Abstract
Background
Exacerbations of chronic obstructive pulmonary disease (COPD) are a major burden to patients and to society. Little is known about the possible role of day-to-day patient-reported outcomes during an exacerbation. This study aims to describe the day-to-day course of patient-reported health status during exacerbations of COPD and to assess its value in predicting clinical outcomes.
Methods
Data from two randomized controlled COPD exacerbation trials (n = 210 and n = 45 patients) were used to describe both the feasibility of daily collection of and the day-to-day course of patient-reported outcomes during outpatient treatment or admission to hospital. In addition to clinical parameters, the BORG dyspnea score, the Clinical COPD Questionnaire (CCQ), and the St George’s Respiratory Questionnaire were used in Cox regression models to predict treatment failure, time to next exacerbation, and mortality in the hospital study.
Results
All patient-reported outcomes showed a distinct pattern of improvement. In the multivariate models, absence of improvement in CCQ symptom score and impaired lung function were independent predictors of treatment failure. Health status and gender predicted time to next exacerbation. Five-year mortality was predicted by age, forced expiratory flow in one second % predicted, smoking status, and CCQ score. In outpatient management of exacerbations, health status was found to be less impaired than in hospitalized patients, while the rate and pattern of recovery was remarkably similar.
Conclusion
Daily health status measurements were found to predict treatment failure, which could help decision-making for patients hospitalized due to an exacerbation of COPD.
Keywords: health status, Clinical COPD Questionnaire, hospital, admission, prediction
Introduction
Exacerbations in patients with chronic obstructive pulmonary disease (COPD) are known to be associated with high inhospital mortality as well as in the following years.1,2 The number of exacerbations strongly influences mortality.3 Other consequences of a higher frequency of exacerbations are high socioeconomic cost,4 deterioration in quality of life,5–8 and increased decline in lung function.9
The definition and management of exacerbations of COPD remains a challenge. Diagnosis of an exacerbation is based exclusively on clinical parameters that have an important day-to-day variation.10 However, because the way clinicians interpret clinical information varies, the clinical decisions may also vary.11,12 To facilitate decision-making, models have been developed that predict outcome in exacerbations of COPD at the emergency department and the intensive care unit.13,14 Some of these models also use patient-reported outcomes, ie, the modified Medical Research Council scale for the “usual” severity of dyspnea.15 Health status/health-related quality of life, an important patient-reported outcome, has not been included in current models even though health-related quality of life measurements at first consultation for an exacerbation may yield important information for stratification of the patients.16 Recently updated Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines have for the first time divided patients diagnosed with COPD into categories based on annual number of exacerbations, GOLD stage, Medical Research Council and health status as assessed with the COPD Assessment Test (CAT) or the Clinical COPD Questionnaire (CCQ).10 However, the role of evaluation of health status in assessing and managing exacerbations has been limited so far to follow-up visits after discharge from hospital.10
New treatment paradigms in COPD are proposed, shifting from best current control only to incorporate future risk estimation and reduction.17 Patient-reported outcomes as assessed with health-related quality of life tools may be used to guide treatment in COPD, especially if they can be proven to predict future events. This would require simple and easy to complete health status questionnaires, such as the CCQ.18,19
The present study evaluates the feasibility of daily patient-reported outcome measurements during exacerbations either treated at home or in hospital, describes the course of patient-reported outcomes during exacerbations, and assesses whether patient reported-outcomes on the CCQ, St George’s Respiratory Questionnaire (SGRQ), and BORG scale could help decision-making in exacerbation of COPD. It also describes their predictive value for time to next exacerbation and mortality.
Materials and methods
Patients
The present study used data from two randomized clinical trials. Study one was a randomized, double-blind, double-dummy, placebo-controlled clinical trial assessing the noninferiority of oral and intravenous corticosteroids during hospitalization because of an exacerbation of COPD, labeled the inhospital study.20 Study two was a randomized, double-blind, placebo-controlled clinical trial evaluating the effects of 14 days of combined high-dose budesonide/formoterol, prednisolone, or placebo during an exacerbation of COPD in an outpatient setting, labeled the outpatient study.21
In both trials, an exacerbation of COPD was defined as a history of increased breathlessness and the presence of at least two of the following symptoms for at least 24 hours: increased cough frequency or severity; increased sputum volume or purulence; and increased wheeze. In both trials, therapy has started immediately after an exacerbation was recorded. In the inhospital study, patients hospitalized for an exacerbation of COPD received either oral or intravenous prednisolone (double-dummy) for five days followed by tapering of the dosage using oral prednisolone.20 The primary endpoint was treatment failure, defined as all-cause mortality, intensive care unit admission, intensification of pharmacological treatment, or rehospitalization within 14 days (early treatment failure) or 90 days (late treatment failure). Excluded were patients with signs of a very severe exacerbation on admission (arterial pH < 7.26 or PaCO2 > 9.3 kPa), those with significant or instable comorbidity, and those with a history of asthma.
To assess exacerbations and survival status, hospital and general practitioner records were reviewed as an extension of the initial study. The second outpatient study evaluated the effects of 14 days of combined high-dose inhaled budesonide/formoterol, oral prednisolone, or placebo (double-dummy) during an exacerbation of COPD.21 Patients were recruited, taken off inhaled steroids, and were randomized to one of the three treatment arms at the start of an exacerbation. Patients were treated at home. In this second study, we used their original data to make assessments on health status and dyspnea but we did not have access to survival status data. Both studies were ethically approved and registered at ClinicalTrials.gov (I:NCT00311961, II:NCT00239278).
Measurements
Lung function was measured according to American Thoracic Society/European Respiratory Society guidelines at admission, and on days 3, 5, and 7. An absolute difference of 100 mL in forced expiratory flow in one second (FEV1) has been suggested to be clinically relevant.22 Arterial blood gases were obtained on the day of admission, and on days 2, 3, 5, and 7 (inhospital study). C-reactive protein and erythrocyte sedimentation rate were measured on admission. Lung function was assessed twice during the run-in period in the outpatient study and on days 1, 7, and 14 of the inhospital study.
Patient-reported outcomes
All tests have measurement uncertainties, and small changes in results might not be clinically meaningful. For many tests, the cut-off for clinically meaningful changes is the minimal clinically important difference (MCID). The MCID helps to interpret changes in scores and can be used to assess the percentage of patients that benefit from an intervention.
The BORG dyspnea score measures dyspnea on a scale from 0 (“nothing at all”) to 10 (“maximal”).23,24 The MCID for the BORG is 1 point.25 The BORG score was measured on days 1–5 and day 7 in the inhospital study.
The CCQ is a 10-item health status scale measuring symptoms, functional status, and mental state in patients with COPD. Scores range from 0 (best) to 6 (worst).18 The MCID is 0.4.26 The CCQ has a 24-hour and a one-week version. It was administered in hospital on days 1–5 and 7 using the diary version and at six weeks using the standard week version. In the outpatient exacerbation study, the CCQ was administered similarly at baseline, two months after stopping inhaled corticosteroids, on the first exacerbation day, and on days 3, 7, and 14.
The SGRQ is a 50-question, 76-item health status scale for both asthma and COPD patients.27 The SGRQ has three subscales, ie, symptoms, activities, and impact. In the present study, the standard three-month version was used.27 The score ranges from 0 (best) to 100 (worst).20 The MCID is 4 points.28 The standard SGRQ (three-month recall period) was administered on days 1 and 7.
Statistical analysis
Differences in patient characteristics between the two studies were tested by chi-square or independent t-tests. To compare the course of patient-reported outcomes and FEV1, all scores were normalized to the number of times the MCID. For example, a mean change in SGRQ score of 3 points resulted in a “number of MCID change” of (3 units/4 units = MCID) 0.75. For the FEV1, an MCID of 100 mL was taken.22 We computed this number of MCID changes in all patient-reported outcomes and FEV1 for each day during the hospitalization to represent the change graphically. To evaluate responsiveness, the number of patients that changed more than the MCID after seven days of treatment was calculated. Although the recall period is different in the patient-reported outcomes used, by standardization into the change in MCID, we have tried to reduce the influence of the different recall periods.
Cox regression models were used to assess predictors of treatment failure. Based on their clinical relevance and on the literature, the following variables were included in the univariate Cox models: treatment arm, age, gender, smoking status, pack years, baseline FEV1% predicted, number of hospitalizations during the year prior to the exacerbation, long-term oxygen use, body mass index, SGRQ, and CCQ total and domain scores, BORG dyspnea score, blood gases, C-reactive protein, and erythrocyte sedimentation rate. Further, the change between day of admission and the next day for blood gases, BORG dyspnea score, and CCQ total and domain scores were also tested. Next, multivariate Cox regression models were used. In these models, treatment arm, FEV1% predicted, age, gender, smoking status, and all variables with a P value < 0.1 in the univariate model were entered. Because the CCQ, SGRQ, and BORG scores measure a similar construct, these were not entered in the models simultaneously, but their effects were estimated in three separate multivariate Cox models. Data were analyzed on an intention-to-treat basis. Statistical analyses were performed using Statistical Package for Social Sciences version 19 software (SPSS Inc, Chicago, IL, USA).
Results
The demographic characteristics of the patients with inhospital and outpatient exacerbations are shown in Table 1. Patients who were admitted to hospital were generally older, had more severe airways obstruction, and had higher/worse CCQ scores (all P < 0.0001).
Table 1.
Patient demographics
| Hospital | Outpatient | |
|---|---|---|
| Patients (n) | 210 | 45 |
| Age, years | 70.6 (8.4) | 64.1 (8.1) |
| Male gender | 158 (75.2) | 37 (82.2) |
| FEV1% predicted | 36.9 (14.73) | 52.1 (12.9) |
| GOLD stage | ||
| I | 4 (2) | |
| II | 33 (16.1) | 26 (57.8) |
| III | 99 (48.3) | 17 (37.8) |
| IV | 69 (33.7) | 2 (4.4) |
| Pack-years (median [IQR]) | 35 (24–50) | 38 (26–48.5) |
| Current smokers | 49 (23.7) | 21 (46.7) |
| BORG score | 4.6 (2.0) | N/A |
| CCQ total score | 3.3 (0.93) | 2.6 (0.79) |
| SGRQ total score | 63.1 (13.9) | N/A |
Note: Values are given as mean (standard deviation), or n (%).
Abbreviations: FEV1, forced expiratory flow in one second; GOLD, The Global initiative for chronic Obstructive Lung Disease; CCQ, Clinical COPD Questionnaire; SGRQ, St George’s Respiratory Questionnaire; IQR, interquartile range.
Feasibility
On the first day of hospital admission, 198 patients completed the BORG, 196 completed the CCQ, 197 completed the SGRQ, and 193 performed spirometry. Reasons for not completing the patient-reported outcomes were being too dyspneic to answer the questionnaires, not in the mood for completing questionnaires, not having reading glasses, or no clarified reason. There were no missing data in the outpatient group except for one patient for whom a CCQ was not recorded at the beginning of the exacerbation.
Course of patient-reported outcomes (dyspnea, health status, and domain scores)
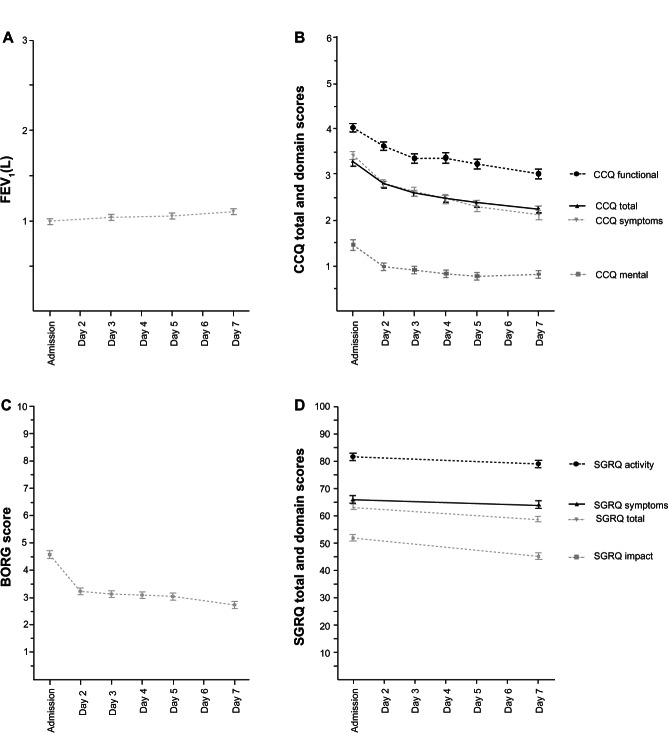
Figure 1 shows the course of the FEV1, BORG, CCQ, and SGRQ total and domain scores in the inhospital study as assessed for each day within the first week. The FEV1 improved only slightly (Figure 1A). The CCQ shows rapid improvement in total score on the first day, as well as in the functional, mental and symptoms domains, and continued to improve on the following days (Figure 1B). In the SGRQ, as in the CCQ, the domain measuring functional status, ie, the SGRQ activity domain and the CCQ functional state domain, were the most impaired. Scores on the symptom domains of both health status scales were less impaired, but still very high. The least impaired was the CCQ mental status domain and the SGRQ impact domain. However, 23 patients still scored above 3 points on the CCQ mental state domain at admission and nine patients scored above 3 points on day 7.
Figure 1.
Course of the mean FEV1, BORG, CCQ total and domain scores, and SGRQ total and domain scores.
Abbreviations: FEV1, forced expiratory flow in one second; CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease; SGRQ, St George’s Respiratory Questionnaire.
The BORG score for dyspnea decreased (improved) quickly during the first day of treatment in hospital, then stabilized (Figure 1C). Within the SGRQ, the activity and impact scores improved most (Figure 1D). To compare the course and responsiveness of the measurements, the absolute mean scores were also shown normalized as number of MCIDs of the measurements (Figure 2).
Figure 2.
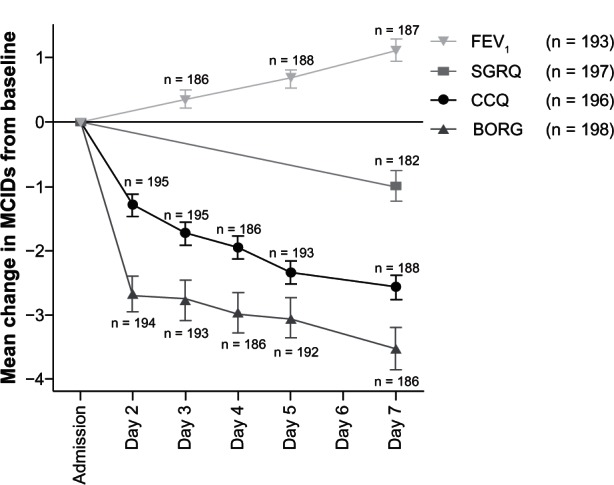
Mean change in minimal clinically important differences of FEV1, BORG, CCQ, and SGRQ.
Note: Error bars represent the standard error of measurement.
Abbreviations: FEV1, forced expiratory flow in one second; CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease; SGRQ, St George’s Respiratory Questionnaire; n, number of patients completed the measurement; MCID, minimal clinically important difference.
The improvement between admission and day 7 represents the responsiveness of the FEV1and the patient-reported outcomes, and is expressed as mean change in absolute score, percentage change, and percentage of patients improving more than the MCID of the measurement (Table 2).
Table 2.
Responsiveness of patient-reported outcomes and lung function on day 7
| n | MCID | Change score | Change % from baseline | Δ > MCID % responders | |
|---|---|---|---|---|---|
| BORG dyspnea | 182 | 1 | −1.76 (2.27) | −32.2 (52.4) | 72.1 |
| CCQ hospital | 181 | 0.4 | −1.03 (1.04) | −29.7 (31.2) | 73.5 |
| CCQ home | 37 | 0.4 | −0.71 (0.83) | −26.2 (28.1) | 59.5 |
| SGRQ | 179 | 4 | −3.99 (13.23) | −5.09 (22.8) | 50.3 |
| FEV1 (mL) hospital | 179 | 100 mL | 111 (9.0) | 13.9 (10.1) | 48.6 |
| FEV1 (mL) home | 45 | 100 mL | 2.2 (17.8) | 2.4 (11.3) | 23.8 |
Notes: Values are presented as the mean (standard deviation). Change in patient-reported outcomes and lung function between admission and day 7, including the percentage of patients improving more than the MCID.
Abbreviations: n, number of patients who completed the measurement on both admission and day 7; MCID, minimal clinically important difference; CCQ, Clinical COPD Questionnaire; SGRQ, St George’s Respiratory Questionnaire; FEV1, forced expiratory flow in one second.
Course of CCQ on inhospital and outpatient treatment
The mean CCQ scores for the two study populations are shown in Figure 3.29 CCQ scores increased (deteriorated) significantly between stable status (before and after the two-month run-in period) and exacerbation in the outpatient group. No data were available on pre-exacerbation CCQ in the hospital study because patients were enrolled during an exacerbation. The slope of the patients treated in hospital was −0.16 points/day in the first 7 days, while the mean change (ie, improvement) for the patients treated at home was −0.12 points/day. The rate of improvement did not differ significantly between the two groups.
Figure 3.
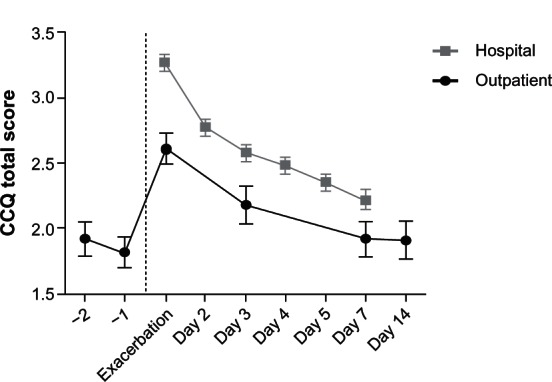
Course of Clinical COPD Questionnaire total scores in hospitalized patients and outpatients during recovery from an exacerbation of COPD.
Notes: Time points −2 and −1 represent run-in visits.Reprinted with permission from Kocks JWH, Kerstjens HAM, van den Berg JWK, van der Molen T. The course of health status during an exacerbation of COPD in hospitalized patients versus outpatient treated patients. Prim Care Respir J. 2010;19:A8–A9.29
Abbreviation: COPD, chronic obstructive pulmonary disease.
Treatment failure
Thirty-eight of 210 patients admitted to hospital had early treatment failure, ie, within the first 14 days after admission. The models including patient-reported outcomes are shown in Table 3 and Table A1. In the models with the BORG or SGRQ score, the patient-reported outcome did not predict treatment failure. However, in the model with the CCQ, treatment failure was predicted by change in CCQ symptom score on the first day and FEV1% predicted on admission. To facilitate the decision rule for clinicians, we performed an additional analysis and calculated the hazard ratios (HR) for patients who did not improve in their symptoms as measured by the CCQ. A lack of improvement in CCQ symptom score in the first day of hospitalization had an HR of 2.6 (95% confidence interval [CI]: 1.2–5.8) and in that model the FEV1% predicted had an HR of 0.95 (95% CI: 0.91–0.99) in predicting time to treatment failure within 14 days after admission. All other clinical variables tested, including blood gases, were not predictive of time to treatment failure within 14 days, whether tested as continuous variables or dichotomously as normal or abnormal.
Table 3.
Cox regression models describing prediction of treatment failure
| Variable | Univariate analysis
|
Multivariate analysis BORG
|
Multivariate analysis CCQ
|
Multivariate analysis SGRQ
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Treatment arm | 0.980 | (0.519–1.851) | 0.950 | 1.159 | (0.69–3.666) | 0.276 | 1.086 | (0.472–2.502) | 0.846 | 1.228 | (0.549–2.749) | 0.616 |
| FEV1% predicted | 0.967 | (0.939–0.996) | 0.024 | 0.636 | (0.927–1.001) | 0.590 | 0.949 | (0.907–0.993) | 0.024 | 0.971 | (0.935–1.009) | 0.129 |
| Age | 1.007 | (0.969–1.047) | 0.720 | 1.010 | (0.956–1.068) | 0.715 | 1.003 | (0.952–1.056) | 0.918 | 1.019 | (0.963–1.078) | 0.512 |
| Male gender | 1.026 | (0.486–2.169) | 0.945 | 0.763 | (0.264–2.209) | 0.618 | 1.239 | (0.429–3.575) | 0.692 | 1.094 | (0.369–3.249) | 0.871 |
| Smoking status | 0.831 | (0.381–1.812) | 0.641 | 0.513 | (0.183–1.435) | 0.203 | 0.632 | (0.217–1.839) | 0.400 | 0.534 | (0.195–1.461) | 0.222 |
| O2 use at home | 2.923 | (1.449–5.897) | 0.003 | 2.430 | (0.923–6.398) | 0.072 | 0.952 | (0.312–2.905) | 0.932 | 1.821 | (0.702–4.725) | 0.218 |
| pCO2 | 1.451 | (1.123–1.874) | 0.004 | 1.304 | (0.933–1.822) | 0.120 | 1.207 | (0.855–1.705) | 0.286 | 1.396 | (0.998–1.953) | 0.052 |
| BORG ∆ 1–2 | 1.171 | (0.997–1.375) | 0.055 | 1.143 | (0.924–1.415) | 0.218 | ||||||
| CCQ symptom ∆1–2 | 0.624 | (0.471–0.828) | 0.001 | 0.636 | (0.427–0.947) | 0.026 | ||||||
| SGRQ total | 0.978 | (0.959–0.999) | 0.037 | 0.979 | (0.954–1.004) | 0.098 | ||||||
Note: Significant P-values (P < 0.05) are shown in bold.
Abbreviations: HR, hazard ratio; CCQ, Clinical COPD Questionnaire; CI, confidence interval; SGRQ, St George’s Respiratory Questionnaire; FEV1 forced expiratory flow in one second; A 1-2, change in score between day 1 (admission) and day 2.
Time to re-exacerbation
Sixty-six percent of the 164 patients in whom complete data could be obtained from the hospital study had a re-exacerbation within the first year, beginning at six weeks after hospitalization. Time to first re-exacerbation was predicted by CCQ total score at six weeks (HR 1.21, 95% CI: 1.01–1.46, Table A2). Patients in the highest tertile of the CCQ (>2.6) score had a hazard ratio of 1.88 (95% CI: 1.18–3.0) compared with the lowest tertile (CCQ < 1.8, Figure 4). Lung function, smoking status, age, GOLD stage, and SGRQ score did not predict time to re-exacerbation.
Figure 4.
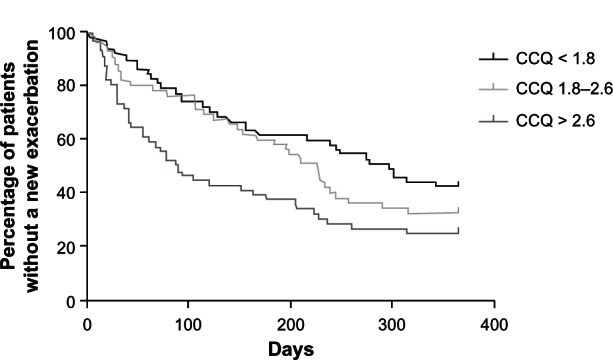
Cox survival curve for re-exacerbation within a year, beginning at six weeks after hospitalization.
Notes: Groups are divided by CCQ score tertiles (CCQ < 1.8; 1.8–2.6; > 2.6).
Abbreviations: CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease.
Mortality
The five-year mortality of the hospital-based study population was 54.9% with a median follow-up of 4.8 (interquartile range 4.2–5.2) years after initial admission. At day 42, the factors predicting increased all-cause mortality were age (HR 1.05, 1.02–1.08), a body mass index < 21 (HR 1.85, 1.02–3.36) and CCQ score (HR 1.41, 1.18–1.69, Table A3). The SGRQ at day 42 after admission did not predict mortality (HR 1.01, 0.99–1.02), nor did the BORG dyspnea score. In the models with SGRQ and BORG, the FEV1% predicted became predictive for mortality, but not in the CCQ model. After adjusting for age, smoking status, gender, and FEV1% predicted, patients in the highest tertile of the CCQ score had an HR for mortality of 3.10 (1.64–5.87) compared with the lowest CCQ tertile (Figure 5).
Figure 5.
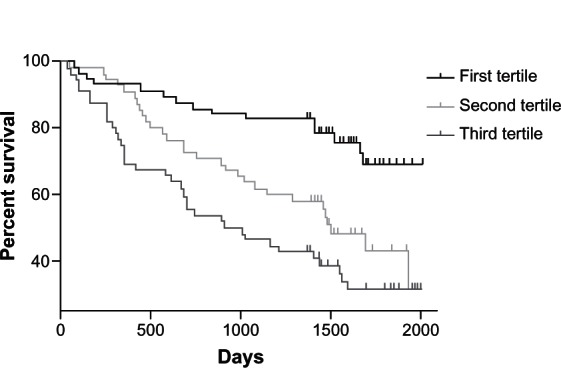
Proportion of patients alive after hospitalization for an exacerbation of COPD (CCQ score < 1.8; 1.8–2.6;>2.6).
Abbreviations: CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease.
Discussion
In this study we demonstrated that daily measurement of patient-reported outcomes is feasible in patients experiencing an exacerbation of COPD. Health status measured by the CCQ predicted treatment failure and may therefore be useful in decision-making. Further, CCQ scores measured six weeks after admission predicted time to re-exacerbation and mortality.
Feasibility
Most patients were able to complete the patient-reported outcomes, even at admission when their health was severely impaired. Patients completed the questionnaires themselves, assisted by the researchers only when needed; support was strictly limited to reading out the questions. SGRQ has been used as the gold standard for measurement of health status in clinical studies and exacerbation of COPD.5,30–32 However, exacerbation studies have assessed differences in health status over longer periods, from 1–6 months to 3 years.5,7,30,31 However, the length and complex scoring algorithm required for the SGRQ makes it less feasible in routine daily practice. During hospitalization, the SGRQ was administered twice within seven days, although seven days was too soon considering the recall period of the SGRQ which might have resulted in a smaller response in the symptoms domain. To be able to use the SGRQ during short follow-up studies, others have discarded its symptom domain.5 A total score should no longer be calculated without this domain, and as a result, the scores are no longer comparable with those from other studies.
Course of patient-reported outcomes
The four measurements (FEV1, BORG, CCQ, and SGRQ) showed a different course of improvement during the first week of recovery, with the CCQ showing a profile in improvement similar to BORG dyspnea during home and hospital exacerbations. FEV1 improved by 13% compared with baseline, and almost 50% of patients improved above the MCID for FEV1.32,33 This improvement (111 mL) is more than previously described,32,33 but still not large. However, one needs to be aware that the suggested MCID is debated because the absolute number in mL is not related to age, gender, and height.
The BORG score showed the most improvement on the first day and remained fairly stable during the following days. In a similar study, Maltais et al found a change in score of 2.6 ± 2.3 points after three days of oral treatment with prednisolone, compared with 1.4 points within three days in our study.33
The CCQ total score improved by more than one MCID within the first day and continued to improve up to day 7, which is comparable with previous data.34 The SGRQ was measured only at admission and on day 7 and the mean score almost reached the MCID. However, the CCQ was more sensitive in assessing differences within days, given that it definitely reached the MCID from the first day.
Hospital versus outpatient health status scores
The health status of patients admitted to hospital during exacerbations was worse than that of those treated at home, which was expected because the decision for hospital admission is often made when patients present with more symptoms and more severe impairment of health status. The most important difference between the two groups was their health status and lung function on the first treatment day of treatment. However, both treatment groups showed a remarkably similar pattern in health status improvement. To our best knowledge, no other studies have shown a detailed course of change in health status during an exacerbation in COPD in both the hospital and outpatient settings, and that was the main reason we decided to present both study populations.
Domain scores in health status questionnaires
The domains that measure functional status, ie, the CCQ functional state domain and the SGRQ activity domains, were the most impaired. Next on the scale of impairment came the symptom domains. The fact that functional status domains were most impaired and the gradual recovery of the functional status domains as compared with breathlessness recorded by the BORG is interesting, because the recently developed Exacerbations of Chronic Pulmonary Disease Tool (EXACT) a patient-reported outcomes questionnaire, focuses on symptoms and not on functional status.35 Although symptom worsening is the key feature in exacerbations, limitations in functional status is what matters most to patients.36 The least impaired were the mental status domain and the SGRQ impact domain. However, 23 patients still scored above 3 points on the CCQ mental state domain at admission and nine patients scored above 3 points on day 7. A CCQ score above 3 is a strong predictor (odds ratio 15.17 [3.19 –72.07]) for depressive symptoms assessed using the Hospital Anxiety and Depression Scale in primary care patients.37 This is an important finding because depression is associated with a worse outcome in patients with COPD, even affecting readmission to hospital.38 This advantage of the CCQ in containing different domains could be important in the holistic management of COPD because these patients need extra attention.
Most studies describe the course of symptoms alone during exacerbation and recovery,39–41 and in the event that health status is measured, generally it is evaluated at admission and after two weeks to three months.16 Health status provides more information than symptoms alone. In our study, dyspnea measured using the BORG score did not predict treatment failure, whereas SGRQ impact scores did in the univariate analysis. Although dyspnea improved rapidly after initiation of treatment, which is in accordance with other studies,42,43 improvement in dyspnea did not predict which patients would face treatment failure. On the other hand, in the model using the CCQ, treatment failure was predicted by a change in CCQ symptom score on the first day. Therefore, the CCQ is a useful tool for predicting treatment failure because it has the additional advantage of providing information not only on (dyspnea) symptom score but also on functional and mental state.
Treatment failure
Treatment failure in our study was predicted by a range of factors in the univariate analyses, ie, the FEV1% predicted, home oxygen, pCO2 and acidosis at admission, SGRQ total and impact score, and CCQ changes within one day. Several non-patient-reported outcomes have been reported as predictors of inhospital mortality or treatment failure.44,45 Additional factors reported in the literature are gender, physical activity, Medical Research Council dyspnea score, O2 oxygen tension, body mass index, neurological impairment, and use of inspiratory accessory muscles.14 Many of these studies report different predictors, probably because of use of different populations, measurements, and analyses; however, none of these studies have reported use of daily measurement of health status. In our multivariate models, only the FEV1% predicted and change in CCQ symptom score were significant independent predictors. Health status measurement with the CCQ is a useful candidate because it is feasible and inexpensive.
Mortality
Our study showed a five-year mortality rate of 55%. A recently published cohort study using data from 73,106 patients hospitalized with COPD also showed high mortality of 50% and 75% at 3.6 and 7.7 years, respectively.46 Again, patient-reported outcomes were not included.
The five-year mortality rate in the study by Soler-Cataluna et al3 was 38.2% after hospital admission for an exacerbation of COPD and 27% in the study by Nishimura.47 Patients in those studies were of approximately similar age, and had a higher FEV1 predicted (46.4% and 41.1%, respectively) compared with 36.9% in our study. In the study by Nishimura, 33% were current smokers versus 49% in the current study. Smoking status and more severe obstruction might explain the high mortality rate in the current study. Unfortunately, the patient-reported outcome measures were different in the Soler-Cataluna et al and Nishimura et al studies, so the health status level cannot be compared, but CCQ in the adjusted model was still shown to be able to predict mortality in our study.
Strengths and limitations
The strength of this study is that we could collect daily patient-reported outcomes in two well-controlled environments, resulting in few missing data. However, the hospital exacerbation study used data from a single-center study and patients with respiratory failure were not included. To some degree, this limits generalization of the data to more severe exacerbations. The outpatient study is small, and the available information is different, so there are concerns about the generalization of the results in this group, but the data are of high quality by design and add important information for the interpretation of results. Future studies are needed to confirm our findings and assess the predictive value of measurement of daily health status in a prospective algorithm in a broader group of patients. In the time to next exacerbation analyses, the number of previous exacerbations was not significant, contrary to a report that exacerbation history is a strong predictor of future exacerbations.48 Unfortunately, we only had history of hospital admissions available, and not exacerbations treated at home, which might cause the signal in the univariate analysis (P = 0.101), but above our predefined threshold for inclusion in the models. Secondly, in our study, it was not possible to calculate the Charlson comorbidity index, so this was not included in our models, although comorbid diseases are predictors of worse outcome in exacerbations. Next, the currently widely promoted CAT test was not available at the time of these studies, so could not be included. A recently published study suggests that the CAT can be used to assess severity of exacerbations and shows higher baseline scores in frequent exacerbators49 as does the CCQ,42 but the responsiveness of CAT is difficult to interpret because its MCID is not yet known.
Conclusion
In conclusion, it is possible and feasible to perform daily measurements of patient-reported outcomes even during inhospital exacerbations. The absence of improvement on the first day in the CCQ symptoms score (an easily measured variable even during an exacerbation) predicts treatment failure. We suggest that patients who do show an improvement of 1 MCID in the CCQ (0.4) might be eligible for early discharge, but this needs to be tested in a prospective algorithm. There is a marked difference in health status between patients treated at home or in hospital for an exacerbation of COPD, while the rate of recovery of health status is similar. Health status as measured by the CCQ after an exacerbation of COPD predicts mortality and time to next exacerbation. Therefore, patient-reported outcomes could help decision-making in patients with an exacerbation of COPD.
Acknowledgments
The authors thank Nico Schouwstra for generating reports from the hospital records and Erik Bathoorn for fruitful discussions. The second study was financially supported by AstraZeneca in The Netherlands.
Appendix
Table A1.
Cox regression models: treatment failure
| Variable | Univariate analysis
|
Multivariate analysis CCQ
|
Multivariate analysis SGRQ
|
Multivariate analysis BORG
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| Treatment arm | 0.980 | (0.519–1.851) | 0.950 | 1.086 | (0.472–2.502) | 0.846 | 1.228 | (0.549–2.749) | 0.616 | 1.159 | (0.690–3.666) | 0.276 | |
| FEV1% predicted | 0.967 | (0.939–0.996) | 0.024 | 0.949 | (0.907–0.993) | 0.024 | 0.971 | (0.935–1.009) | 0.129 | 0.636 | (0.927–1.001) | 0.590 | |
| Age | 1.007 | (0.969–1.047) | 0.720 | 1.003 | (0.952–1.056) | 0.918 | 1.019 | (0.963–1.078) | 0.512 | 1.010 | (0.956–1.068) | 0.715 | |
| Male gender | 1.026 | (0.486–2.169) | 0.945 | 1.239 | (0.429–3.575) | 0.692 | 1.094 | (0.369–3.249) | 0.871 | 0.763 | (0.264–2.209) | 0.618 | |
| Smoking status | 0.831 | (0.381–1.812) | 0.641 | 0.632 | (0.217–1.839) | 0.400 | 0.534 | (0.195–1.461) | 0.222 | 0.513 | (0.183–1.435) | 0.203 | |
| Packyears | 0.990 | (0.974–1.007) | 0.258 | ||||||||||
| Previous admission | 1.328 | (0.878–2.009) | 0.180 | ||||||||||
| Previous admission > 2 | 2.283 | (0.810–6.438) | 0.119 | ||||||||||
| O2 use at home | 2.923 | (1.449–5.897) | 0.003 | 0.952 | (0.3 12–2.905) | 0.932 | 1.821 | (0.702–4.725) | 0.218 | 2.430 | (0.923–6.398) | 0.072 | |
| BMI | 0.986 | (0.925–1.052) | 0.671 | ||||||||||
| BMI < 2I | 0.872 | (0.340–2.233) | 0.775 | ||||||||||
| O2 sat | 1.000 | (0.969–1.032) | 0.997 | ||||||||||
| O2 sat t1t2 | 0.957 | (0.879–1.042) | 0.307 | ||||||||||
| pH | 0.038 | (0.001–1.323) | 0.071 | ||||||||||
| pH t1t2 | 0.000 | (0.000–1.463) | 0.059 | ||||||||||
| Pa, O2 | 0.939 | (0.804–1.097) | 0.429 | ||||||||||
| Pa, O2t1t2 | 0.837 | (0.686–1.021) | 0.079 | ||||||||||
| Pa, CO2 | 1.451 | (1.123–1.874) | 0.004 | 1.207 | (0.855–1.705) | 0.286 | 1.396 | (0.998–1.953) | 0.052 | 1.304 | (0.933–1.822) | 0.120 | |
| Pa, CO2t1t2 | 1.003 | (0.717–1.403) | 0.986 | ||||||||||
| HBCO | 0.946 | (0.711–1.260) | 0.705 | ||||||||||
| HbCO t1t2 | 1.153 | (0.830–1.602) | 0.395 | ||||||||||
| Hypoxaemia | 1.071 | (0.477–2.405) | 0.869 | ||||||||||
| Hypercapnea | 0.544 | (0.165–1.793) | 0.317 | ||||||||||
| Acidotic | 0.378 | (0.145–0.988) | 0.047 | ||||||||||
| CRP | 1.000 | (0.996–1.004) | 0.921 | ||||||||||
| ESR | 1.007 | (0.995–1.018) | 0.255 | ||||||||||
| Glucose | 0.988 | (0.953–1.023) | 0.493 | ||||||||||
| SGRQ total score | 0.978 | (0.959–0.999) | 0.037 | 0.979 | (0.954–1.004) | 0.098 | |||||||
| SGRQ symptoms | 0.989 | (0.972–1.008) | 0.250 | ||||||||||
| SGRQ impact | 0.980 | (0.961–1.000) | 0.045 | ||||||||||
| SGRQ activity | 0.987 | (0.972–1.003) | 0.100 | ||||||||||
| CCQ total | 0.217 | (0.943–1.757) | 0.294 | ||||||||||
| CCQ symptoms | 0.927 | (0.678–1.267) | 0.633 | ||||||||||
| CCQ functional status | 1.264 | (0.942–1.695) | 0.119 | ||||||||||
| CCQ mental status | 1.144 | (0.932–1.402) | 0.198 | ||||||||||
| CCQ tt1t2 | 0.062 | (0.072–0.929) | 0.017 | ||||||||||
| CCQ st1t2 | 0.624 | (0.471–0.828) | 0.001 | 0.636 | (0.427–0.947) | 0.026 | |||||||
| CCQ ft1t2 | 0.783 | (0.610–1.004) | 0.054 | ||||||||||
| CCQ mt1t2 | 1.077 | (0.860–1.349) | 0.518 | ||||||||||
| CCQ st1t2 > 0 | 0.339 | (0.171–0.671) | 0.002 | ||||||||||
| BORG | 1.151 | (0.989–1.340) | 0.070 | ||||||||||
| BORG t1t2 | 1.171 | (0.997–1.375) | 0.055 | 1.143 | (0.924–1.415) | 0.218 | |||||||
| BORG t1t2 > 0 | 0.278 | (0.038–2.029) | 0.207 | ||||||||||
Abbreviations: BMI, body mass index; CI, confidence interval; CCQ, Clinical COPD Questionnaire; CSR, C-reactive protein; ESR, erythrocyte sedimentation rate; HR, hazard ratio; SGRQ, St George’s Respiratory Questionnaire; FEV1 forced expiratory flow in one second.
Table A2.
Cox regression models: time to exacerbation
| Variable | Univariate analysis
|
Multivariate analysis CCQ
|
Multivariate analysis SGRQ
|
Multivariate analysis BORG
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Treatment arm | 1.243 | (0.857–1.804) | 0.252 | 1.170 | (0.793–1.727) | 0.428 | 1.056 | (0.673–1.657) | 0.812 | 1.179 | (0.783–1.177) | 0.431 |
| FEV1% predicted | 0.992 | (0.980–1.004) | 0.212 | 0.996 | (0.983–1.009) | 0.561 | 0.994 | (0.979–1.008) | 0.377 | 0.992 | (0.979–1.005) | 0.249 |
| Age | 1.018 | (0.996–1.042) | 0.114 | 1.016 | (0.989–1.042) | 0.245 | 1.007 | (0.977–1.038) | 0.646 | 1.015 | (0.988–1.044) | 0.278 |
| Male gender | 1.624 | (1.026–2.517) | 0.038 | 1.636 | (0.985–2.718) | 0.057 | 1.766 | (0.968–3.221) | 0.064 | 1.566 | (0.937–2.216) | 0.087 |
| Smoking status | 0.769 | (0.492–1.201) | 0.249 | 0.888 | (0.557–1.418) | 0.620 | 0.698 | (0.400–1.221) | 0.208 | 0.892 | (0.553–1.439) | 0.640 |
| Packyears | 1.004 | (0.995–1.013) | 0.384 | |||||||||
| Previous admission | 1.295 | (0.910–1.765) | 0.101 | |||||||||
| Previous admission > 2 | 1.305 | (0.573–2.972) | 0.527 | |||||||||
| O2 use at home | 1.373 | (0.753–2.503) | 0.301 | |||||||||
| BMI | 0.975 | (0.936–1.016) | 0.226 | |||||||||
| BMI < 2I | 1.137 | (0.649–1.199) | 0.654 | |||||||||
| SGRQ total score | 0.995 | (0.984–1.007) | 0.440 | 0.995 | (0.983–1.007) | 0.423 | ||||||
| SGRQ symptoms | 1.000 | (0.988–1.013) | 0.938 | |||||||||
| SGRQ impact | 0.995 | (0.985–1.006) | 0.358 | |||||||||
| SGRQ activity | 0.997 | (0.989–1.006) | 0.558 | |||||||||
| CCQ total | 1.233 | (1.047–1.452) | 0.012 | 1.213 | (1.007–1.462) | 0.042 | ||||||
| CCQ symptoms | 1.215 | (1.049–1.407) | 0.009 | 1.178** | (1.008–1.376) | 0.040 | ||||||
| CCQ functional status | 1.158 | (I.0I7–I.3I9) | 0.027 | |||||||||
| CCQ mental status | 1.041 | (0.907–1.194) | 0.570 | |||||||||
| BORG | 0.981 | (0.912–1.054) | 0.595 | 0.996 | (0.924–1.074) | 0.912 | ||||||
Note: **Using CCQ symptoms instead of total score.
Abbreviations: BMI, body mass index; CI, confidence interval; CCQ, Clinical COPD Questionnaire; HR, hazard ratio; SGRQ, St George’s Respiratory Questionnaire; FEV1 forced expiratory flow in one second.
Table A3.
Cox regression models: mortality
| Variable | Univariate analysis
|
Multivariate analysis CCQ
|
Multivariate analysis SGRQ
|
Multivariate analysis BORG
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Treatment arm | 1.042 | (0.673–1.612) | 0.855 | 1.079 | (0.689–1.691) | 0.740 | 0.987 | (0.597–1.632) | 0.959 | 1.015 | (0.642–1.606) | 0.949 |
| FEV1% predicted | 0.986 | (0.972–1.001) | 0.062 | 0.989 | (0.971–1.006) | 0.199 | 0.976 | (0.958–0.995) | 0.012 | 0.980 | (0.964–0.996) | 0.015 |
| Age | 1.046 | (1.016–1.076) | 0.002 | 1.083 | (1.046–1.121) | 0.000 | 1.080 | (1.037–1.125) | 0.000 | 1.088 | (1.048–1.130) | 0.000 |
| Male gender | 1.199 | (0.710–2.025) | 0.498 | 0.888 | (0.498–1.582) | 0.686 | 1.156 | (0.595–2.248) | 0.668 | 0.940 | (0.529–1.672) | 0.833 |
| Smoking status | 1.166 | (0.709–1.919) | 0.545 | 1.922 | (1.113–3.316) | 0.019 | 2.186 | (1.155–4.136) | 0.016 | 1.797 | (1.042–3.100) | 0.035 |
| Packyears | 1.001 | (0.991–1.011) | 0.870 | |||||||||
| Previous admission | 1.330 | (0.935–1.892) | 0.112 | |||||||||
| Previous admission > 2 | 1.755 | (0.763–4.036) | 0.186 | |||||||||
| O2 use at home | 1.787 | (0.945–3.382) | 0.074 | 1.537 | (0.702–3.361) | 0.282 | 1.798 | (0.431–4.422) | 0.201 | 1.976 | (0.881–4.436) | 0.099 |
| BMI | 0.965 | (0.918–1.015) | 0.168 | |||||||||
| BMI < 2I | 1.852 | (1.022–3.357) | 0.042 | 1.896 | (0.958–3.752) | 0.066 | 1.359 | (0.602–3.066) | 0.460 | 1.561 | (0.793–3.069) | 0.197 |
| SGRQ total score | 1.001 | (0.987–1.015) | 0.905 | 1.005 | (0.989–1.021) | 0.530 | ||||||
| SGRQ symptoms | 0.999 | (0.986–1.011) | 0.821 | |||||||||
| SGRQ impact | 1.003 | (0.991–1.015) | 0.586 | |||||||||
| SGRQ activity | 0.998 | (0.988–1.008) | 0.688 | |||||||||
| CCQ total | 1.410 | (1.176–1.69I) | 0.000 | 1.383 | (1.103–1.733) | 0.005 | ||||||
| CCQ symptoms | 1.242 | (1.048–1.472) | 0.012 | |||||||||
| CCQ functional status | 1.443 | (1.236–1.685) | 0.000 | 1.402** | (1.169–1.68I) | 0.000 | ||||||
| CCQ mental status | 1.027 | (0.874–1.207) | 0.746 | |||||||||
| BORG | 0.982 | (0.900–1.071) | 0.677 | 1.017 | (0.927–1.115) | 0.723 | ||||||
Note:
Using CCQ functional status instead of total score.
Abbreviations: BMI, body mass index; CI, confidence interval; CCQ, Clinical COPD Questionnaire; HR, hazard ratio; SGRQ, St George’s Respiratory Questionnaire; FEV1 forced expiratory flow in one second.
Appendix
Footnotes
Disclosure
TvdM contributed to the development of the CCQ. The other authors have no conflicts of interest in this work.
References
- 1.Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD – a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–223. doi: 10.2147/copd.s3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almagro P, Calbo E, Ochoa de Echagüen A, et al. Mortality after hospitalization for COPD. Chest. 2002;121:1441–1448. doi: 10.1378/chest.121.5.1441. [DOI] [PubMed] [Google Scholar]
- 3.Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wouters EF. The burden of COPD in The Netherlands: Results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S51–S59. doi: 10.1016/s0954-6111(03)80025-2. [DOI] [PubMed] [Google Scholar]
- 5.Bourbeau J, Ford G, Zackon H, et al. Impact on patients’ health status following early identification of a COPD exacerbation. Eur Respir J. 2007;30:907–913. doi: 10.1183/09031936.00166606. [DOI] [PubMed] [Google Scholar]
- 6.Doll H, Grey-Amante P, Duprat-Lomon I, et al. Quality of life in acute exacerbation of chronic bronchitis: results from a German population study. Respir Med. 2002;96:39–51. doi: 10.1053/rmed.2001.1208. [DOI] [PubMed] [Google Scholar]
- 7.Anzueto A, Leimer I, Kesten S. Impact of frequency of COPD exacerbations on pulmonary function, health status and clinical outcomes. Int J Chron Obstruct Pulmon Dis. 2009;4:245–251. doi: 10.2147/copd.s4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:698–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.[No authors listed]From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013 Available from: http://www.goldcopd.org/Accessed March 30. 2013
- 11.Wildman MJ, O’Dea J, Kostopoulou O, et al. Variation in intubation decisions for patients with chronic obstructive pulmonary disease in one critical care network. QJM. 2003;96:583–591. doi: 10.1093/qjmed/hcg104. [DOI] [PubMed] [Google Scholar]
- 12.Escher M, Perneger TV, Chevrolet JC. National questionnaire survey on what influences doctors’ decisions about admission to intensive care. BMJ. 2004;329:425. doi: 10.1136/bmj.329.7463.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche N, Rabbat A, Zureik M, Huchon G. Chronic obstructive pulmonary disease exacerbations in emergency departments: predictors of outcome. Curr Opin Pulm Med. 2010;16:112–117. doi: 10.1097/MCP.0b013e328335f039. [DOI] [PubMed] [Google Scholar]
- 14.Wildman MJ, Sanderson C, Groves J, et al. Predicting mortality for patients with exacerbations of COPD and asthma in the COPD and asthma outcome study (CAOS) QJM. 2009;102:389–399. doi: 10.1093/qjmed/hcp036. [DOI] [PubMed] [Google Scholar]
- 15.Roche N, Zureik M, Soussan D, et al. Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Eur Respir J. 2008;32:953–961. doi: 10.1183/09031936.00129507. [DOI] [PubMed] [Google Scholar]
- 16.Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics. 2005;23:345–363. doi: 10.2165/00019053-200523040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Postma D, Anzueto A, Calverley P, et al. A new perspective on optimal care for patients with COPD. Prim Care Respir J. 2011;20:205–209. doi: 10.4104/pcrj.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Molen T, Willemse BW, Schokker S, et al. Development, validity and responsiveness of the clinical COPD questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cave AJ, Atkinson L, Tsiligianni I, Kaplan A. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J COPD. 2012;7:447–456. doi: 10.2147/COPD.S29868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Jong YP, Uil SM, Grotjohan HP, et al. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest. 2007;132:1741–1747. doi: 10.1378/chest.07-0208. [DOI] [PubMed] [Google Scholar]
- 21.Bathoorn E, Liesker JJ, Postma DS, et al. Anti-inflammatory effects of combined budesonide/formoterol in COPD exacerbations. COPD. 2008;5:282–290. doi: 10.1080/15412550802363360. [DOI] [PubMed] [Google Scholar]
- 22.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 23.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 24.Grant S, Aitchison T, Henderson E, et al. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest. 1999;116:1208–1217. doi: 10.1378/chest.116.5.1208. [DOI] [PubMed] [Google Scholar]
- 25.Ries AL. Minimally clinically important difference for the UCSD shortness of breath questionnaire, Borg scale, and visual analog scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 26.Kocks JW, Tuinenga MG, Uil SM, et al. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respir Res. 2006;7:62. doi: 10.1186/1465-9921-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 28.Jones PW. Interpretation of health status measurements: from clinical trials to routine practice. Eur Respir Rev. 2002;12:87. [Google Scholar]
- 29.Kocks JWH, Kerstjens HAM, van den Berg JWK, van der Molen T. The course of health status during an exacerbation of COPD in hospitalized patients versus out-patient treated patients. Prim Care Respir J. 2010;19:A8–A9. [Google Scholar]
- 30.Ferrari R, Tanni SE, Caram LM, et al. Predictors of health status do not change over three-year periods and exacerbation makes difference in chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2011;9:112. doi: 10.1186/1477-7525-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura K, Sato S, Tsukino M, et al. Effect of exacerbations on health status in subjects with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2009;7:69. doi: 10.1186/1477-7525-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson NJ, Walker PP, Costello RW, Calverley PM. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:1510–1516. doi: 10.1164/rccm.200504-595OC. [DOI] [PubMed] [Google Scholar]
- 33.Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2002;165:698–703. doi: 10.1164/ajrccm.165.5.2109093. [DOI] [PubMed] [Google Scholar]
- 34.Trappenburg JC, Monninkhof EM, Bourbeau J, et al. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax. 2011;66:977–984. doi: 10.1136/thoraxjnl-2011-200071. [DOI] [PubMed] [Google Scholar]
- 35.Leidy NK, Wilcox TK, Jones PW, et al. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183:323–329. doi: 10.1164/rccm.201005-0762OC. [DOI] [PubMed] [Google Scholar]
- 36.Celli BR, Vestbo J. The EXACT-PRO: measuring exacerbations of COPD. Am J Respir Crit Care Med. 2011;183:287–288. doi: 10.1164/rccm.201009-1401ED. [DOI] [PubMed] [Google Scholar]
- 37.Cleland JA, Lee AJ, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract. 2007;24:217–223. doi: 10.1093/fampra/cmm009. [DOI] [PubMed] [Google Scholar]
- 38.Ng TP, Niti M, Tan WC, et al. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60–67. doi: 10.1001/archinte.167.1.60. [DOI] [PubMed] [Google Scholar]
- 39.Seemungal TA, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 40.Aaron SD, Donaldson GC, Whitmore GA, et al. Time course and pattern of COPD exacerbation onset. Thorax. 2012;67:238–243. doi: 10.1136/thoraxjnl-2011-200768. [DOI] [PubMed] [Google Scholar]
- 41.van den Berge M, Hop WC, van der Molen T, et al. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group Prediction and course of symptoms and lung function around an exacerbation in chronic obstructive pulmonary disease. Respir Res. 2012;13:44. doi: 10.1186/1465-9921-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koutsokera A, Kiropoulos TS, Nikoulis DJ, et al. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med. 2009;103:919–926. doi: 10.1016/j.rmed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Pinto-Plata VM, Livnat G, Girish M, et al. Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest. 2007;131:37–43. doi: 10.1378/chest.06-0668. [DOI] [PubMed] [Google Scholar]
- 44.Nevins ML, Epstein SK. Predictors of outcome for patients with COPD requiring invasive mechanical ventilation. Chest. 2001;119:1840–1849. doi: 10.1378/chest.119.6.1840. [DOI] [PubMed] [Google Scholar]
- 45.Gunen H, Hacievliyagil SS, Kosar F, et al. Factors affecting survival of hospitalized patients with COPD. Eur Respir J. 2005;26:234–241. doi: 10.1183/09031936.05.00024804. [DOI] [PubMed] [Google Scholar]
- 46.Suissa S, Dell’aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura K. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 48.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 49.Mackay AJ, Donaldson GC, Patel AR, et al. Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185:1218–1224. doi: 10.1164/rccm.201110-1843OC. [DOI] [PubMed] [Google Scholar]