Abstract
Background:
Various histopathological changes have been observed following neoadjuvant chemotherapy in individual tumors in the literature.
Aims and Objectives:
To observe histopathologic changes seen after neoadjuvant chemotherapy in breast malignancies, squamous cell carcinomas, adenocarcinomas, and Wilms′ tumor using breast cancer predominantly as the model.
Materials and Methods:
The present prospective study was carried out on 60 patients including 40 patients with carcinoma breast and 20 patients with other malignancies who received neoadjuvant chemotherapy.
Results:
Post neoadjuvant chemotherapy, mastectomy specimens revealed nuclear enlargement, nuclear shrinkage, necrosis, vacuolation of nucleus, vacuolation of cytoplasm, dyscohesion, and shrinkage of tumor cells with nuclear changes of nonviability like karyorrhexis, karyolysis, and pyknosis. Stromal reactions manifested as fibrosis, elastosis, collagenization, hyalinization, microcalcification, and neovascularization. Areas of necrosis included both vascular and avascular pattern. The stroma also revealed fibrinoid necrosis and mucinous change. Hyalinization of the blood vessel wall was a common finding. The most common inflammatory host response observed in the present study was lymphocytic; others included mixed inflammation, plasmacytic, prominent histiocytic, and giant cell types. Giant cell reaction was significantly correlated to all types of tumor responses (P < 0.05). Similar changes were also observed in other malignancies. A detailed review of the literature has also been done and presented.
Conclusion:
The tumor grade decreases and differentiation improves, in addition to the retrogressive changes and increase in stromal component, as a result of chemotherapy in carcinoma breast as well as in other malignancies.
Keywords: Breast carcinoma, changes following chemotherapy, neoadjuvant chemotherapy
INTRODUCTION
Emergence of new concepts in the management of oncologic malignancies and the fast-changing trends of modern medicine necessitate the development of strategies for customized treatment suited to the patient's variable requirements. Response to chemotherapy cannot be predicted with certainty in an individual presently. Moreover, the histopathological changes after chemotherapy vary in patients.
Studies have systematically described various histopathological changes seen after neoadjuvant chemotherapy in a variety of tumors including breast carcinoma, rectal carcinoma, ovarian carcinoma, head and neck carcinomas, esophageal carcinoma, Wilms’ tumor, and non-small-cell lung carcinoma. However, the emphasis of these studies varied and no study described the pathologic changes following chemotherapy comprehensively.[1]
This study describes the various histopathologic changes seen after neoadjuvant chemotherapy in breast malignancies, squamous cell carcinomas, adenocarcinomas, and Wilms’ tumor.
MATERIALS AND METHODS
The present prospective study was carried out on 60 patients including 40 patients with carcinoma breast and 20 patients with other malignancies who received neoadjuvant chemotherapy. During a study period of one-and-a half years, a total of 355 patients were treated for breast carcinoma with neoadjuvant chemotherapy. Of these, 40 patients fulfilling the inclusion criteria as per the protocol of the thesis project were included in the study. Inclusion criteria was patients with prechemotherapy clinicoimaging assessment of tumor size, with an established histological diagnosis of carcinoma on biopsy, and who had received at least two cycles of neoadjuvant chemotherapy.
The study group was divided into three subgroups; group A (n = 30) and group B (n = 10) included breast carcinoma patients with initial pathologic material submitted as trucut or wedge biopsy in group A and lumpectomy specimens in Group B. Group C (n = 20) included cases of other malignancies besides breast carcinoma who received neoadjuvant chemotherapy.
After initial tissue diagnosis, the patients were clinically examined in detail and investigated including radiological, imaging, and other laboratory tests to decide the stage of the cancer. The initial biopsies were subjected to routine formalin fixation and paraffin processing with microscopic analysis on hematoxylin- and eosin-stained sections supplemented by special stains including immunohistochemistry to decide histological type and grade of the tumor according to the World Helath Organization (WHO) classification.[2,3] Detailed histopathological examination was carried out especially looking for chemotherapy-induced histopathologic changes like necrosis, fibrosis, inflammatory reactions, and other retrogressive changes.
For carcinoma breast, neoadjuvant chemotherapy regimens either included cyclophosphamide 50 to 60 mg/m2 IV (IV: Intravenous), doxorubicin 40 to 50 mg/m2 IV, and 5-fluorouracil 500 to 800 mg/m2 IV (CAF), or cyclophosphamide, epirubicin, and 5-fluorouracil (CEF), 21 days apart. Number of chemotherapy cycles varied from two to six depending on the initial size of the tumor to make them operable. The drugs and doses of neoadjuvant chemotherapy given to the patient were recorded in the pro forma.
The tumor cells were evaluated for dissociation, dyscohesion, and loss of organization of the tumor cells and necrobiotic changes such as necrosis, vacuolation of nucleus and cytoplasm, karyorrhexis, pyknosis, and karyolysis. Any change in pattern or type of carcinoma was noted.
The stroma was examined for host response including fibrosis, elastosis, and collagenization, and infiltration by lymphocytes, plasma cells, fibroblasts, histiocytes, and giant cell formation was observed. Similar changes in tumor cells and stroma were observed in the lymph nodes.
Epithelial-to-stromal ratio was calculated as the mean of readings in all sections and viable-to-nonviable tumor cell ratios were calculated in both pretreatment biopsies and postchemotherapy specimens, viability being defined as distinct nuclear chromatin with intact nuclear and cytoplasmic membrane in the absence of the criteria of necrosis (karyorrhexis, karyolysis, pyknosis).
A note was made on the tumor type and any change after chemotherapy; tumor grading was done based on the Elston and Ellis modification of Bloom-Richardson (MRB) grading system and Nottingham Prognostic Index (NPI) was calculated.[4]
Lymphocytic response was graded as: Grade 1, scattered lymphocytes between tumor cells; grade 2, formation of microaggregates of lymphocytes; grade 3, dense infiltration of lymphocytes destroying tumor cells or forming masses. The presence of lymphovascular embolization and in situ disease/cancerization of ducts were separately noted.
Twenty cases of other malignancies (group C) besides breast included 12 cases of squamous cell carcinoma (seven from head and neck, four of cervix, and one of esophagus), five cases of adenocarcinoma, and three of Wilms’ tumor. Neoadjuvant chemotherapy for squamous cell carcinoma of head and neck included chemotherapy based on docetaxel, cisplatin, and 5-fluorouracil (TPF regimen). Patients with carcinoma cervix received paclitaxel and carboplatin regimen, and for esophageal carcinoma, cisplatin and 5-fluorouracil was given. Chemotherapy given for ovarian carcinoma was paclitaxel and carboplatin, and patients with anorectal carcinoma received a 5-fluorouracil- and oxaliplatin-based regimen (FOLFOX). Children with Wilms’ tumor received vincristine and dactinomycin cycles varying from three to four cycles, as was considered appropriate for reducing the bulk of the tumor.
These were separately evaluated for changes induced by neoadjuvant chemotherapy. Histologic sections from all the malignancies were observed for any change in differentiation, changes in architecture, necrobiotic changes, and host tissue response.
The results obtained were interpreted and correlated statistically. Pearson's coefficient of correlation was applied to correlate the three types of response patterns with all the parameters observed. A P value ≤ 0.05 was considered as significant.
RESULTS
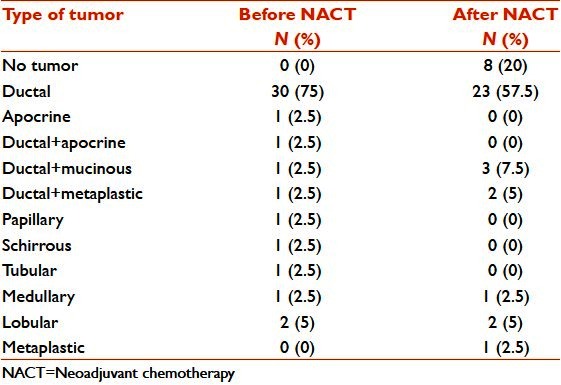
Breast tumors which showed complete response after neoadjuvant chemotherapy included five of ductal carcinoma-not otherwise specified (NOS) type, and one each of schirrhous, apocrine, and tubular types. Apocrine change and tubular and papillary pattern disappeared in postchemotherapy specimens; mucinous change and metaplastic change appeared in two each of ductal NOS types. Ductal NOS with metaplastic change differentiated to completely metaplastic in mastectomy specimens [Table 1].
Table 1.
Carcinoma breast: Histological types of the tumor before and after chemotherapy
Number of cases with metastasis was 22; number of lymph nodes with metastatic deposits was 88. The most common pathologic changes noted were necrosis, elastosis/collagenization, and lymphocytic response. There was no relation of lymphocytic response with the clinical or pathologic response irrespective of their presence in the pre or postchemotherapy specimens. Giant cell response was significantly correlated to all the types of response grades (P < 0.05). Collagenization was significantly correlated to pathologic and tumor regression grade (P < 0.05) [Tables 2 and 3].
Table 2.
Carcinoma breast: Inflammatory and stromal response seen after neoadjuvant chemotherapy
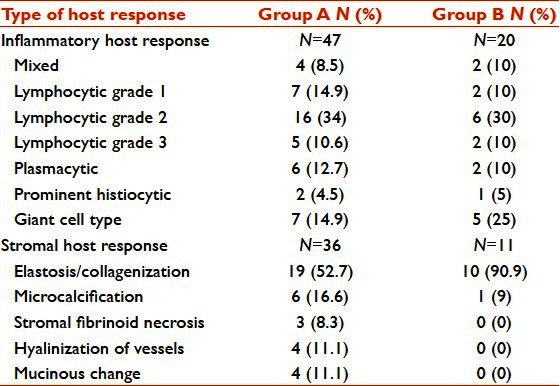
Table 3.
Carcinoma breast: Inflammatory and stromal response seen after neoadjuvant chemotherapy in axillary lymph nodes

In the present study, of the total 20 cases of group C, 12 cases were of squamous cell carcinoma (seven from head and neck, four of cervix, and one of esophagus), five cases were of adenocarcinoma, and three cases were of Wilms’ tumor. Of the 12 cases of squamous cell carcinoma in the present study, there was improvement in histological differentiation from moderate to well-differentiated carcinoma in two cases of head and neck cancers, whereas there was complete pathologic disappearance of tumor in another two cases comprising one case each of carcinoma tongue and cervical carcinoma. Other histological changes observed were increase in keratinization with formation of keratin pearls, acellular keratin with islands of nonviable tumor cells, histiocytic giant cells, and increase in lymphocytes surrounding residual tumor cells.
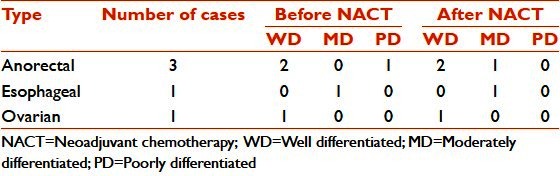
Of the five cases of adenocarcinoma, a change in histological differentiation was seen in a case of anorectal carcinoma which revealed poor differentiation with solid sheets, a small amount of mucin in the initial biopsy, and moderately differentiated mucin-secreting adenocarcinoma with large mucin pools after chemotherapy. In serous papillary adenocarcinoma of the ovary, degenerative changes were observed with karyorrhexis, pyknosis, smudging, dyscohesion, and loss of papillary architecture in some areas. In a single case of esophageal adenocarcinoma, an increase in signet ring cells was noticed [Table 4].
Table 4.
Adenocarcinoma: Histological differentiation before and after chemotherapy
Of the three Wilms’ tumors, one was biphasic and two were triphasic. In the two cases revealing triphasic Wilms’ tumor in the pretreatment biopsy, blastemal component decreased with marked necrobiotic changes and totally disappeared in the one with biphasic Wilms’ tumor. Epithelial component in all of them showed squamous differentiation. Mesenchymal component revealed rhabdoid differentiation in one and chondroid differentiation in the other with the presence of smooth and skeletal muscles.
DISCUSSION
In the present prospective study, a spectrum of histopathologic changes was observed with the use of chemotherapy. The effects have been divided into two, as pathologic changes in tumor cells and changes in the stroma.
In the tumor cells, nuclear enlargement, nuclear shrinkage, necrosis, vacuolation of nucleus, cytoplasm, pyknotic nuclei, and degenerative changes have been described in the literature.[1,5–9] In the present study, loss of architecture, dyscohesion, and shrinkage of tumor cells with retrogressive changes like karyorrhexis, karyolysis, and pyknosis were observed in addition to the above-mentioned changes. In view of the presence of a large variety of retrogressive changes, the emphasis was more on trying to recognize viable tumor cells, morphologically identified as cells with distinct nuclear chromatin with intact nuclear and cytoplasmic membrane in the absence of criteria of necrosis (karyorrhexis, karyolysis, pyknosis). Necrosis was the most common event observed.
In the stroma, fibrosis, elastosis, collagenization, hyalinization, microcalcification, and neovascularization have been observed and described.[5,6,8–13] In the present study, in addition, some prominent findings were elastosis/ collagenization of the stroma, hyalinization of the walls of the blood vessels, and atrophy of the adjacent breast parenchyma and cancerization of ducts even in atrophic lobules. Other changes included stromal fibrinoid necrosis and mucinous change. Collagenization was found to be significantly correlated to pathologic response and tumor regression grade (P < 0.05).
Lymphocytic reaction and the presence of plasma cells and macrophages with the formation of histiocytic giant cells observed in many studies may be indicative of host tissue response to necrobiotic tumor.[5,10–12,14] The most common inflammatory host response observed in the present study was lymphocytic; others included mixed inflammation, plasmacytic, prominent histiocytic, and giant cell types. Giant cell reaction was significantly correlated to all types of tumor responses (P < 0.05). Pathologically similar changes were observed in response to chemotherapy in draining lymph nodes but not as pronounced as in the primary site.
Though these changes have been observed by many workers, positive correlation of the presence of these changes with the effect on chemotherapy has not been significant in many studies. According to them, the presence of fibrosis, elastosis, collagenization, hyalinization, necrosis, inflammatory infiltrate, lymphocytic response, giant cell reaction, plasma cells, foamy macrophages, microcalcification, and neovascularization were not significantly related to the response to chemotherapy.[1,9] Honkoop et al. concluded that none of the pretreatment pathologic or biologic characteristics were predictive of a good pathologic response.[11]
In the present study, the most common type was ductal carcinoma NOS; mucinous change appeared in three patients after chemotherapy who were diagnosed with ductal carcinoma NOS type in the initial biopsy, and a transformation from ductal to metaplastic carcinoma and from papillary pattern to ductal NOS was seen in one case each. A change to either a higher or lower grade was noted by Rasbridge et al.[8] However, no postchemotherapy change in tumor grade was seen by Gazet et al.[10]
Complete response was seen in one case of apocrine carcinoma and disappearance of apocrine component in mixed ductal with apocrine differentiation in another. One case each of schirrhous and tubular carcinoma had complete pathologic response. The response in the case of ductal carcinoma NOS was not significantly different from the response in invasive lobular carcinoma. In the present study, the clinical and pathologic response was not found to have a significant correlation with the prechemotherapy grade. However, significant correlation was observed between the postchemotherapy grade and the pathologic and tumor regression grades but not with clinical response. So, a better response to chemotherapy was observed in the poorly differentiated malignancies with higher MRB and NPI grades and a poorer response with well-differentiated ones (P < 0.05) [Figure 1]. In contrast, Sanchez et al. observed a poor response in poorly differentiated carcinomas.[15]
Figure 1.
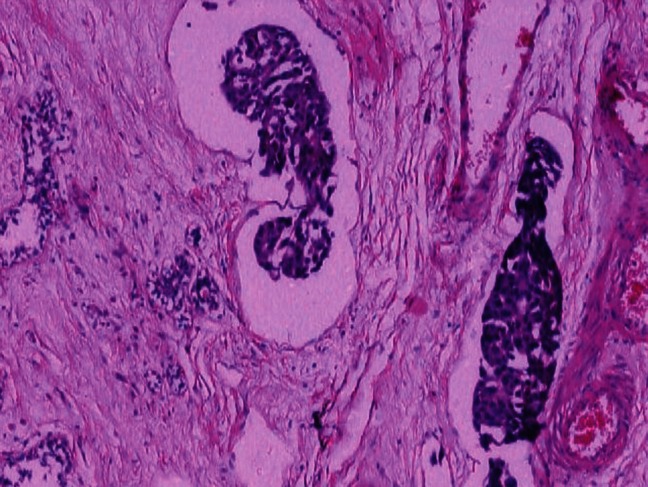
Carcinoma breast: Occasional nests of viable tumor cells left post chemotherapy (H and E; ×100)
Post neoadjuvant chemotherapy, hyalinization of the blood vessel wall was a common finding. The adjoining breast showed atrophy of parenchyma; even then, terminal duct lobular units (TDLUs) revealed ductal carcinoma in situ (DCIS)/ cancerization of ducts by surviving tumor cells in some cases. No correlation of size with the response grades was observed, that is, size reduction after chemotherapy was seen in both small and large tumors and was not restricted to any one group.
In squamous cell carcinoma, changes observed were improvement in histologic differentiation in some, complete pathologic disappearance of the tumor in a few, an increase in keratinization with formation of keratin pearls, acellular keratin with islands of nonviable tumor cells, histiocytic giant cells, and increase in lymphocytes surrounding residual tumor cells in most cases [Figure 2]. In adenocarcinoma, an improvement in histologic differentiation was seen with large mucin pools after chemotherapy, degenerative changes were observed in serous papillary adenocarcinoma of the ovary, and an increase in signet ring cells was noticed in an esophageal carcinoma [Figure 3]. In Wilms’ tumor, there was an almost complete disappearance of blastemal component and retrogressive changes in the epithelial component. Mesenchymal component revealed rhabdoid and chondroid differentiation in some [Figure 4].
Figure 2.
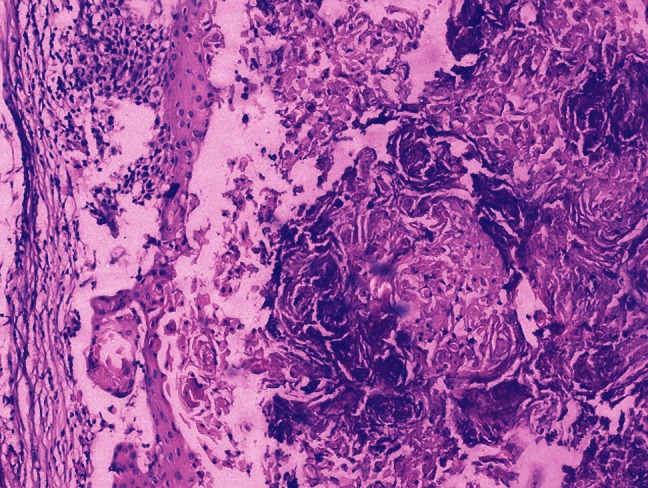
Squamous cell carcinoma: After chemotherapy, only necrotic masses of keratin with areas of calcification are left (H and E; ×100)
Figure 3.
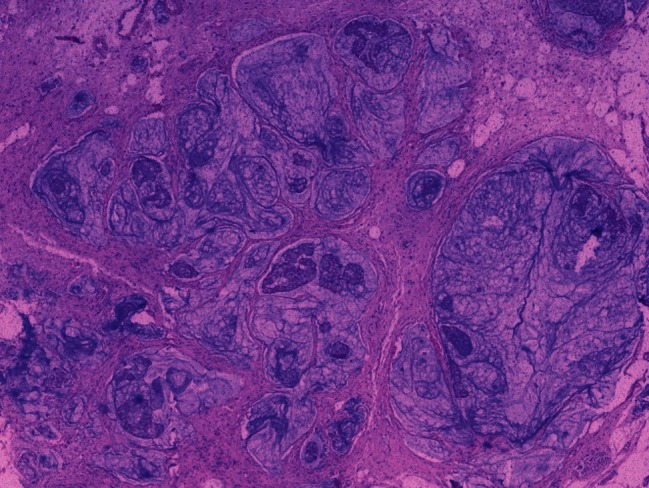
Adenocarcinoma: Abundant pools of mucin with occasional scattered tumor cells after chemotherapy (H and E; ×40)
Figure 4.
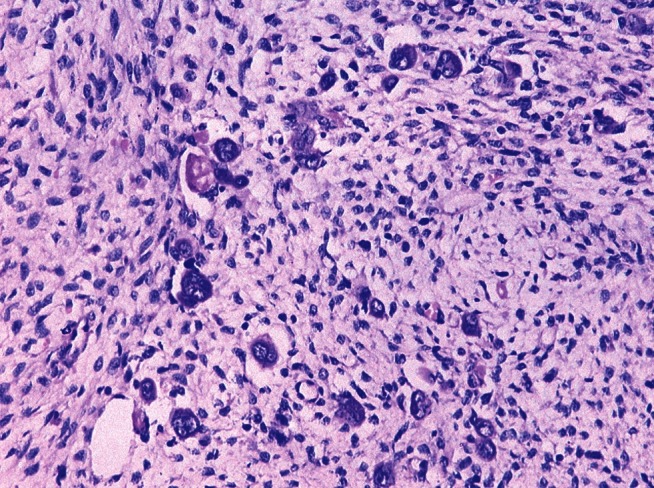
Wilms’ tumor: Only few bizarre tumor cells persisting along with the mesenchymal component with complete disappearance of the blastemal component which was a triphasic tumor before chemotherapy (H and E; ×200)
The results of this study again reveal that the response to chemotherapy may be markedly variable in patients. The desired response in some may be achieved with a fewer number of cycles, whereas in other patients, the tumor may resist even with the maximum number of neoadjuvant chemotherapy cycles presently employed, thus defeating the very purpose even at the potential risk of toxicity. It was also concluded that the tumor grade decreases and differentiation improves, in addition to the retrogressive changes and increase in stromal component as a result of chemotherapy in carcinoma breast as well as in other malignancies.
ACKNOWLEDGMENT
Dr. S. K Mathur, Dr. Sunita Singh, Dr. Nisha Marwah, Dr. Rajnish Kalra, Dr. Sanjay Kumar, Dr. Veena Gupta, Dr. Meenu Gill, Dr. S. P. Kataria, Dr. Sumiti Gupta, Dr. Sonia Chhabra, Dr. Gajender for their opinions and suggestions in the reporting of the cases.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Khanna AK, Saxena SK, Khanna S, Kumar A. Histopathological changes following anterior chemotherapy in advanced breast cancer. Indian J Cancer. 1990;272:109–15. [PubMed] [Google Scholar]
- 2.Rosai J. Breast. In: Rosai J, editor. Rosai and Ackerman's Surgical Pathology. 9th ed. Missouri: Mosby; 2004. pp. 1763–876. [Google Scholar]
- 3.Gamble M, Wilson I. The hematoxylins and eosin. In: Bancroft D, Gamble M, editors. Theory and Practice of Histological techniques. 5th ed. Philadelphia: Churchill Livingstone; 2001. pp. 125–38. [Google Scholar]
- 4.Connolly JL, Jacobs TW. The breast. In: Silverberg SG, De Llelis RA, Frable WJ, LiVolsi VA, Wick MR, editors. Silverberg's principles and practice of surgical pathology and cytopathology. 4th ed. Philadelphia: Churchill Livingstone; 2006. pp. 419–504. [Google Scholar]
- 5.Morrow M, Braveman A, Thelmo W, Sohn CK, Sand J, Mora M, et al. Multimodal therapy for locally advanced breast cancer. Arch Surg. 1986;121:1291–6. doi: 10.1001/archsurg.121.11.1291. [DOI] [PubMed] [Google Scholar]
- 6.Faneyte IF, Schrama JG, Peterse JL, Remijnse PL, Rodenhuis S, van de Vijver MJ. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer. 2003;88:406–12. doi: 10.1038/sj.bjc.6600749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burcombe RJ, Makris A, Richman PI, Daley FM, Noble S, Pittam M, et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer. 2005;92:147–55. doi: 10.1038/sj.bjc.6602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasbridge SA, Gillett CE, Seymour AM, Patel K, Richards MA, Rubens RD, et al. The effects of chemotherapy on morphology, cellular proliferation, apoptosis and oncoprotein expression in primary breast carcinoma. Br J Cancer. 1994;70:335–41. doi: 10.1038/bjc.1994.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aktepe F, Kapucuoglu N, Pak I. The effects of chemotherapy on breast cancer tissue in locally advanced breast cancer. Histopathology. 1996;29:63–7. doi: 10.1046/j.1365-2559.1996.d01-485.x. [DOI] [PubMed] [Google Scholar]
- 10.Gazet JC, Coombes RC, Ford HT, Griffin M, Corbishley C, Makinde V, et al. Assessment of the effect of pretreatment with neoadjuvant therapy on primary breast cancer. Br J Cancer. 1996;73:758–62. doi: 10.1038/bjc.1996.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honkoop AH, Pinedo HM, De Jong JS, Verheul HM, Linn SC, Hoekman K, et al. Effects of chemotherapy on pathologic and biologic characteristics of locally advanced breast cancer. Am J Clin Pathol. 1997;107:211–8. doi: 10.1093/ajcp/107.2.211. [DOI] [PubMed] [Google Scholar]
- 12.Moreno A, Escobedo A, Benito E, Serra JM, Gumà A, Riu F. Pathologic changes related to CMF primary chemotherapy in breast cancer. Pathological evaluation of response predicts clinical outcome. Breast Cancer Res Treat. 2002;75:119–25. doi: 10.1023/a:1019607924403. [DOI] [PubMed] [Google Scholar]
- 13.Hasebe T, Tsuda H, Hirohashi S, Shimosato Y, Tsubono Y, Yamamoto H, et al. Fibrotic focus in infiltrating ductal carcinoma of the breast: A significant histopathological prognostic parameter for predicting the long-term survival of the patients. Breast Cancer Res Treat. 1998;49:195–208. doi: 10.1023/a:1006067513634. [DOI] [PubMed] [Google Scholar]
- 14.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–30. [PubMed] [Google Scholar]
- 15.Sánchez MF, Dominguez AG, Uribe N, Ulloa AC, Estrada DF, Candelaria M, et al. Clinical and pathological predictors of the response to neoadjuvant anthracycline chemotherapy in locally advanced breast cancer. Med Oncol. 2006;23:171–83. doi: 10.1385/MO:23:2:171. [DOI] [PubMed] [Google Scholar]


