds and Col lesion by 2.23, 2.35 and 2.35 folds, respectively, on the 7th, 14th and 28th days. Pre-treatment with A. indica significantly increased the SOD levels. In IB lesion, A. indica pre-treatment increased the SOD level by 28.44%, 25.71% and 28.08% on the 7th, 14th and 28th days, respectively. In Col lesion, A. indica pre-treatment raised the levels by 30.02%, 29.56% and 30.00% on the 7th, 14th and 28th days, respectively. The results of MDA and SOD changes show that A. indica appears to have an important antioxidant property. Basak and Chakravorty have isolated quercetin, a bioflavonoid with potent antioxidant activity and β-sitosterol, from A. indica leaves.[54] Yanpallewar et al. have shown that A. indica effectively stabilizes SOD activity in cerebral ischemia–reperfusion injury. Pongtip Sithisarn et al. have shown that extracts from leaf, flower and stem bark of the siamese neem tree have a strong antioxidant potential.[55] The central dogma of AD, the cholinergic deficiency, has been proven beyond doubt by various investigators in the past. In the present study, no significant difference was seen between any of the study groups. However, in the IB and Col lesions, there was reduced acetylcholine esterase activity. This supports the fact that the neurotoxins IB and Col damage the cholinergic neurons. A. indica pre-treatments had no effect in reversing the levels of ACh. Nevertheless, more detailed experiments involving acetylcholine turn over study would have been fruitful to pinpoint the exact protective role of this indigenous product on the cholinergic cascade of AD hypothesis.
CONCLUSION
From the above discussion, it is well evident that the Ayurvedic rasayana, A. indica, is effective in reversing the neurobehavioral changes, attenuating the cognitive deficits and decreasing the oxidative stress in experimental AD models. Being a preliminary study, the present study cannot accreditate the exact mechanism to its protective role in experimental AD, but the observed beneficial effects might be explained on the basis of oxidative theory of this neurodegenerative disorder. Therefore, A. indica can be tested for its safety and efficacy as monotherapy or add-on therapy for prophylaxis or symptomatic improvement (both cognitive and behavioral) in AD by randomized, multicentric clinical trials.
ACKNOWLEDGMENTS
The authors would like to thank Prof. V.K. Joshi, Department of Dravyaguna, Faculty of Ayurveda, Institute of Medical Sciences, Banaras Hindu University, Varanasi for his kind help in the authentication of the A. indica leaves, HPTLC and gas chromatographic analysis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Parnetti L, Senin U, Mecocci P. Cognitive enhancement therapy for Alzheimer's disease. The way forward. Drugs. 1997;53:752–68. doi: 10.2165/00003495-199753050-00003. [DOI] [PubMed] [Google Scholar]
- 2.Geldmacher DS, Whitehouse PJ., Jr Differential diagnosis of Alzheimer's disease. Neurology. 1997;48:S2–9. doi: 10.1212/wnl.48.5_suppl_6.2s. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JL, Mendez MF. Alzheimer's disease: Cognitive and behavioral pharmacotherapy. Conn Med. 1997;61:543–52. [PubMed] [Google Scholar]
- 4.Max W. Drug treatments for Alzheimer's disease: Shifting the burden of care. CNS Drugs. 1999;11:363–72. [Google Scholar]
- 5.Byrne GJ. Treatment of cognitive impairment in Alzheimer's disease. Aust J Hosp Pharm. 1998;28:261–6. [Google Scholar]
- 6.Christie JE, Shering A, Ferguson J, Glen AI. Physostigmine and arecoline: Effects of intravenous infusions in Alzheimer presenile dementia. Br J Psychiatry. 1981;138:46–50. doi: 10.1192/bjp.138.1.46. [DOI] [PubMed] [Google Scholar]
- 7.Little A, Levy R, Chuaqui-Kidd P, Hand D. A double-blind, placebo controlled trial of high-dose lecithin in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1985;48:736–42. doi: 10.1136/jnnp.48.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grill JD, Cummings JL. Current therapeutic targets for the treatment of Alzheimer's disease. Expert Rev Neurother. 2010;10:711–28. doi: 10.1586/ern.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams P, Sorribas A, Howes MJ. Natural products as a source of Alzheimer's drug leads. Nat Prod Rep. 2011;28:48–77. doi: 10.1039/c0np00027b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galluzzi KE, Appelt DM, Balin BJ. Modern care for patients with Alzheimer disease: Rationale for early intervention. J Am Osteopath Assoc. 2010;110:S37–42. [PubMed] [Google Scholar]
- 11.Osborn GG, Saunders AV. Current treatments for patients with Alzheimer disease. J Am Osteopath Assoc. 2010;110:S16–26. [PubMed] [Google Scholar]
- 12.Battacharya SK. Intraditional medicine. In: Mukherjee B, editor. Traditional Medicine. New Deihi: IBH Publishing Co; 1993. p. 320. [Google Scholar]
- 13.Sharma PV. Dravyaguna Vijnan. Varanasi, India: Chaukhamba Sanskrit Sansthan; 1978. ‘Nimba: Nimbati syasthyamdadati’; p. 231. [Google Scholar]
- 14.Rastogi RP, Mehrotra BN. Compendium of Indian Medicinal Plants. Vol. 2. Lucknow: CDRI; 1991. p. 87. [Google Scholar]
- 15.Khanna N, Goswami M, Sen P, Ray A. Antinociceptive action of Azadirachta indica (neem) in mice: Possible mechanisms involved. Indian J Exp Biol. 1995;33:848–50. [PubMed] [Google Scholar]
- 16.Sen P, Mediratta PK, Ray A. Effects of Azadirachta indica A Juss on some biochemical, immunological and visceral parameters in normal and stressed rats. Indian J Exp Biol. 1992;30:1170–5. [PubMed] [Google Scholar]
- 17.Jaiswal AK, Bhattacharya SK, Acharya SB. Anxiolytic activity of Azadirachta indica leaf extract in rats. Indian J Exp Biol. 1994;32:489–91. [PubMed] [Google Scholar]
- 18.Acharya SB, Yanpallewar SU, Singh RK. A preliminary study on the effect of Azadirachta indica on bronchial smooth muscle and mast cells. J Nat Rem. 2003;3:78–82. [Google Scholar]
- 19.Yanpallewar SU, Sen S, Tapas S, Kumar M, Raju SS, Acharya SB. Effect of Azadirachta indica on paracetamol-induced hepatic damage in albino rats. Phytomedicine. 2003;10:391–6. doi: 10.1078/0944-7113-00230. [DOI] [PubMed] [Google Scholar]
- 20.Njiro SM, Kofi-Tsekpo MW. Effect of an aqueous extract of Azadirachta indica on the immune response in mice. Onderstepoort J Vet Res. 1999;66:59–62. [PubMed] [Google Scholar]
- 21.Verma S, Hamdard ME, Dandiya PC. A note on neuropsychopharmacological studies of Melia azadarach leaves. Indian J Pharmacol. 1989;21:46–50. [Google Scholar]
- 22.Chattopadhyay RR. Possible biochemical mode of anti-inflammatory action of Azadirachta indica A. Juss. in rats. Indian J Exp Biol. 1998;36:418–20. [PubMed] [Google Scholar]
- 23.Khosla P, Bhanwra S, Singh J, Seth S, Srivastava RK. A study of hypoglycaemic effects of Azadirachta indica (Neem) in normaland alloxan diabetic rabbits. Indian J Physiol Pharmacol. 2000;44:69–74. [PubMed] [Google Scholar]
- 24.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 25.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 26.Bouic PJ. Sterols and sterolins: New drugs for the immune system? Drug Discov Today. 2002;7:775–8. doi: 10.1016/s1359-6446(02)02343-7. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan JS, Kumar S, Chaturvedi R. A new flavanonol glycoside from Adansonia digitata roots. Planta Med. 1984;50:113. doi: 10.1055/s-2007-969642. [DOI] [PubMed] [Google Scholar]
- 28.Committee for the Purpose of Control and Supervision on Experiments on Animals. CPCSEA guidelines for laboratory animal facility. Indian J Pharmacol. 2003;35:257–74. [Google Scholar]
- 29.Bhattacharya SK, Kumar A, Jaiswal AK. Effect of mentat: A herbal formulation, on experimental models of Alzheimer's disease and central cholinergic markers in rats. Fitoterapia. 1995;66:216–22. doi: 10.1089/acm.1997.3.327. [DOI] [PubMed] [Google Scholar]
- 30.Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46:321–40. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 31.Walsh RN, Cummins RA. The Open-Field Test: A critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 32.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 33.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 34.Pellow S, Chopin P, File SE, Briley M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 35.Willner P. The validity of animal models of depression. Psychopharmacology (Berl) 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 36.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 37.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharya SK, Kumar A, Ghosal S. Effects of glycowithanolides from Withania somnifera on an animal model of Alzheimer's disease and perturbed central cholinergic markers of cognition in rats. Phytother Res. 1995;9:110–13. [Google Scholar]
- 39.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 41.Augustinson KB. Methods of Biochemical Analysis. New York: Interscience Publishers Inc; 1957. Assay methods of cholinesterases; pp. 43–7. [DOI] [PubMed] [Google Scholar]
- 42.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 43.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JM, Kilts CD. Animal models of depression and schizophrenia. In: Nemeroff CB, Schatzberg AF, editors. Textbook of Psychopharmacology. New York: American Psychiatric Association Press; 1998. pp. 88–123. [Google Scholar]
- 45.Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer's disease: Phenomenology and treatment. J Clin Psychiatry. 1987;48:9–15. [PubMed] [Google Scholar]
- 46.Yanpallewar S, Rai S, Kumar M, Chauhan S, Acharya SB. Neuroprotective effect of Azadirachta indica on cerebral post-ischemic reperfusion and hypoperfusion in rats. Life Sci. 2005;76:1325–38. doi: 10.1016/j.lfs.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 47.Murray CL, Fibiger HC. Learning and memory deficits after lesions of the nucleus basalis magnocellularis: Reversal by physostigmine. Neuroscience. 1985;14:1025–32. doi: 10.1016/0306-4522(85)90273-8. [DOI] [PubMed] [Google Scholar]
- 48.Dunnett SB, Whishaw IQ, Jones GH, Bunch ST. Behavioural, biochemical and histochemical effects of different neurotoxic amino acids injected into nucleus basalis magnocellularis of rats. Neuroscience. 1987;20:653–69. doi: 10.1016/0306-4522(87)90117-5. [DOI] [PubMed] [Google Scholar]
- 49.Dekker AJ, Connor DJ, Thal LJ. The role of cholinergic projections from the nucleus basalis in memory. Neurosci Biobehav Rev. 1991;15:299–317. doi: 10.1016/s0149-7634(05)80008-9. [DOI] [PubMed] [Google Scholar]
- 50.Emerich DF, Walsh TJ. Cholinergic cell loss and cognitive impairments following intraventricular or intradentate injection of colchicine. Brain Res. 1990;517:157–67. doi: 10.1016/0006-8993(90)91021-8. [DOI] [PubMed] [Google Scholar]
- 51.Kumar Veerendra MH, Gupta YK. Intracerebroventricular administration of colchicine produces cognitive impairment associated with oxidative stress in rats. Pharmacol Biochem Behav. 2002;73:565–71. doi: 10.1016/s0091-3057(02)00838-9. [DOI] [PubMed] [Google Scholar]
- 52.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halliwell B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med. 1991;91:145–225. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 54.Basak SP, Chakravorty DP. Chemical investigation of Azadirachta indica leaf. J Indian Chem Soc. 1968;45:466–7. [Google Scholar]
- 55.Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209) J Ethnopharmacol. 2005;99:109–12. doi: 10.1016/j.jep.2005.02.008. [DOI] [PubMed] [Google Scholar]


