Abstract
Suboptimal antiretroviral adherence is associated with poorer HIV outcomes. Psychosocial factors, including life stress, depression and coping, may influence adherence behavior. This prospective investigation sought to examine the impact of life stress (acute life events, chronic stress, and perceived stress), depression, and coping style on adherence to HIV treatment regimes over time. Participants were 87 treatment-seeking HIV-infected individuals recruited from an urban HIV clinic. They completed clinician-administered interviews and self-report questionnaires at baseline and 3-month follow-up. Acute life events and chronic stress prospectively predicted decreases in treatment adherence more strongly among individuals in a major depressive episode (n =21) compared to non-depressed individuals (n =66). Coping style did not appear to be the mechanism by which life stress influenced adherence among depressed HIV-infected individuals. These findings demonstrate that life stress has toxic effects for depressed individuals and suggest that treatment adherence interventions with depressed individuals could be enhanced via development of stress management skills.
Keywords: HIV/AIDS, Antiretrovirals, Treatment adherence, Depression, Life stress, Coping
Due to the ongoing success of highly active antiretroviral therapy (HAART), HIV is now considered to be a chronic illness (Gifford and Groessl 2002). Although availability of HAART has resulted in better health, quality of life, and life expectancy for HIV-infected individuals (Liu et al. 2006), successful response to HAART requires high levels of adherence to treatment regimens (Lucas 2005). Unfortunately, recent reports indicate average adherence rates of 60–70% among HIV-infected individuals (Simoni et al. 2006), which are far below recommended guidelines. Life stress likely adds to the ongoing challenge of appropriate adherence to one’s antiretroviral treatment regime.
Previous research has suggested that HIV-infected individuals experience a wide variety of stress, both related and unrelated to their illness (Gore-Felton and Koopman 2002; Koopman et al. 2000; Roberts et al. 2001). To date, only a few studies have investigated the influence of life stress on treatment adherence to HAART. Acute life events assessed by self-reported checklists (Catz et al. 2001; Leserman et al. 2008; Mellins et al. 2003) and perceived stress (Bottonari et al. 2005; French et al. 2005; Murphy et al. 2002) have been associated with poorer adherence among HIV-infected individuals. Although previous studies suggest that life stress influences medication adherence, it is possible that different aspects of life stress vary in their impact on adherence. One aspect of “stress” is acute life events, which are “events that have significant threat and unpleasantness for an extended period of time” (McQuaid et al. 2000), have a clear start point (e.g., the death of a loved one or the loss of a job), and negatively impact one’s life. Alternatively, chronic stress has been defined as “persistent difficulties” (Hammen et al. 1987) or “ongoing difficulties” (Brown and Harris 1978), such as long-term poverty or long-standing relationship dissatisfaction. These stressors often lack a clear onset but by definition have a long-standing impact on one’s life. Finally, while both acute life events and chronic stress are generally defined by the presence of an external agent, some researchers argue that stress is best conceptualized as the individual’s subjective perception of stress (Cohen et al. 1983). As stress researchers have repeatedly criticized the use of self-report measures of life events as being overly subjective and vulnerable to reporting biases (McQuaid et al. 2000; Monroe and Roberts 1990), a methodologically sound investigation of life stress could provide a clearer picture about whether stress influences adherence outcomes via experience of acute life events, chronic stress, or one’s overall perception of stress.
While past research has demonstrated that life stress is associated with poorer adherence, it seems likely that HIV-infected individuals vary in terms of the degree to which life stress disrupts adherence to their treatment regimes. In the present study, we focus on depression as a potential moderator. Depressive symptoms are common among HIV-infected individuals and rates of Major Depressive Disorder (MDD) are two times higher among HIV-infected individual than among non-infected individuals (Ciesla and Roberts 2001). Depression has been associated with maladaptive functioning, poor self-regulation, and disease progression among HIV-infected individuals (Rabkin 2008). Moreover, depression has also been associated with poor HIV treatment adherence (Ammassari et al. 2004; Gonzalez et al. 2004; Lima et al. 2007; Safren et al. 2001), though there have been some mixed findings (Catz et al. 2000; Deschamps et al. 2004; Komiti et al. 2003). It is possible that results have varied across previous studies due to the differential impact of distress versus clinical depression on treatment adherence (Coyne 1994; Fechner-Bates et al. 1994). Furthermore, if depression does influence the impact of life stress on adherence, it would also be important to explore mechanisms that account for this relationship, such as coping style.
Lazarus and Folkman’s stress appraisal and coping theory (Lazarus and Folkman 1984) suggests that coping style may be the mechanism by which life stress influences behavior, health, and well-being. Coping has been defined as “thoughts and behaviors that people use to deal with the internal and external demands of situations that are appraised as stressful” (Folkman and Moskowitz 2004). Whereas active methods of coping will either attempt to address the problem or lead to expression of emotion, avoidant methods of coping are aimed at distracting the individual from the issue or emotion they are experiencing. Given evidence that coping style influences the health, well-being, and adherence behavior of HIV-infected individuals (Jia et al. 2004; Safren et al. 2002; Weaver et al. 2005), it is likely that coping style could be the underlying mechanism by which depression and life stress influence treatment adherence among HIV-infected individuals.
The present study extends our previous work on the impact of life stress on treatment adherence by HIV-infected individuals (Bottonari et al. 2005), utilizing a prospective time frame and methodologically-sound measures of life stress, depression, and coping style. We aimed to replicate our previous finding that life stress has a greater adverse impact on treatment adherence among more highly depressed individuals and wanted to examine whether maladaptive coping was responsible for this effect. In an effort to address limitations of previous work, we examined several aspects of life stress (i.e., acute life events, chronic stress, and perception of stress) and depression (both diagnosis and severity of symptoms). It was hypothesized that life stress would be associated with decreases in treatment adherence over a 3 month prospective interval, that depression would moderate this effect, and that coping style would be the mechanism by which stress and depression influence treatment adherence. In particular, we predicted that life stress would have a greater impact on patients with greater depression. Given evidence that depression and stress interact to predict adherence, we hypothesized that maladaptive coping would mediate this relationship.
Methods
Participants
Eligible participants were HIV-seropositive males and females (aged 18 and over), who were undergoing treatment for HIV infection with HAART at an urban HIV clinic. One hundred and one individuals were recruited for the first research session (T1), and 87 (86%) returned for a 2nd research session (T2, which was 3 months later). Forty-four percent of the participants were female; 63% ethnic minority, 40% sexual minority, 40% in romantic relationship, 82% high school education or greater, and on average were middle-aged (M = 46 ± 8 years). Dropouts did not complete the study for the following reasons: “too busy to return (n = 3)”, “incarcerated (n = 2)”, “too sick to attend session (n = 4)”, and “could not be contacted (n = 5)”. There were no significant differences in T1 variables between dropouts and completers. Overall, the sample was made up of experienced HIV patients (75% AIDS-defined, CD4: 563 ± 309 cells/mm3, 62% undetectable viral loads). They reported an average of 11.7 years since diagnosis, 10 years on antiretrovirals, and a range of transmission modalities: men having sex with men (29.9%), heterosexual sex (29.9%), intravenous drug use (18.4%), blood transfusion (3.4%), and multiple methods (e.g., sexual and drug use behavior; 18.4%). The literacy screen (REALM) (Davis et al. 1993) estimated that the majority of the sample (79.3%) read at a high school level. Participants were compensated for their time with $15 gift cards and free participation in psycho-educational workshops. All participants signed informed consents approved by the University’s Institutional Review Board (IRB) prior to the initiation of any research procedures.
Measures
Literacy screen
Rapid estimate of adult literacy in medicine (REALM)
(Davis et al. 1993). The REALM is a brief screening instrument that was used to assess whether the participants would be able to read the self-report questionnaires. The REALM has been shown to correlate highly (r = 0.88 to 0.97) with standardized reading tests. Participants were required to read at or above a 4th–6th grade level to be eligible for the study.
Life stress
Chronic Strain Interview (CSI)
(Hammen et al. 1987). The CSI is a five-item clinician-administered measure that was used to assess for the presence of ongoing chronic stress involving finances, personal health, family health, relationship status, and employment status. Ratings are made in each area to reflect a dimensional view of stress: 0 = “poor conditions” (e.g., extreme poverty), 1 = “problematic” (e.g., financially strapped), 2 = “below average” (e.g., budgeting required to make ends meet), 3 = “average” (e.g., has enough money to pay bills but little extra) and 4 = “exceptionally positive” (e.g., wealthy). Scores from each area are summed to create a composite stress score; however, personal health ratings were excluded from the composite stress score as CSI coded participants’ health in similar way given HIV-positive status. Previous research has demonstrated that overall reliability across all areas of functioning is high (α = 0.97), with high inter-rater reliability as well (0.93 to 0.99) (Hammen et al. 1987). Interrater reliability of the CSI in the present study was good (ICC = 0.85 at T2; n = 25).
Life events and difficulties schedule (LEDS)
(Brown and Harris 1978). The LEDS is an interview-based measure that was used to assess for acute life events experienced during the 3-month prospective interval. Interviews were conducted by the first author with a standardized series of prompts to guide the interview. The emphasis is on measuring objective, quantifiable aspects of stressful experiences (e.g., cost, injury, changes in frequency of contacts with supports) rather than subjective impressions. The interviewer asked probing questions about potential events in order to elicit a narrative about the context surrounding each event and the impact that the event had on the participant’s life. Following completion of the interview, the interviewer presented the information gathered about the events to a panel of trained raters. Their presentation included clarifying details of the context surrounding the event but did not include information on the participant’s subjective response to the event. Consensus threat ratings were based on a standardized manual of over 5,000 examples of events, with threat scores ranging from 1 “little threat” to 5 “marked threat”, with 5 representing the most severe threat (Bilfulco et al. 1989). Training was provided to the consensus team such that all consensus members and the interviewer rated in agreement (κ > 0.80) prior to assigning ratings for study data. Formal inter-rater reliability ratings are not available for this investigation due to limitations of rater availability and time burden of rating process. However, our lab has previously reported high reliability in our ratings of event severity (κ = 0.85) (Bottonari et al. 2007). Analyses utilized ratings of cumulative threat experienced as a result of these events (i.e., the sum of the threat ratings across each participant’s events; however, HIV-related health events were excluded).
Perceived stress scale-4 item version (PSS)
(Cohen and Williamson 1988). The PSS is a 4-item questionnaire that assesses thoughts and feelings associated with subjective levels of distress (e.g., ‘felt nervous or stressed’, ‘felt you were on top of things’). The directions were revised to ask about ‘your feelings and thoughts during the last 3 months’ to correspond with the study time period. Items are rated on the following scale: 0 = ‘Never’, 1 = ‘Almost never’, 2 = ‘Sometimes’, 3 = ‘Fairly often’, and 4 = ‘Very often’. Reliability has been shown to be adequate in past research (α = 0.60) and in the present sample (α = 0.67 at T2).
Coping style
Brief COPE inventory (COPE)
(Carver 1997). The COPE is a 28-item self-report instrument that assesses 14 narrow facets of coping with 2-item scales. Participants are asked to rate the extent to which they used each coping mechanism to deal with the stress they had experienced over the past 3 months. Participants responded to questions using a 4-point Likert scale from 0 = “I haven’t been doing this at all” to 3 = “I’ve been doing this a lot”. As we were interested in broader dimensions of coping, an exploratory factor analysis was conducted. Results indicated that the coping data were best represented by two factors using loadings that were >0.35 on one factor and <0.35 on the other factor. Factor 1 accounted for 21% of the variance and was named ‘Active Coping’ (see Table 1). The “Active Coping” factor consisted of the following scales: Active coping, Emotional support, Instrumental support, Planning, Positive reframing, Humor, Acceptance, and Religion (α = 0.79). Factor 2 accounted for 16.3% of the variance and was named ‘Avoidant Coping’ (see Table 1). The “Avoidant Coping” factor consisted of the following scales: Denial, Substance use, Behavioral disengagement, Venting, and Self-blame (α = 0.74). Both of the coping factors were normally distributed; however, they were not significantly correlated with each other (T1: r = 0.04, P = 0.74).
Table 1.
Distributional properties
| Measures | Mean (SD) | Range | Skew | Kurtosis |
|---|---|---|---|---|
| Treatment adherence | ||||
| T2 30-day adherence | 2.9 (6.6) | 0–30 | 3.2 | 9.1 |
| T2 Transformed 30-day adherence | 6.9e + 06 (2.3e + 06) | 1–8533825 | −2.0 | 3.6 |
| Life stress | ||||
| T2 LEDS | 5.6 (4.8) | 0–23 | 1.0 | 1.0 |
| T2 CSI | 13.6 (2.3) | 9–22 | 0.7 | 1.0 |
| T2 PSS | 6.7 (2.7) | 0–13 | −0.1 | −0.1 |
| Depression | ||||
| T1 MADRAS | 17.8 (11.4) | 0–47 | 0.2 | −1.0 |
| Coping style | ||||
| T1 ACTIVE | 28.1 (9.4) | 9–47 | 0.0 | −0.8 |
| T1 AVOIDANT | 11.4 (6.5) | 0–26 | 0.4 | −0.7 |
T1 time 1, T2 time 2, LEDS cumulative threat associated with acute life events, as measured by life events and difficulties schedule, CSI chronic stress, as measured by Chronic Strain Interview, PSS perceived stress, as measured by Perceived Stress Scale-4 item measure, MADRAS clinician-assessed depression, as measured by the Montgomery-Asberg Depression Rating Scale, ACTIVE active coping style, as measured by Brief COPE, AVOIDANT avoidant coping style, as measured by Brief COPE
Depression
Depression severity
The Montgomery-Asberg Depression Rating Scale (MADRAS) (Montgomery and Asberg 1979) is a 10-item clinician-administered interview that examines depressive severity over the previous week. Internal consistency was good in the current sample (α = 0.85 at T1), with high inter-rater reliability as well (ICC = 0.90 at T1; n = 25).
Depression diagnosis
The Mini International Neuropsychiatric Interview-July 1, 2005 Version (MINI) (Sheehan et al. 1997) is a brief structured interview for the major Axis I psychiatric disorders, shown to be valid and reliable when compared to the Structured Clinical Interview for DSM-III-R and the CIDI (Lecrubier et al. 1997; Sheehan et al. 1998). The MINI was used to assess for the presence of clinically significant depression [i.e., meeting criteria for a DSM-IV Major Depressive Episode (MDE)]; interrater reliability of the MINI MDE module was excellent (κ = 1.00; n = 25).
Adherence/health measures
Treatment adherence
(Modified Adult AIDS Clinical Trial Group (AACTG) Treatment adherence measure) (Chesney et al. 2000). Self-report methods can be used to effectively measure HIV treatment adherence, particularly in observational studies as opposed to adherence intervention studies (Simoni et al. 2006). In addition to the standard AACTG measure, we also asked about the number of days with missed doses over the last 30-days in order to assess adherence over the previous month. Adherence was measured at both T1 and T2 so that the T1 measure could be used as a control variable.
Surrogate markers
HIV infection was documented for all participants by Enzyme-Linked ImmunoSorbent Assay (ELISA) and confirmed by Western blot. Absolute CD4 cell counts were calculated using the total white blood cell count. HIV RNA levels (viral load) were measured by Roche Amplicor assay where the level of undetectability was <50 copies/ml. The most recent CD4 cell count and viral load associated with each research session were recorded after being gathered from the participants’ medical records.
Procedures
Participants completed two research sessions (T1 and T2), separated by 3 months in time (M = 93 days, SD = 5). During the sessions, participants completed self-report questionnaires and clinician-administered interviews. Clinical health status measures (e.g., CD4 count and viral load) were obtained by clinic staff as part of their regular medical visits.
Data analytic strategy
Analyses were conducted using R version 2.9.1 (R Development Core Team 2009). First, we conducted preliminary analyses to examine the distributional properties of the variables of interest. Box-Cox transformations (Box and Cox 1964) were used when necessary to transform distributions towards normality. Next, we explored whether potentially confounding third variables, such as demographic variables, literacy level, duration of HIV illness, length of time on HAART, experience of side effects, clinical health status (e.g., CD4 + count), comorbid medical illness, current level of substance use, and time until T2 research session were associated with the primary independent and dependent variables. If they were significantly correlated with both the independent and dependent variables, they were included as covariates in the analyses. Subsequently, multiple regression was used to examine the primary hypotheses. Separate set-wise regressions examined whether T2 stress (i.e., stress that has occurred during the 3-month study period prior to T2) predicted T2 adherence, controlling for T1 adherence and the confounding variables. We tested the hypothesis that T1 depression moderated (Aiken and West 1991) the relationship between T2 life stress and T2 treatment adherence. Given evidence of an interaction between depression and stress, we planned to examine whether T1 coping style (two analyses to examine active versus avoidant coping) would mediate the relationship between the T2 stress × T1 depression interaction and T2 adherence (e.g., Barron and Kenny 1986).
Results
Preliminary analyses
Self-reported treatment adherence
The measure of 30-day adherence was chosen for the dependent variable as it had the highest correlation with viral load (T1: r = −0.44, P <0.01; T2: r = −0.29, P < 0.01), had the highest test–retest reliability (r = 0.48, P < 0.05), and corresponded well to the assessment periods. Moreover, there is empirical evidence to suggest that 30-day self-reported estimates of treatment adherence may be more accurate than shorter time periods (Lu et al. 2008). Forty-seven percent of the sample (n = 41) reported 100% adherence at either assessment and 33% (n = 29) reported perfect adherence for both. On average, participants reported missing 3 days of medication over the 30 days before each assessment (range = 0–30 missed days). Subsequent analyses utilized Box-Cox transformed variables to improve the normality of the distribution of 30-day adherence. The Box-Cox transformation (Box and Cox 1964) of the 30-day adherence variable was negatively related to the raw variable, such that higher values on the Box-Cox transformed 30-day adherence variable indicate better adherence.
Life stress
Approximately 85% of the participants (n = 73) experienced at least 1 LEDS event (n = 209 LEDS events) over the course of the 3-month study. Each of the life stress variables was normally distributed (see Table 1). Furthermore, the life stress variables were all significantly correlated, yet only modestly related (0.25 < r < 0.45), suggesting that they each represented a unique aspect of life stress (see Table 2 for correlations between main study variables).
Table 2.
Correlation matrix of major variables
| LEDS | CSI | PSS | MADRAS | MDE | ACTIVE | AVOID | T1 ADH | T2 ADH | |
|---|---|---|---|---|---|---|---|---|---|
| LEDS | 0.45** | 0.28** | 0.33** | 0.28** | 0.17 | 0.21* | −0.11 | −0.26* | |
| CSI | 0.25* | 0.43** | 0.27* | 0.18 | 0.24* | −0.15 | −0.14 | ||
| PSS | 0.23* | 0.02 | 0.02 | 0.03 | 0.14 | −0.06 | |||
| MADRAS | 0.60** | 0.01 | 0.54** | −0.36** | −0.22* | ||||
| MDE | −0.15 | 0.33* | −0.31* | −0.19 | |||||
| ACTIVE | 0.04 | −0.05 | −0.22* | ||||||
| AVOID | −0.20 | −0.06 | |||||||
| T1 ADH | 0.48** | ||||||||
| T2 ADH |
P < 0.05;
P < 0.01
LEDS Cumulative threat associated with acute life events, as measured by Life Events and Difficulties Schedule, CSI Chronic stress, as measured by Chronic Strain Interview, PSS perceived stress, as measured by Perceived Stress Scale-4 item measure, MADRAS Clinician-assessed depression, as measured by the Montgomery-Asberg Depression Rating Scale, MDE Presence/absence of current Major Depressive Episode, ACTIVE active coping style, as measured by Brief COPE, AVOIDANT avoidant coping style, as measured by Brief COPE, T1 ADH time 1 treatment adherence, T2 ADH time 2 treatment adherence
Moderators and mediators
There was wide variability in depression (see Table 1), with 24% (n = 21) of the sample meeting criteria for a diagnosis of clinically significant depression (i.e., met criteria for a current MDE) at baseline. Depression diagnosis and T1 depressive severity were significantly correlated (point-biserial r = 0.60, P < 0.05). Avoidant coping at T1 was correlated with both depression severity (r = 0.54, P < 0.01), and MDE (point-biserial r = 0.33, P < 0.05). In contrast, active coping at T1 was not significantly associated with either depression severity (r = −0.01, P = 0.95) or MDE (point-biserial r = −0.15, P = 0.17).
Control variables
Preliminary correlational analyses were conducted to identify potential confounding variables. Gender, age, and experience of side effects were significantly associated with the independent and dependent variables and were subsequently used as control variables. In addition, T1 treatment adherence was used as a control variable in the analyses.
Test of hypotheses
Cumulative threat of acute LEDS events
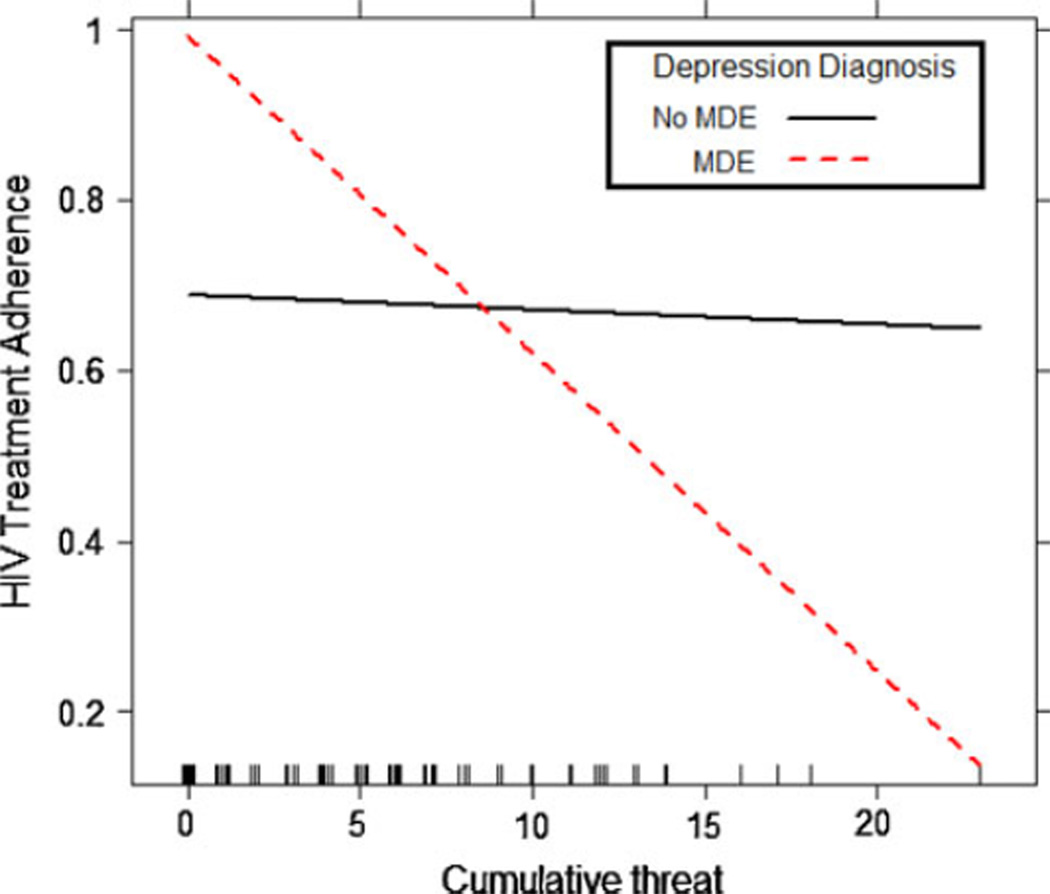
As seen in Table 3, there was no evidence of a main effect of acute life events on HIV treatment adherence at Step 1. Likewise, at Step 2, neither proposed moderator (i.e., depression diagnosis or depressive severity) predicted HIV treatment adherence, controlling for the impact of acute life events. At Step 3, there was a statistically significant interaction between acute life events and depression diagnosis (B = −0.04, P < 0.05), but not for acute life events × depressive severity. Consistent with the graphical display of the interaction in Fig. 1, simple slope analyses indicated that acute life events predicted decreases in adherence for participants with a MDE (B = −0.04, P < 0.05), but did not predict changes in adherence for individuals without diagnosed depression (B = 0.00, P = 0.81).
Table 3.
Hierarchical regression of the impact of stress on HIV treatment adherence
| Type of stress |
LEDS |
||||||
|---|---|---|---|---|---|---|---|
| Moderators |
DEP SEVERITY |
MDE |
|||||
| B | SE | ΔR2 | B | SE | ΔR2 | ||
| Step 1 | T1 ADH | 0.40** | 0.10 | 0.33# | 0.40 | 0.10 | 0.33# |
| GEND | −0.13* | 0.06 | −0.13* | 0.06 | |||
| AGE | 0.01 | 0.00 | 0.01 | 0.00 | |||
| SIDE | −0.04 | 0.04 | −0.04 | −0.04 | |||
| STRESS | −0.01 | 0.01 | −0.01 | 0.01 | |||
| Step 2 | MOD | 0.01 | 0.00 | 0.003 | 0.05 | 0.08 | 0.003 |
| Step 3 | MOD × STRESS | 0.00 | 0.00 | 0.02 | −0.04* | 0.02 | 0.04* |
| Type of stress |
CSI |
||||||
| Moderators |
DEP SEVERITY |
MDE |
|||||
| B | SE | ΔR2 | B | SE | ΔR2 | ||
| Step 1 | T1 ADH | 0.40** | 0.10 | 0.32# | 0.40** | 0.10 | 0.32# |
| GEND | −0.16* | 0.06 | −0.16* | 0.06 | |||
| AGE | 0.01 | 0.00 | 0.01 | 0.00 | |||
| SIDE | −0.04 | 0.04 | −0.04 | 0.04 | |||
| STRESS | 0.00 | 0.01 | 0.00 | 0.01 | |||
| Step 2 | MOD | 0.00 | 0.00 | 0.003 | 0.03 | 0.08 | 0.001 |
| Step 3 | MOD × STRESS | 0.00 | 0.01 | 0.008 | −0.07 | 0.03 | 0.04* |
| Type of stress |
PSS |
||||||
| Moderators |
DEP SEVERITY |
MDE |
|||||
| B | SE | ΔR2 | B | SE | ΔR2 | ||
| Step 1 | T1 ADH | 0.41** | 0.10 | 0.34# | 0.41** | 0.10 | 0.34# |
| GEND | −0.13* | 0.06 | −0.13* | 0.06 | |||
| AGE | 0.01 | 0.00 | 0.01 | 0.00 | |||
| SIDE | −0.05 | 0.04 | −0.05 | 0.04 | |||
| STRESS | −0.01 | 0.01 | −0.01 | 0.01 | |||
| Step 2 | MOD | 0.00 | 0.00 | 0.004 | 0.04 | 0.08 | 0.002 |
| Step 3 | MOD × STRESS | 0.00 | 0.00 | 0.0001 | −0.02 | 0.03 | 0.00 |
Due to space constraints, we have not shown the variables from the previous step in each subsequent step though these were included in the analyses; T1 ADH time 1 treatment adherence, GEND Gender of participant, AGE age of participant, SIDE side effects, STRESS relevant form of stress as indicated in column headings, LEDS LEDS threat, CSI chronic stress, PSS perceived stress, DEP SEVERITY clinician-assessed depressive severity, MDE presence/absence of current Major Depressive Episode, MOD relevant moderator; MOD × STRESS interaction between moderator and life stress;
P <0.05;
P <0.01
Fig. 1.
Interactive effect of MDE status and acute life events on HIV treatment adherence
Given this finding, we began mediation analyses (Barron and Kenny 1986) to examine coping as the mechanism by which acute life events influence adherence among individuals in an MDE. Inconsistent with mediation, the acute life events × depression diagnosis did not predict either active (B = 0.41, P = 0.49) or avoidant coping (B = 0.22, P = 0.55). Further, the acute life events × depression diagnosis interaction remained statistically significant even after statistically controlling for T1 avoidant coping (B = −0.04, P < 0.05) and the T1 avoidant coping × acute life events interaction (B = −0.04, P < 0.05). We observed similar results with the active coping at the trend level, controlling for T1 active coping (statistical trend; B = −0.03, P < 0.06), as well as the T1 active coping × acute life events interaction B = −0.03, P < 0.06).
Chronic stress
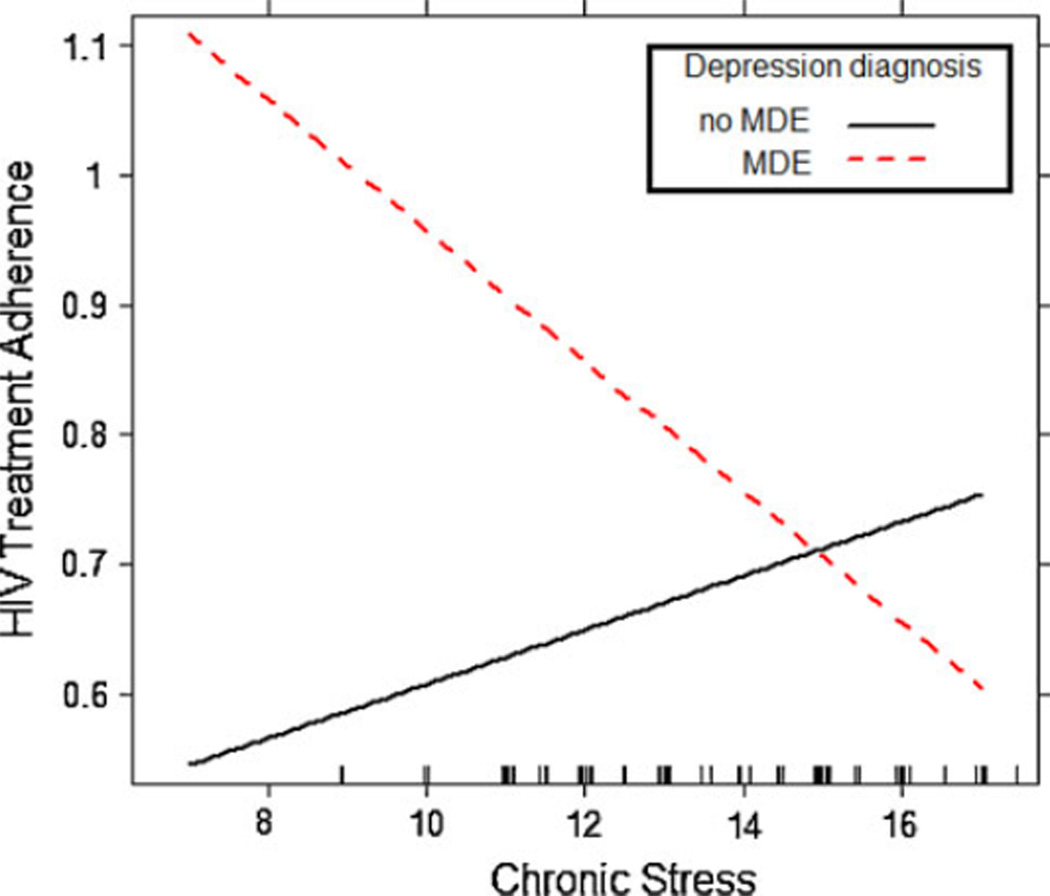
As seen in Table 3, there was no evidence of a main effect of chronic stress on HIV treatment adherence. At Step 2, neither moderator was statistically significant, controlling for the influence of T2 chronic stress and the control variables. At Step 3, there was a statistically significant depression diagnosis × chronic stress interaction (B = −0.07, P < 0.05), controlling for the influence of chronic stress, depression diagnostic status, and the control variables. Consistent with the graphical display in Fig. 2, simple slope analyses indicated that chronic stress was marginally associated with decreases in treatment adherence for participants with a MDE (B = −0.05, P < 0.07), but did not predict changes in adherence for non-depressed individuals (B = 0.02, P = 0.19). Finally, mediation analyses revealed that the chronic stress × depression diagnosis did not predict either active (B = −0.02, P = 0.99) or avoidant coping (B = 1.03, P = 0.12). Further, the chronic stress × depression diagnosis interaction remained statistically significant even after statistically controlling for T1 active (B = −0.06, P < 0.05) and T1 avoidant coping (B = −0.07, P < 0.05), as well as the active coping × chronic stress interaction (B = −0.07, P < 0.05) and the avoidant coping × chronic stress interaction (B = −0.09, P < 0.05).
Fig. 2.
Interactive effect of MDE status and chronic stress on HIV treatment adherence
Perceived stress
As seen in Table 3, the main effect of perceived stress was not statistically significant. Moreover, neither of the proposed moderators interacted with perceived stress to predict changes over time in HIV treatment adherence.
Discussion
The present study examined whether life stress (i.e., acute life events, chronic stress, and perceived stress) would prospectively predict changes in treatment adherence over a 3-month period, and whether depression (both symptom severity and diagnostic status) would influence the strength and form of the relationship between life stress and HIV treatment adherence. Our results illustrated that, among clinically depressed individuals, higher levels of threat associated with acute life events prospectively predicted decreases in treatment adherence. Likewise, we observed a marginal trend suggesting a similar process with chronic stress predicting decreases in treatment adherence among clinically depressed adults, but not being predictive of changes in adherence among non-depressed patients. In contrast, perceived stress did not prospectively predict treatment adherence, regardless of depression diagnostic status. In an effort to explain the interactive effect of depression and life stress on treatment adherence, we examined whether coping style would be the mechanism by which stress and depression influence treatment adherence. However, results demonstrated that neither active nor avoidant coping style explained this relationship. Finally, contrary to our hypotheses, the effect of life stress on treatment adherence was not moderated by depressive severity.
Findings from the present study, as well as our previous research (Bottonari et al. 2005), have important implications concerning anticipating both who is more likely to experience adherence lapses and when those lapses will occur. These studies suggest that depression-prone HIV-infected patients are at greater risk for problematic adherence, but that particular lapses in adherence will likely occur in the context of life stress. In other words, while practitioners need to be attentive to adherence issues among their depressed patients, they should be most focused on difficulties that may arise when these patients are confronted with life stress. While these studies are informative about the “who” and “when” of problematic adherence, they are unfortunately silent on the issue of how these lapses take place. Psychological interventions to increase adherence have been designed to reduce depression and to increase general stress management skills (e.g., Safren et al. 2009). Future interventions may also want to incorporate a more detailed and individualized functional analysis of these lapses and design interventions based on the specific nature of the lapses.
Overall, our sample experienced a wide range of stress over the course of the study, including divorce, eviction, loss of employment, arguments with family members, and health problems among family members. These findings mirror previous results (Gore-Felton and Koopman 2002; Koopman et al. 2000; Roberts et al. 2001), suggesting that many HIV-infected individuals experience substantial stress and chaos in their lives. However, life stress did not uniformly predict changes in treatment adherence across participants over time; effects were largely limited to patients suffering from episodes of major depression. Our work suggests that studies that involve samples with a relatively high proportion of depressed patients will be more likely to detect main effects of stress on adherence compared to those with relatively low proportions of depressed patients. In contrast to episodes of major depression, severity of depressive symptoms did not moderate the relationship between life stress and HIV treatment adherence in the present study. These divergent results are consistent with the viewpoint that there is something distinct about a diagnosis of clinical depression relative to elevated depressive symptomatology (Coyne 1994; Ruscio et al. 2007, 2009).
The present investigation has a number of strengths as well as noteworthy limitations. First, a major strength of the present investigation is the use of multiple measures of life stress and depressive symptomatology, allowing us to examine the unique impact of each type of stress and to parse apart the influence of various aspects of depression on our outcomes. First, use of the 3 life stress measures allowed us to examine the differential impact of acute life events, chronic stress, and perception of stress on treatment adherence, which was important as each type of life stress was only modestly associated with one another. Further, examination of the presence of diagnosable clinical depression and clinician-assessed severity of depressive symptoms allowed us to parse apart the influence of various aspects of depression on our outcomes. Second, the present study built on previous investigations of the stress and coping model among HIV + populations (e.g., Weaver et al. 2005) by measuring both life stress and coping style. Limitations of the present investigation include reliance on self-reported treatment adherence when electronic medication monitoring systems and unannounced pill counts may provide more accurate estimates of treatment adherence and our modest sample size which may not fully account for the variability in experience of life stress, depression, coping style, and treatment adherence among the general HIV-infected population.
In conclusion, among our clinically depressed HIV-infected participants, higher levels of acute life events prospectively predicted decreases in adherence over time to their medication regimens. This finding, combined with our previous work (Bottonari et al. 2005), suggests that life stress has a relatively stronger effect on treatment adherence when HIV-infected individuals are depressed compared to when they are psychologically well. Nonetheless, it will be important for future studies to replicate these findings with more objective measures of adherence, such as bottle cap openings and unexpected pill counts and with larger sample sizes. Moreover, although depression is common among HIV-infected individuals, it is absent from many models of health behavior related to HIV-infection and prevention (Mimiaga et al. 2008). Our results highlight the need for future models of various health behaviors and interventions for HIV-infected individuals to account for depression (see Crepaz et al. 2008 for recent meta-analysis of stress management interventions for HIV-infected individuals). Providers must strive to improve identification of clinically significant depression and high levels of stress among their patients in order to further improve treatment adherence intervention efforts.
Acknowledgments
This research was conducted at the University at Buffalo: State University of New York as part of the first author’s dissertation. This research was supported in part by a National Research Service Award from the Office of AIDS, National Institute of Mental Health (F31MH072551) to Kathryn A. Bottonari and John E. Roberts. We thank Christine A. Calmes, James J. Prisciandaro, Erica L. Carlos, and Paula K. Yanes, who participated in rating of the life events and reliability analyses.
Contributor Information
Kathryn A. Bottonari, Charlie Norwood VA Medical Center, 1 Freedom Way (261), Augusta, GA 30904, USA, Kathryn.Bottonari@va.gov
Steven A. Safren, Harvard Medical School/Massachusetts General Hospital, Boston, MA, USA
John R. McQuaid, San Francisco VA Medical Center, University of California San Francisco, San Francisco, CA, USA
Chiu-Bin Hsiao, Department of Medicine, University at Buffalo: State University of New York, Buffalo, NY, USA.
John E. Roberts, Department of Psychology, University at Buffalo: State, University of New York, Buffalo, NY, USA
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45:394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- Barron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bilfulco A, Brown G, Edwards A, Harris T, Neilson E, Richards C, et al. Life events and difficulties schedule (LEDS-2): Volume 1. Life events manual. London: Royal Halloway and Bedford College, University of London; 1989. [Google Scholar]
- Bottonari KA, Roberts JE, Ciesla JA, Hewitt RG. Life stress and adherence to antiretroviral therapy among HIV + individuals: A preliminary investigation. AIDS Patient Care & STDs. 2005;19:17–25. doi: 10.1089/apc.2005.19.719. [DOI] [PubMed] [Google Scholar]
- Bottonari KA, Roberts JE, Kelly MAR, Kashdan TB, Ciesla JA. A prospective investigation of the impact of attachment style on stress generation among clinically depressed individuals. Behavior Research &Therapy. 2007;45:179–188. doi: 10.1016/j.brat.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society: Series B. 1964;26:211–252. [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. New York: Free Press; 1978. [Google Scholar]
- Carver CS. You want to measure coping but your protocol’s too long: Consider the Brief COPE. International Journal of Behavioral Medicine. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- Catz SL, Heckman TG, Kochman A, DiMarco M. Rates and correlates of HIV treatment adherence among late middle-aged and older adults living with HIV disease. Psychology, Health, & Medicine. 2001;6:47–58. [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19:124–133. [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford NL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG Adherence Instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson GR. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Coyne J. Self-reported distress: Analog or ersatz depression? Psychological Bulletin. 1994;116:29–45. doi: 10.1037/0033-2909.116.1.29. [DOI] [PubMed] [Google Scholar]
- Crepaz N, Passin WF, Herbst JH, Rama SM, Malow RM, Purcell DW, et al. Meta-analysis of cognitive-behavioral interventions on HIV-positive persons’ mental health and immune functioning. Health Psychology. 2008;27:4–14. doi: 10.1037/0278-6133.27.1.4. [DOI] [PubMed] [Google Scholar]
- Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, et al. Rapid estimate of adult literacy in medicine: A shorthand screening instrument. Family Medicine. 1993;25:391–395. [PubMed] [Google Scholar]
- Deschamps NE, Graeve VD, van Wijngaerden E, De Saar V, Vandamme NM, van Vaerenbergh K, et al. Prevalence and correlates of nonadherence to antiretroviral therapy in a population of HIV patients using medication event monitoring system. AIDS Patient Care & STDS. 2004;18:644–657. doi: 10.1089/apc.2004.18.644. [DOI] [PubMed] [Google Scholar]
- Fechner-Bates S, Coyne JC, Schwenk TL. The relationship of self-reported distress to psychopathology. Journal of Consulting and Clinical Psychology. 1994;62:550–559. doi: 10.1037//0022-006x.62.3.550. [DOI] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Coping: pitfalls and promise. Annual Review of Psychology. 2004;55:745–774. doi: 10.1146/annurev.psych.55.090902.141456. [DOI] [PubMed] [Google Scholar]
- French T, Weiss L, Waters M, Tersoriero J, Finkelstein R, Agins B. Correlation of a brief perceived stress measure with nonadherence to antiretroviral therapy over time. Journal of Acquired Immune Deficiency Syndromes. 2005;38:590–597. doi: 10.1097/01.qai.0000135960.88543.8d. [DOI] [PubMed] [Google Scholar]
- Gifford NL, Groessl EJ. Chronic disease self-management and adherence to HIV medications. Journal of Acquired Immune Deficiency Syndromes. 2002;31:S163–S166. doi: 10.1097/00126334-200212153-00016. [DOI] [PubMed] [Google Scholar]
- Gonzalez JS, Penedo FJ, Antoni MH, Dura´n RE, McPherson-Baker S, Ironson G, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology. 2004;23:413–418. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- Gore-Felton C, Koopman C. Traumatic experiences: Harbinger of risk behavior among HIV-positive adults. Journal of Trauma & Dissociation. 2002;3:121–135. [Google Scholar]
- Hammen CL, Adrian C, Gordon D, Jaenicke C, Hiroto D. Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- Jia H, Uphold C, Wu S, Reid K, Findley K, Duncan P. Health-related quality of life among men with HIV infection: Effects of social support, coping, and depression. AIDS Patient Care & STDs. 2004;18:594–603. doi: 10.1089/apc.2004.18.594. [DOI] [PubMed] [Google Scholar]
- Komiti A, Judd F, Grech P, Mijch A, Hoy J, Williams B, et al. Depression in people living with HIV/AIDS attending primary care and outpatient clinics. Australian and New Zealand Journal of Psychiatry. 2003;37:70–77. doi: 10.1046/j.1440-1614.2003.01118.x. [DOI] [PubMed] [Google Scholar]
- Koopman C, Gore-Felton C, Marouf F, Butler LD, Field N, Gill M, et al. Relationships of perceived stress to coping, attachment and social support among HIV-positive persons. AIDS Care. 2000;12:663–672. doi: 10.1080/095401200750003833. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer Publishing; 1984. [Google Scholar]
- Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonora I, Sheehan K, et al. The MINI International Neuropsychiatric Interview (M.I.N.I.) a short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–231. [Google Scholar]
- Leserman J, Ironson G, O’Cleirigh C, Fordiani JM, Balbin E. Stressful life events and adherence in HIV. AIDS Patient Care & STDS. 2008;22:403–411. doi: 10.1089/apc.2007.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21:1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- Liu C, Weber K, Robison E, Hu Z, Jacobson LP, Gange SJ. Assessing the effect of HAART on change in quality of life among HIV-infected women. AIDS Research and Human Retroviruses. 2006;3:6–16. doi: 10.1186/1742-6405-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS and Behavior. 2008;12:86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- Lucas GM. Antiretroviral adherence, drug resistance, viral fitness, and HIV disease progression: A triangle web is woven. Journal of Antimicrobial Chemotherapy. 2005;55:413–416. doi: 10.1093/jac/dki042. [DOI] [PubMed] [Google Scholar]
- McQuaid J, Monroe SM, Roberts JE, Kupfer DJ, Frank E. A comparison of two life stress assessment approaches: Prospective prediction of treatment outcome in recurrent depression. Journal of Abnormal Psychology. 2000;109:787–791. doi: 10.1037//0021-843x.109.4.787. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Kang E, Leu C, Havens JF, Chesney MA. Longitudinal study of mental health and psychosocial predictors of medical treatment adherence in mothers living with HIV Disease. AIDS Patient Care & STDs. 2003;17:407–416. doi: 10.1089/108729103322277420. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Raisner S, Reilly LC, Soroudi N, Safren SA. Individual interventions. In: Mayer KH, Pizer H, editors. HIV-prevention. NY: Academic Press; 2008. pp. 203–239. [Google Scholar]
- Monroe SM, Roberts JE. Conceptualizing and measuring life stress: Problems, principles, procedures, progress. Stress Medicine. 1990;6:209–216. [Google Scholar]
- Montgomery SA, Asberg M. A new depression rating scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Greenwell L, Hoffman D. Factors associated with antiretroviral adherence among HIV-infected women with children. Women and Health. 2002;36:97–111. doi: 10.1300/J013v36n01_07. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Rabkin JG. HIV and depression: 2008 review and update. Current HIV/AIDS Reports. 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Ciesla JA, Direnfeld DM, Hewitt RG. Emotional distress among HIV-positive individuals: The roles of acute negative life events and psychological diatheses. Personality and Individual Differences. 2001;30:241–257. [Google Scholar]
- Ruscio J, Brown TA, Ruscio NM. A taxometric investigation of DSM-IV major depression in a large outpatient sample: Interpretable structural results depend on the mode of assessment. Assessment. 2009;16:127–144. doi: 10.1177/1073191108330065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio J, Zimmerman M, McGlinchey JB, Chelminski I, Young D. Diagnosing major depressive disorder: XI. A taxometric investigation of the categorical/dimensional debate on the structure underlying DSM-IV symptoms. Journal of Nervous & Mental Disorders. 2007;195:10–19. doi: 10.1097/01.nmd.0000252025.12014.c4. [DOI] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychology. 2009;28:1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Otto MW, Worth JL, Salomon E, Johnson W, Mayer K, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-steps and medication monitoring. Behavior Research & Therapy. 2001;39:1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Safren SA, Radomsky NS, Otto MW, Salomon E. Predictors of psychological well-being in a diverse sample of HIV-positive patients receiving highly active antiretroviral therapy. Psychosomatics. 2002;43:478–485. doi: 10.1176/appi.psy.43.6.478. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Bonora LI, et al. Reliability and validity of the MINI International Neuropsychiatric Interview (M.I.N.I.): According to the SCID-P. European Psychiatry. 1997;12:232–241. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Simoni J, Kurth A, Pearson C, Pantalone D, Merrill J, Frick P. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and Behavior. 2006a;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. Journal of Acquired Immune Deficiency Syndromes. 2006b;43:S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KE, Llabre MM, Duran RE, Antoni MH, Ironson G, Penedo FJ, et al. A stress and coping model of medication adherence and viral load in HIV-positive men and women on highly active antiretroviral therapy (HAART) Health Psychology. 2005;24:385–392. doi: 10.1037/0278-6133.24.4.385. [DOI] [PubMed] [Google Scholar]