Abstract
In recent years the etiopathology of a number of debilitating diseases such as type 2 diabetes, arthritis, atherosclerosis, psoriasis, asthma, cystic fibrosis, sepsis, and ulcerative colitis has increasingly been linked to runaway cytokine-mediated inflammation. Cytokine-based therapeutic agents play a major role in the treatment of these diseases. However, the temporospatial changes in various cytokines are still poorly understood and attempts to date have focused on the inhibition of specific cytokines such as TNF-α. As an alternative approach, a number of preclinical studies have confirmed the therapeutic potential of targeting alpha7 nicotinic acetylcholine receptor-mediated anti-inflammatory effects through modulation of proinflammatory cytokines. This “cholinergic anti-inflammatory pathway” modulates the immune system through cholinergic mechanisms that act on alpha7 receptors expressed on macrophages and immune cells. If the preclinical findings translate into human efficacy this approach could potentially provide new therapies for treating a broad array of intractable diseases and conditions with inflammatory components.
Keywords: Nicotinic, Acetylcholine, Alpha7, Inflammation, Diabetes
Role of cytokines in chronic inflammation and disease
Cytokines and inflammation
In recent years an explosion of research into the underlying causes of inflammation and its effects on human health has begun to yield a deeper understanding of how inflammation works and how it is connected to many of the chronic debilitating diseases of our time. Researchers are discovering that inflammation may be at the root of diseases as diverse as heart disease, diabetes, cancer, asthma, inflammatory bowel disease, and Alzheimer’s disease. Any organ or tissue of the body can become inflamed, involving a cascade of events starting with vasodilation and increased vascular permeability induced in part by the release of histamine from ruptured or activated mast cells, circulating basophils and platelets, and by circulating vascular cell adhesion molecule-1 (VCAM-1), endothelial-leukocyte adhesion molecule-1 (E-selectin), and intercellular adhesion molecule-1 (ICAM-1) [1]. Increased vascular permeability leads to local edema, during which phagocytes and humoral factors are released into the intravascular space, including proteins of the complement system; eicosanoids, which are products of arachidonic acid metabolism; kinins; cytokines; and platelet-activating factor [2]. Cytokines include tumor necrosis factor, interleukins, lymphokines, monokines, interferons, colony stimulating factors, and transforming growth factors produced by monocytes, T-cells, platelets, and endothelial cells. Monocytes (macrophages and dendritic cells) and neutrophils are key components of the innate immune system that, when activated, potentiate inflammation. Monocytes are recruited to sites of infection in part by neutrophil degranulation products released in response to invading organisms.
The ability of the immune system to recognize and respond to bacteria and other insults is mediated to a large extent by the innate immune system through a family of transmembrane receptors, the Toll-like receptors (TLRs), which signal the production of proinflammatory cytokines when stimulated by their specific ligands [3]. For example, activation of TLR4 on macrophages by lipopolysaccharide (LPS), which is found in the outer membrane of Gram-negative bacteria, triggers the synthesis of inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β and activates the production of the immunoregulatory cytokine IL-12, which is required for the adaptive immune response [4]. Once activated by inflammatory mediators, macrophages are long-lived and produce large quantities of proinflammatory cytokines, including TNF-α, IL-1, IL-6, IL-12/IL-23, IL-18, and the high mobility group box chromosomal protein 1 (HMGB1) [5]. These cytokines amplify the inflammatory response and trigger adaptive immune responses. Circulating cytokines interact with specific receptors on various cell types and activate Janus kinase/signal transduction and transcription protein (JAK-STAT), nuclear factor kappaB (NF-κB), and transforming growth factor beta (TGF-β) signaling pathways leading to an inflammatory response involving cell adhesion, permeability, and apoptosis, and an increased production of reactive oxygen species [6]. While inflammatory responses have certain beneficial effects, persistent inflammation has a degrading effect that can lead to debility and death. In this regard, an imbalance between the pro- and anti-inflammatory cytokines can play a prominent role in a number of debilitating diseases, several of which are summarized briefly below.
Obesity and diabetes
Obesity and type 2 diabetes are associated with a state of abnormal inflammatory response [7]. It appears that obesity is associated with a low-grade inflammation of adipose tissue resulting from chronic activation of the innate immune system which can subsequently lead to insulin resistance, impaired glucose tolerance, and even diabetes [8]. In obesity, there is an increased production and secretion of a wide range of inflammatory molecules, including TNF-α and IL-6, which have local effects on adipose physiology and systemic effects on other organs such as liver and muscle. When TNF-α activity is blocked in obesity, either biochemically or genetically, the result is improved insulin sensitivity [9]. Infiltration of adipose tissue by macrophages may be the major source of locally produced proinflammatory cytokines. In obesity, the proinflammatory effects of cytokines primarily involve the NF-κB and c-Jun N-terminal kinase (JNK) intracellular signaling pathways [10]. Several other factors derived from adipocytes and macrophages including leptin and resistin are also overproduced during obesity and probably contribute to the pathogenesis of insulin resistance. Adipocyte-derived resistin is a circulating protein implicated in insulin resistance in rodents and is produced largely by macrophages [11]. Inflammation is a hyper-resistinemic state in humans, and cytokine induction of resistin may contribute to insulin resistance in obesity [12]. Circulating adiponectin levels are also decreased in subjects with obesity-related insulin resistance and type 2 diabetes. Adiponectin has been shown to counteract the proinflammatory effects of TNF-α, inhibit liver neoglucogenesis, and promote fatty acid oxidation in skeletal muscle [13]. Elevated C-reactive protein (CRP) levels have consistently been demonstrated in overweight and obese adults, both young and older, and studies have shown that elevated levels of CRP and other inflammatory markers such as ferritin, uric acid, white cell counts, and fibrinogen are positively and independently associated with insulin resistance [14].
Alzheimer’s disease
Oxidative damage and inflammation are important features of the brain pathology of Alzheimer’s disease (AD). Oxidative damage can be found in membranes (lipid peroxidation), proteins (nitrosylation and other post-translational changes), and nucleic acids [15]. In AD there is increased deposition of amyloid peptide A-beta as well as an increased presence of monocytes and macrophages in the vessel walls and activated microglial cells in the brain that are found in close association with A-beta deposits [16]. Histological examination of AD brains as well as cell culture studies have shown that the interaction of microglia with fibrillar A-beta leads to their phenotypic activation. The conversion of these cells into a classically “activated” phenotype results in production of chemokines, neurotoxic cytokines, and reactive oxygen and nitrogen species that are deleterious to the central nervous system (CNS) [16]. Inflammatory changes from activation of microglia and astrocytes manifest as increased levels of proinflammatory cytokines in AD patients [17]. Middle-aged offspring in families with a parental history of late-onset AD also show increased production of proinflammatory cytokines in lipopolysaccharide-stimulated whole blood samples [15]. A direct correlation has been established between the A-beta-induced neurodegeneration and cytokine production and its subsequent release. In effect, neuroinflammation is responsible for an abnormal secretion of proinflammatory cytokines that trigger signaling pathways that activate brain tau hyperphosphorylation in residues that are not modified under normal physiological conditions [18].
Arthritis
Cytokines play a central role in joint inflammation and destruction in rheumatoid arthritis (RA) [19]. In particular, the proinflammatory cytokines IL-1, IL-6, and TNF-α are reported to play important roles in cartilage and bone degradation and in initiating and perpetuating inflammatory and destructive processes in the rheumatoid joint. The rationale for the use of anticytokine therapy in inflammatory joint diseases is based largely on evidence from studies in vitro and in vivo that implicate these major cytokines in RA pathogenesis [20]. These cytokines regulate many NF-κB inducible genes that control expression of other cytokines, cell adhesion molecules, immunoregulatory molecules, and proinflammatory mediators. The importance and central role of cytokines in the disease process is attested to by the significant improvements achieved therapeutically with antibodies directed against TNF-α [21]. TNF-α occupies a primary position in the cytokine cascade through its ability to upregulate production of other cytokines, including IL-1, GM-CSF (granulocyte–macrophage colony stimulating factor), IL-6, IL-8, and IL-10 [5]. The pathogenesis of RA is complex and includes many cell types, including T cells, B cells, mast cells, and synoviocytes. Among the T lymphocytes, Th17 cells have been strongly implicated in RA. IL-17A contributes to the pathogenesis of arthritis as has been shown in several experimental arthritis models [22]. The role of B cells in RA is also significant because not only can they produce potentially pathologic autoantibodies and proinflammatory cytokines, but they also can present antigens to T cells and provide co-stimulatory signals essential for T-cell activation, clonal expansion, and effector function [23]. Increased numbers of mast cells are found in the synovial tissues and fluids of patients with rheumatoid arthritis and at sites of cartilage erosion [24]. Because the mast cell contains potent mediators, including histamine, heparin, proteinases, leukotrienes, and multifunctional cytokines, its potential contributions to the processes of inflammation and matrix degradation have become evident. Mast cells also contribute to initiation of autoantibody-mediated arthritis via IL-1 [25]. Fibroblast-like synoviocytes in the synovial intimal lining also play a key role by producing cytokines that perpetuate inflammation and proteases that contribute to cartilage destruction [26]. More recently, antibodies specific for citrullinated protein epitopes have been discovered, offering a hallmark for the diagnosis and prognosis of RA and a useful tool for understanding the fundamental pathologic processes. It has been proposed that inflammation in RA may be directed to cartilaginous joints and be self-perpetuating through recognition of post-translational modifications (glycosylation and citrullination) on CII by T and B cells that can have arthritogenic consequences [27].
Asthma
A primary risk factor for atopic asthma is sensitization to perennial aeroallergens resulting from a failure to generate protective immunologic tolerance. Dendritic cells are professional antigen presenting cells that mediate the response to inhaled allergens. In animal models, the induction and maintenance of allergic airway inflammation is primarily a function of myeloid dendritic cells, whereas tolerance to inhaled allergens is likely a function of plasmacytoid dendritic cells [28]. This tolerance process is orchestrated by airway mucosal dendritic cells and normally results in programming of regulatory T cells, which inhibit activation of the Th2 memory cells that, among other activities, drive IgE production and prime the effector populations responsible for IgE-mediated tissue damage [29]. In addition, inflammatory cells such as activated eosinophils and neutrophils are associated with increased levels of numerous cytokines and other proinflammatory mediators [30]. Viruses, but also endotoxin or allergen exposure, are able to recruit neutrophils via IL-8 production by activated macrophages or epithelial cells [31]. Together, these inflammatory mediators contribute to diffuse bronchial inflammation. Bronchial provocation with allergens induces a subset of activated T-lymphocytes resulting in production of interleukins IL-4, IL-5, and IL-13. IL-4 in conjunction with IL-13 signals the switch from IgM to IgE antibodies [32]. The cross-linkage of two IgE molecules by allergen causes mast cells to degranulate, releasing histamine, leukotrienes, and other mediators that perpetuate the airway inflammation. IL-5 activates the recruitment and activation of eosinophils. Since eosinophil development from hematopoietic progenitor cells is regulated by IL-5, which influences maturation, differentiation, mobilization, activation, and survival, a fully humanized anti-IL-5 monoclonal IgG1 antibody (Mepolizumab) has been shown to reduce airway and blood eosinophils and prevent asthma exacerbations [33].
Atherosclerosis
The process of inflammation is now believed to be the etiological event that precedes the development and the continual process of atherosclerosis and appears to be associated with a significant prothrombotic status and endothelial dysfunction [34]. Most risk markers for cardiovascular disease have a proinflammatory component that stimulates the release of a number of active molecules such as inflammatory mediators, reactive oxygen species (ROS), nitric oxide, and peroxynitrite from endothelial, vascular smooth muscle, and immune cells in response to injury [35]. The mediators of the inflammatory response, such as TNF-α, the interleukins IL-1 and IL-6, GM-CSF, and others, all increase the binding of LDL to the endothelium and smooth muscle to further upregulate the inflammatory response [36]. Cytokine-induced activation of inflammatory pathways in endothelial cells modifies the production/activity of vasodilatory mediators such as nitric oxide, prostacyclin, endothelium-derived hyperpolarizing factor, and bradykinin, as well as vasoconstrictive mediators such as endothelin and angiotensin II [6]. Cytokines also interact with mitochondria to increase the production of ROS [37], and unquenched intracellular ROS induce monocytes to differentiate into macrophages. It is well known that during the development of atherosclerosis, macrophages interact with vascular endothelial cells, medial smooth muscle cells and infiltrated inflammatory cells, particularly T cells and dendritic cells [38]. These cells then accumulate cholesterol esters in the cytoplasm, which leads to foam cell formation in lesion development [39]. Further recruitment of macrophages can occur by the release of cytokines from the endothelium and vascular smooth muscle as part of the inflammatory cycle.
Cancer
Inflammation can promote all stages of tumor development through multiple mechanisms, which include enhanced proliferation and resistance to apoptosis of initiated cells, induction of DNA mutations, promotion of angiogenesis, invasion and metastasis, and components of the tumor microenvironment, including tumor cells themselves, may promote an inflammatory state by producing inflammatory mediators [40]. Inflammatory responses play decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis [41]. Inflammatory components are present in the microenvironment of tumors, including leukocytes, cytokines, and complement components, and are orchestrated by transcription factors, such as NFκB and STAT3 [42]. Studies indicate the upregulation of TNF-α, CRP, and tissue factor in patients with malignancy, further suggesting that the pathogenesis associated with malignancy is associated with an inflammatory component [43]. CD4(+) regulatory (Treg) cells can restore immune homeostasis during chronic inflammation and may function to reduce cancer risk by efficiently downregulating inflammation through IL-10-dependent processes. However, under proinflammatory conditions, Tregs may fail to provide anti-inflammatory protection and instead contribute to a Th-17-driven procarcinogenic process. Consequently, individuals with a weakened IL-10 and Treg-mediated inhibitory loop are highly susceptible to the carcinogenic consequences of elevated IL-6 and IL-17 and show more frequent inflammation-associated cancers [44]. During chronic inflammatory processes, excess free radicals and DNA-reactive aldehydes from lipid peroxidation are also produced, which can deregulate cellular homeostasis and drive normal cells to malignancy [45]. It has been suggested that the conditions provided by a chronic inflammatory environment are so essential for the progression of the neoplastic process that therapeutic intervention aimed at inhibiting inflammation, reducing angiogenesis, and stimulating cell-mediated immune responses may have a major role in reducing the incidence of common cancers.
Cystic fibrosis
Cystic fibrosis (CF) is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), an anion channel whose dysfunction results in altered epithelial salt and water transport leading to impaired mucociliary clearance, chronic inflammation, and tissue damage [46]. In CF, the damage to airway epithelial cells is exacerbated by the activation of Toll-like receptor 2 (TLR2) by airway pathogens [47]. The result is the production of a cascade of proinflammatory cytokines and chemokines, including IL-8, subsequent inflammatory infiltration of epithelial cells by neutrophils in patients with CF, and exacerbation of symptoms.
Inflammatory bowel diseases
Crohn’s disease and ulcerative colitis are chronic inflammatory bowel diseases (IBDs) of unknown origin. The development of inflammatory bowel disease is associated with the interplay of genetic, bacterial, and environmental factors and dysregulation of the intestinal immune system [48]. The chronic immune stimulation of these conditions is thought to be due to an imbalance of proinflammatory and inhibitory cytokines [49]. It is well known that immune responses in the intestine remain in a state of controlled inflammation, suggesting that not only active suppression by regulatory T cells plays an important role in the normal intestinal homeostasis, but also its dysregulation leads to the development of inflammatory bowel diseases [50]. Abnormal signaling pathways, including mitogen-activated protein kinases (MAPK), JNK, phosphatidylinositol 3-kinase/serin-threonine kinase (PI3K/Akt), and NF-κB, play an important role in the inflammatory process, can lead to dysregulation of the inflammatory response, and are crucial in the pathogenesis of IBDs. Disorders of these signaling pathways can affect the intestinal barrier and cause uninhibited release of effector T cells and uncontrolled inflammation [50]. The latest research is focused on the key role of specific cytokines in IBD. In patients with IBD, a number of recruited monocytes and activated macrophages are the source of cytokines in the inflamed alimentary tract mucosa. The role of proinflammatory cytokines and chemokines such as IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, IL-17, IL-23, TNF-α, and interferons (IFNs) in IBD is associated with the initiation and progression of ulcerative colitis and Crohn’s disease [51].
Psoriatic arthritis and psoriasis
Psoriasis is one of the most common human inflammatory skin diseases characterized by hyperproliferation and aberrant differentiation of keratinocytes. One of the triggers of the typical epidermal changes seen in psoriasis is considered to be a dysregulated immune response with Th-1/Tc1 cells playing a central role [52]. As in rheumatoid arthritis, monocytes and macrophages also play a pathogenic role in psoriatic arthritis. Activated inflammatory cells and subsequent release of proinflammatory cytokines such as TNF-α are at the heart of the production of psoriatic lesions [53]. This pathogenic role of TNF-α is supported by the efficacy of approved TNF-α inhibitors, such as infliximab, etanercept, and adalimumab, in psoriatic arthritis and psoriasis [54]. T helper (Th17) cells, a novel T-cell subset, have also been implicated in the pathogenesis of psoriasis and other autoimmune inflammatory diseases [55]. Specifically, IL-23 stimulates survival and proliferation of Th17 cells and thus serves as a key master cytokine regulator for these diseases. In psoriasis, IL-23 is overproduced by dendritic cells and keratinocytes, and this cytokine stimulates Th17 cells within dermis to make IL-17A and IL-22. IL-22, in particular, drives keratinocyte hyperproliferation in psoriasis [55].
Role of alpha7 nicotinic receptors in inflammation
Structure and function of alpha7 nicotinic receptors
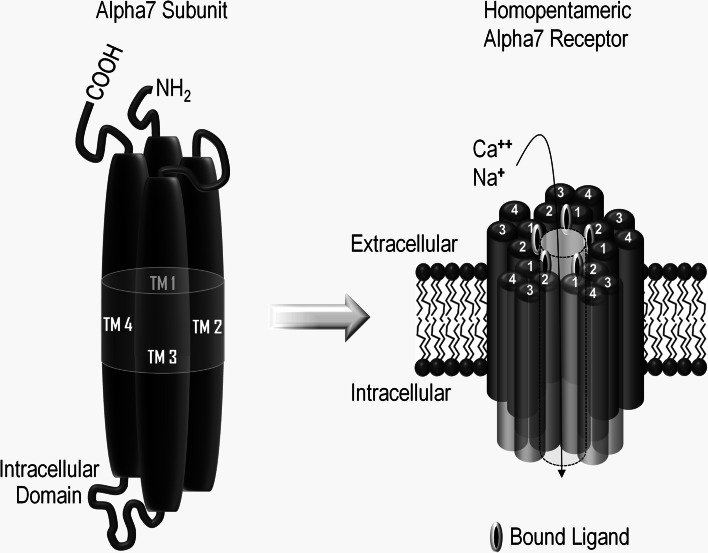
Acetylcholine (ACh) is one of the most widely distributed intercellular signaling molecules in the body. The two classes of receptors that mediate ACh signaling were originally designated nicotinic and muscarinic, a nomenclature based on their differential responsiveness to nicotine and the mushroom toxin muscarine, respectively. Muscarinic receptors are members of the seven transmembrane domain G-protein-coupled receptor type and mediate numerous biologic effects, including heart rate, glandular secretions, and smooth muscle contractility among others [56]. Nicotinic acetylcholine receptors are members of the four transmembrane domain superfamily of neurotransmitter-gated ion channels. These receptors play a central role in modulating synaptic neurotransmission and fundamental intracellular signaling and are in control of neuronal viability and synaptic architecture; recent advances in nicotinic receptor biology have come from an increased understanding of the diverse receptor subtypes and their distinctive combinations of subunits [57]. Neuronal nicotinic acetylcholine receptors are pentameric complexes composed of alpha and beta subunits that assemble like the staves in a barrel to form an ion channel that is gated by the binding of acetylcholine or other nicotinic cholinergic ligands. The diversity of different protein subunits that are available to co-assemble (nine alpha and four beta subunits) provides considerable variation in the possible receptor subtypes, all with different physiological function and ligand binding affinity. The alpha7 subtype is a well-characterized member of the ligand-gated ion channel superfamily [58]. In neurons, alpha7 protein subunits assemble as a pentameric complex composed of five alpha7 subunits. The alpha7 subunit is approximately 56 kDa and is composed of 502 amino acids, including a 22 amino acid N-terminal signal peptide, followed by an extracellular 200 amino acid ligand-binding domain. The assembled subunits form a central ion pore. The binding of ligands at the junctions between subunits initiates conformational changes in the receptor that can lead to channel opening. The transmembrane domains (TM 1–4) of each of the five subunits are composed of four α-helices that span the lipid bilayer and form the ion channel (see Fig. 1). Although nicotinic receptors were originally described as sodium channels, the alpha7 homopentamer is also highly permeable to calcium [59]. The involvement of neuronal alpha7 nicotinic receptors in cognitive processes and their potential as therapeutic targets for schizophrenia, Alzheimer’s, and other cognitive diseases and disorders has been extensively described [60]. More recently an appreciation of the properties and potential therapeutic value of non-neuronal alpha7 nicotinic receptors, present on circulating monocytic and immuno-regulatory cell types, has begun to emerge [61]. The remainder of the present review will focus on the central role played by non-neuronal alpha7 nicotinic receptors in regulating a broad array of inflammatory processes that are relevant to numerous diseases and disorders.
Fig. 1.
Structure of alpha7 nicotinic receptors. Alpha7 nicotinic receptor subunit proteins have an N-terminal signal peptide and ligand-binding domain, four transmembrane domains (TM1–4), and a regulatory intracellular domain located between TM3 and TM4 (left). The alpha7 receptor subunits assemble to form a homopentameric structure with a central ion pore that is permeable to sodium and calcium (right). Binding of ACh or other nicotinic cholinergic ligands to the interfaces between adjacent subunit pairs triggers conformational changes that lead to channel opening and ion flux
The cholinergic anti-inflammatory pathway
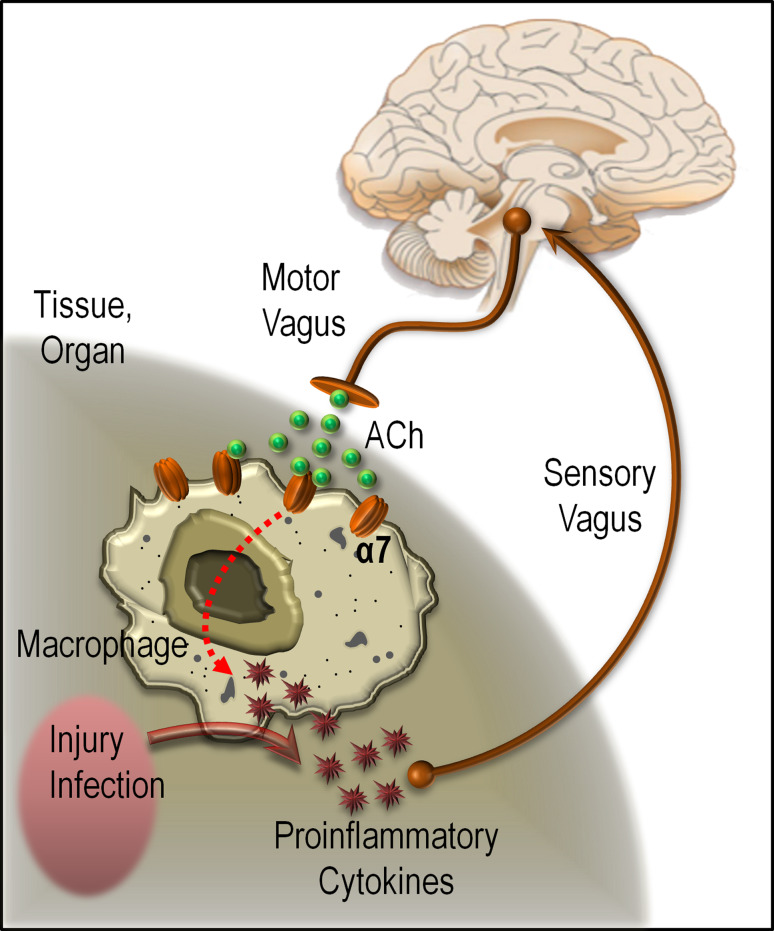
A growing body of evidence has emerged showing that the CNS modulates the immune system through the reticuloendothelial system (RES) [62]. The so-called cholinergic anti-inflammatory pathway and its role in immune responses and inflammatory cascades have attracted substantial interest due to the obvious relevance to a variety of debilitating human diseases [63]. The cholinergic anti-inflammatory pathway inhibits inflammation by suppressing cytokine synthesis via release of acetylcholine in organs of the RES, including the lungs, spleen, liver, kidneys, and gastrointestinal tract. Acetylcholine can interact with alpha7 nicotinic acetylcholine receptors expressed by macrophages and other cytokine-producing cells, downregulate proinflammatory cytokine synthesis, and prevent tissue damage [64]. This modulation is mediated through the vagus nerve, utilizing the major vagal neurotransmitter ACh, which acts on alpha7 nicotinic cholinergic receptors on macrophages to suppress the release of TNF-α and other proinflammatory cytokines [65] (also see Fig. 2). This places the alpha7 nicotinic receptor subtype at the apex of key CNS and peripheral cellular pathways that are involved in anti-inflammatory processes as well as cell survival. Consequently, it opens the door for treating a broad array of intractable diseases and conditions with inflammatory components, such as asthma, rheumatoid and osteoarthritis, inflammatory bowel diseases, obesity, type 2 diabetes, and sepsis.
Fig. 2.
Alpha7 nicotinic receptors and the cholinergic anti-inflammatory pathway. Pathogens and other forms of injury activate macrophages to produce proinflammatory cytokines. Activation of cytokine production, which normally restores health, can cause disease if the response is excessive. If necessary, balance can be restored by efferent signals from the vagus nerve, which can inhibit cytokine production by releasing acetylcholine (ACh), which can bind to alpha7 nicotinic acetylcholine receptors on macrophages and inhibit the production of proinflammatory cytokines
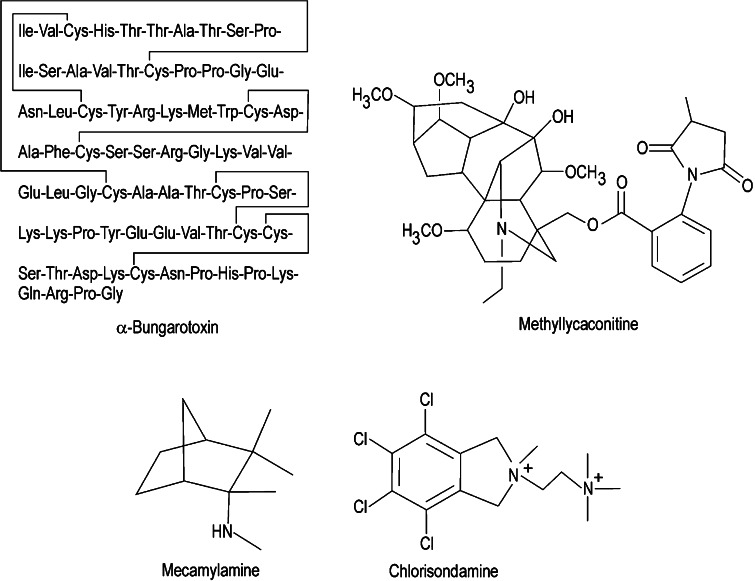
The cholinergic vagal pathway appears to play an important role in modulating inflammatory responses, as evidenced by the observations that vagotomy increases LPS-induced TNF-α serum levels and hepatic TNF-α responses and that electrical stimulation of the vagus nerve or treatment of vagotomized animals with ACh prevents the increase in TNF-α release [66]. The primary cholinergic target for these effects appears to be the alpha7 nicotinic cholinergic receptor. The key role played by alpha7 nicotinic receptors in inflammatory processes is supported by observations that nicotine exerts anti-inflammatory effects on macrophages that can be counteracted by selective alpha7 antagonists such as α-bungarotoxin (see Fig. 3) [61, 66]. In addition, nicotine and alpha7 agonists such as GTS-21 and CAP55 (see Fig. 4) are effective in models of inflammation [67] and have been shown to inhibit local leukocyte recruitment and decrease endothelial cell activation [68].
Fig. 3.
Structures of alpha7 nicotinic receptor antagonists. Compounds considered to be the most selective for the alpha7 nicotinic receptor subtype include α-bungarotoxin and methyllycaconitine (MLA). Compounds considered to be less selective for alpha7 versus other nicotinic receptor subtypes include mecamylamine and chlorisondamine
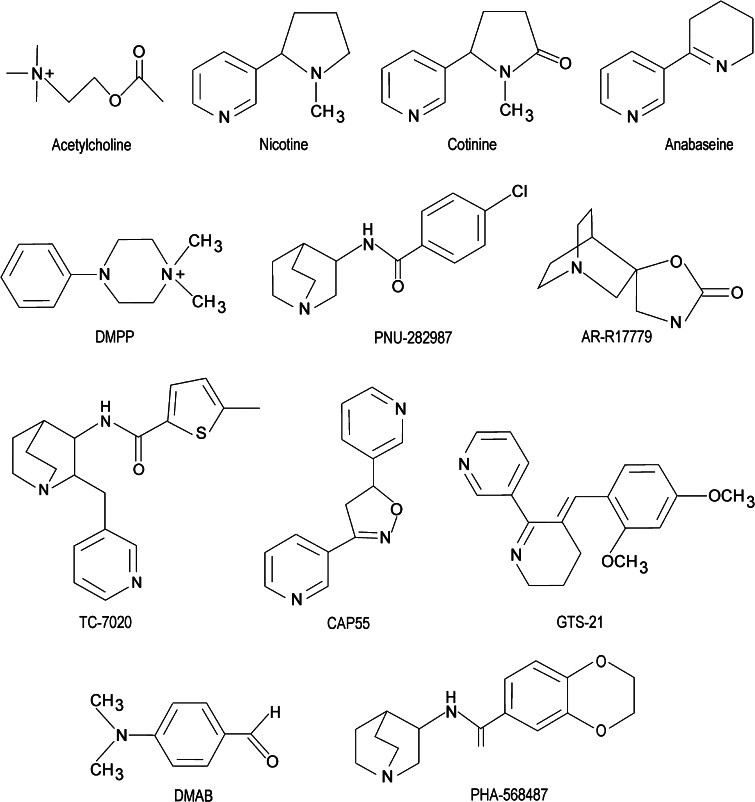
Fig. 4.
Structures of alpha7 nicotinic receptor agonists that demonstrate anti-inflammatory effects. Compounds considered to be the most selective for the alpha7 nicotinic receptor subtype include TC-7020, PNU-282987, AR-R17779, CAP55, GTS-21, DMAB, and PHA-568487. Compounds considered to be less selective for alpha7 versus other nicotinic receptor subtypes include acetylcholine, nicotine, cotinine, anabaseine, and DMPP
Acetylcholine and nicotine were shown to inhibit LPS-induced TNF-α release in murine-derived microglial cells [69] and to prevent the production of ROS in fibrillar beta amyloid peptide (1-42)-stimulated microglia [70], effects that were both attenuated by the alpha7 antagonist α-bungarotoxin. The role of alpha7 nicotinic receptors in cholinergic modulation of TNF-α in LPS-stimulated macrophages has been confirmed using alpha7 knockout models. For example, systemic reduction of TNF-α levels by choline in endotoxin-treated mice was eliminated in alpha7 knockout mice during endotoxemia [71]. Similarly, vagus nerve stimulation does not inhibit TNF-α release in alpha7 knockout mice [72]. Alpha7 agonists, including nicotine and GTS-21, have also proven effective in reducing macrophage cytokine production and inflammation in animal models of pancreatitis [73] and peritonitis [74] and in experimentally induced ileus in mice [75].
Cytokines and alpha7-mediated anti-inflammatory effects
Cells
A growing body of evidence indicates that cells other than neurons throughout the body express nicotinic receptor subtypes. These include lymphocytes, macrophages, dendritic cells, adipocytes, keratinocytes, endothelial cells, and epithelial cells of the intestine and lung [76]. It has been known for nearly 30 years that phagocytic cells express nicotinic receptors [77], but this area has not been significantly explored until the last decade. Polymorphonuclear leukocytes (PMN), which are comprised of neutrophils, eosinophils, and basophils, have been shown to express multiple nicotinic receptor subtypes, including α4β2 and α3β4 [78]. Human lymphocytes contain functional alpha7 nicotinic receptors that modulate lymphocyte activation [79], as do human macrophages [80]. Macrophages mediate physiologic control of cytokine production by auto/paracrine acetylcholine (ACh) through the nicotinic receptors expressed in these cells. Stimulation of macrophages (U937 cell line) with LPS has been shown to upregulate expression of α1, α4, α5, α7, α10, β1, and β3 subunits and downregulate α6 and β2 subunits [81]. However, these changes have not been confirmed for tissue macrophages in vivo.
Distinct nicotinic receptor subtypes show differential regulation of the production of pro- and anti-inflammatory cytokines whereby inhibition of the expression of the TNF-α gene is mediated predominantly by the α-bungarotoxin-sensitive alpha7 receptors and that of the IL-6 and IL-18 genes by other (mecamylamine-sensitive) receptor subtypes [81]. Mecamylamine- and α-bungarotoxin-sensitive receptors were found to regulate expression of the IL-1β gene equally efficiently, but upregulation of IL-10 production by auto/paracrine ACh is mediated predominantly through alpha7 nicotinic receptors. Studies have also shown that the alpha7 subtype is expressed on the surface of CD4(+) T cells and that this expression is upregulated upon immune activation [82]. Nicotine (2 mg kg−1 day−1), administered by mini-pump for 9 days, was shown to reduce T cell proliferation in response to an encephalitogenic antigen as well as the production of Th1 (TNF-α and IFN-γ) and Th17 cytokines (IL-17, IL-17F, IL-21, and IL-22), whereas cells from alpha7 knockout animals were refractory to nicotine. Alpha7-selective compounds have been shown to suppress the expression of a number of different cytokines. For example, GTS-21 attenuates TNF-α and IL-1β levels in human whole blood activated by exposure to endotoxin and inhibits TNF-α production in endotoxin-stimulated primary monocytes from human blood in vitro at the transcriptional level [83]. Similarly, activation of alpha7 receptors on cultured human monocytes by nicotine’s main metabolite cotinine has been shown to abrogate the production of cytokines that are under the transcriptional control of the NF-κB system (TNF-α, IL-1β, IL-6, IL-12/IL-23, p40) and shift the response towards an IL-10-dominated anti-inflammatory profile that is PI3K/GSK (glycogen synthase kinase)-3β-dependent, but NF-κB -independent [84].
Tissues
Adipose
Based on a growing body of evidence it is now believed that low-grade chronic inflammation is associated with the onset of insulin resistance and type 2 diabetes [85]. This chronic inflammation is in turn characterized by an increased number of macrophages in adipose tissue together with the production of inflammatory cytokines, including TNF-α [86]. Increases in TNF-α and concomitant insulin resistance of adipocytes can lead to a cascade of events including mitochondrial damage, increased lipolysis, and redistribution of fatty acids to ectopic triglyceride deposits [87, 88]. Previous studies suggest a direct link between alpha7 nicotinic receptors and regulation of TNF-α in adipocytes, whereby activation of alpha7 receptors reduces TNF-α protein levels [89].
Brain
Regulation of cholinergic tone in the brain can influence CNS inflammation. Acetylcholine receptors are expressed by glial cells. In particular, microglial cells express the alpha7 nicotinic receptor, and its activation attenuates the proinflammatory response of microglial cultures, suggesting that acetylcholine may control brain inflammation, in analogy to what is demonstrated in peripheral tissues [90]. The acetylcholinesterase inhibitor rivastigmine has been shown to reduce neuroinflammation in experimental autoimmune encephalomyelitis (EAE) [91]. In these studies, rivastigmine markedly ameliorated clinical symptoms of EAE and reduced demyelination, microglia activation, axonal damage, the reactivity of encephalitogenic T-cells, and the production of proinflammatory cytokines (TNF-α, IFN-γ, and IL-17) without affecting IL-10 production. These effects were abolished by alpha7 nicotinic acetylcholine receptor antagonists. Dietary supplementation with the low-potency, but selective alpha7 nicotinic receptor agonist choline following traumatic brain injury (TBI) in rats resulted in improved spatial memory in the Morris water maze test, significant cortical tissue sparing, reduced brain inflammation, and normalization of some TBI-induced deficits in receptor expression [92]. Nicotine has been shown to block microglial activation and to reduce TNF-α levels and dopaminergic cell loss following LPS-induced inflammation, both in cell cultures (0.1–1.0 μM) and in rat brain with chronic nicotine administration (5 mg kg−1 day−1 i.p.; 4 weeks) [93]. These effects were blocked by the alpha7-selective antagonist α-bungarotoxin. The mechanism(s) of alpha7-mediated anti-inflammatory effects in the brain are not entirely understood. However, there is evidence that microglial alpha7 receptors drive a signaling process involving the activation of phospholipase C and calcium release from intracellular stores rather than function as conventional ion channels, resulting in a shift of microglial function from proinflammatory to neuroprotective [94].
Joints
Neuroimmune interactions are known to influence several chronic inflammatory and rheumatic diseases, but the underlying mechanisms have been insufficiently elucidated. The expression and localization of alpha7 nicotinic receptors in synovial biopsies from patients with rheumatoid arthritis (RA) and psoriatic arthritis have been investigated [95]. Using alpha7-specific antibodies, alpha7-positive cells were detected mainly in synovial lining cells and vessels and were identified as primarily macrophages and fibroblasts, with the majority of these cells expressing the receptor. Alpha7 was also identified at both the mRNA and protein level in cultured fibroblast-like synoviocytes, and the alpha7 agonists nicotine and AR-R17779 reduced TNF-α, IL-6, and IL-8 production by synoviocytes. In other studies, acetylcholine significantly reduced the production of IL-6, the chemokines IL-8, CCL2, CCL3, CCL5, and GM-CSF by IL-1-stimulated synoviocytes, and these effects were blocked by the alpha7 antagonist MLA (methyllycaconitine) or by using alpha7 siRNA to knock down receptor expression [96]. The selective alpha7 agonist PNU-282987 also decreased the production of IL-6 by IL-1-stimulated synoviocytes.
Lung
Expression of alpha7 nicotinic subunits by epithelial and endothelial cells in the lung has been reported, including primary cultures of human bronchial cultured epithelial cells that have been shown to express alpha7 mRNA and α-bungarotoxin binding that is indicative of receptor expression [97]. Functional nicotinic receptors are also expressed on eosinophils and the nicotinic agonist DMPP (dimethylphenylpiperazinium) has been shown to downregulate eosinophil function in vitro [98]. Based on studies using the alpha7 antagonists MLA and α-bungarotoxin or alpha7 knockout mice, the alpha7 nicotinic receptor appears to be a key regulator of the plasticity of the human airway epithelium by controlling basal cell proliferation and differentiation pathways and is involved in airway remodeling during bronchopulmonary diseases [99]. In studies of endotoxin- and live Escherichia coli-induced acute lung injury (ALI) in mice, alpha7-containing alveolar macrophages and neutrophils were present in bronchoalveolar lavage and injured lungs [100]. Administration of the selective alpha7 agonists dimethylaminobenzaldehyde (DMAB), PNU-282987, and PHA-568487 reduced bronchoalveolar lavage macrophage inflammatory protein (MIP-2) production and transalveolar neutrophil migration and reduced mortality. In addition, alpha7 (−/−) mice developed severe lung injury and had higher mortality compared with alpha7 (+/+) mice. In similar studies, the selective alpha7 receptor agonist GTS-21 was shown to dose-dependently inhibit LPS-induced TNF-α release by MH-S mouse alveolar macrophages in vitro [101]. Intranasal administration of GTS-21 also dose-dependently inhibited TNF-α release into the lung compartment after intrapulmonary delivery of LPS.
Placenta
Previous studies have demonstrated the production of numerous cytokines by the placenta ex vivo following LPS stimulation and anti-inflammatory effects of glucocorticoids on placental cell inflammatory responses from cultured normal and preeclampsia placental explants [102]. Using this model system, it has been shown that cholinergic agonists, including nicotine, exert anti-inflammatory effects on placental inflammatory responses by significantly suppressing placenta cytokine production following stimulation [67]. Treatment of the placental explants with nicotine (100 μM) and the alpha7-selective agonists GTS-21 and CAP55 significantly reduced LPS-induced TNF-α production over a wide range of LPS concentrations and significantly reduced LPS-induced IL-6, IL-1β, and IL-8 production by placental cells. With the use of the nicotinic antagonist mecamylamine, it was further demonstrated in these studies that the anti-inflammatory effects of nicotine and other cholinergic agonists are in part mediated through a nicotinic receptor-mediated pathway. This is consistent with previous studies reporting alpha7 nicotinic receptor expression by the human placenta [103, 104]. It has also been reported that alpha7 protein expression was significantly increased in placentas obtained from severe preeclampsia patients [103]. Thus, the modulation of alpha7 expression within the placenta and the function of this receptor with respect to acetylcholine suggest potentially important placental signaling pathways underlying the effects of nicotine and other cholinergic agonists in placental inflammatory responses.
Skin
The cholinergic system is involved in basic functions of the skin through autocrine, paracrine, and endocrine mechanisms, such as keratinocyte proliferation, differentiation, adhesion and migration; epidermal barrier formation; pigment, sweat, and sebum production; blood circulation; angiogenesis; and a variety of immune reactions [105]. ACh is produced in keratinocytes, endothelial cells, and most notably in immune competent cells invading the skin at sites of inflammation. Alpha7 receptor activity in skin also appears to be under endogenous regulation by secreted lymphocyte antigen-6/urokinase-type plasminogen activator receptor-related protein-1 (SLURP-1), a secreted protein that shows structural similarity to the alpha7 receptor antagonist snake venom toxin α-bungarotoxin [106]. It is localized to human skin, exocervix, gums, stomach, and esophagus and has been implicated in maintaining the physiological and structural integrity of the keratinocyte layers of the skin. The SLURP-1 protein has also been identified in several biological fluids such as sweat, saliva, tears, and urine from normal volunteers [107]. SLURP-1 significantly increases both ACh potency and efficacy via the alpha7 nicotinic receptor, acts as a positive allosteric modulator of alpha7, and is postulated to be involved in regulating TNF-α release from keratinocytes and macrophages via alpha7 nicotinic receptor-mediated pathways [108]. This raises the intriguing possibility that, in addition to acetylcholine, endogenous peptides may actively modulate the alpha7 cholinergic cascade.
Alpha7 nicotinic receptors as therapeutic targets in inflammation
Since the overproduction of TNF-α and other cytokines is associated with the pathophysiology of numerous diseases, controlling cytokine synthesis and release is critical for preventing unrestrained inflammation and maintaining health. In this regard, alpha7 nicotinic receptors appear to play a major role and may thus represent potential therapeutic targets for many diseases that have a strong inflammatory component.
Diabetes
A key factor that underlies the development of diabetes is a characteristic systemic inflammation, marked by increases in the venous blood concentrations of C-reactive protein, IL-6, and TNF-α [109]. TNF-α has been shown not only to evoke the production of other inflammatory cytokines but also to increase the activities of signaling pathways that are believed to lead to insulin resistance [110]. It has been shown that a prototypical selective alpha7 agonist (TC-7020) can reduce the proinflammatory cytokine TNF-α, reduce elevated glucose levels, decrease glycated hemoglobin, reduce triglycerides, and reduce food intake and weight gain in a murine model of type 2 diabetes [111]. These effects were reversed by the alpha7 antagonist MLA. Furthermore, the JAK2 kinase-specific inhibitor AG-490 also inhibited TC-7020-induced weight loss, decreased food intake, and reduced glucose levels. These findings suggest that alpha7-selective drugs could have broad effects on food intake and weight gain as well as on normalization of insulin resistance and decreased inflammatory effects, opening up the possibility to treat not only diabetes but also metabolic syndrome.
Asthma
The nonneuronal cholinergic system is known to be present in airway inflammatory cells, where acetylcholine is predominantly proinflammatory for lymphocytes and epithelial cells, anti-inflammatory for mast cells and macrophages, both pro- and anti-inflammatory for monocytes, and variable in neutrophils and eosinophils [112]. The activity of this system in the lungs may underlie epidemiological evidence suggesting that smokers are less likely to develop some inflammatory and allergic diseases [113]. Alpha7 nicotinic receptors may be implicated since the expression of alpha7 on T lymphocytes from children with asthma is higher than that from normal controls [114]. Alpha7 activity appears to influence the balance of Th1/Th2 cells and may thus play an important role in the development of asthma.
In the rat mast/basophil cell line RBL-2H3, low nanomolar concentrations of nicotine inhibit several parameters of allergic asthma, including the production of Th2 cytokines (TNF-α and IL-1) and the cysteinyl leukotriene LTC4 [115]. Cysteinyl leukotrienes are primarily produced by mast cells, and these cells play a central role in allergic asthma. The mechanism by which nicotine modulates mast cell activation appears to be unrelated to the effects of nicotine on intracellular free calcium concentration but is causally associated with inhibition of cytosolic phospholipase A2 activity and the PI3K/ERK/NF-κB pathway, including phosphorylation of Akt and ERK and nuclear translocation of NF-κB [115]. In these studies the suppressive effect of nicotine on the late-phase response was blocked by the alpha7 antagonists MLA and α-bungarotoxin, implicating the direct involvement of alpha7 receptors.
In studies with epithelial cells from cystic fibrosis lung that show increased production of proinflammatory mediators such as the neutrophil chemokine IL-8, nicotine (10 μM) inhibited Toll-like receptor (TLR2 and TLR4)-induced IL-8 production [116]. Based on studies using alpha7 antagonists or alpha7 knockout mice, the alpha7 nicotinic receptor appears to be a key regulator of the plasticity of the human airway epithelium by controlling basal cell proliferation and differentiation pathways and is involved in airway remodeling during bronchopulmonary diseases [99]. Alpha7 knockout mice develop severe lung injury and have higher mortality, and administration of alpha7 agonists such as DMAB, PNU-282987, and PHA-568487 reduces macrophage inflammatory protein production and transalveolar neutrophil migration and reduces mortality [100]. The selective alpha7 receptor agonist GTS-21 was shown to dose-dependently inhibit LPS-induced TNF-α release by mouse alveolar macrophages in vitro and to inhibit TNF-α release into the lung compartment after intrapulmonary delivery of LPS in mice [101]. Mouse tracheal smooth muscle and epithelium are positive for alpha7 subunits and the nicotinic agonist DMPP induces a dose-dependent relaxation of tracheal smooth muscles precontracted with methacholine [117]. These results suggest that the smooth muscle-relaxing effect of nicotinic agonists could have some utility in the treatment of asthma. Modulation of key inflammatory pathways by alpha7-selective agonists has the potential to further alleviate asthmatic symptoms.
Arthritis
The cholinergic anti-inflammatory pathway plays a central role in the etiology of arthritis. This has been confirmed in numerous animal models. An anti-inflammatory role in arthritis was suggested by studies that showed an anti-inflammatory effect of cholinergic stimulation, both by vagal nerve stimulation and cholinergic agonists, on carrageenan-induced paw inflammation [68]. It has also been shown that vagal nerve stimulation can suppress the development of collagen-induced arthritis in rats [118]. The reduction of inflammation observed may be attributable to downregulation of cytokine production by the reticuloendothelial system. In mice that were subjected to unilateral cervical vagotomy and induction of arthritis with type II collagen, clinical arthritis was exacerbated by vagotomy and ameliorated by nicotine administration (0.4 mg/kg p.o.) [119]. Moreover, nicotine inhibited bone degradation and reduced TNF-α expression in synovial tissue, and the specific alpha7 agonist AR-R17779 ameliorated clinical arthritis and reduced synovial inflammation. This was accompanied by a reduction of TNF-α levels in both plasma and synovial tissue. The effect of AR-R17779 was more potent compared with that of nicotine and was associated with delayed onset of the disease as well as with protection against joint destruction. AR-R17779 poorly crosses the blood–brain barrier, suggesting that the anti-inflammatory effects of this agonist are probably due to the stimulation of peripheral alpha7 receptors. In studies with alpha7 knockout mice, a significant increase in the incidence and severity of collagen-induced arthritis as well as increased synovial inflammation and joint destruction were seen [120]. Exacerbation of the arthritis was associated with elevated systemic proinflammatory cytokines and enhanced T-helper cell 1 (Th1)-cytokine and TNF-α production by spleen cells. These kinds of data provide evidence for a role of the cholinergic anti-inflammatory pathway in rheumatoid arthritis and implicate specific alpha7 cholinergic modulation as a potential anti-inflammatory therapeutic strategy in joint inflammation.
Cystic fibrosis
Activation of TLR2 by airway pathogens and subsequent infiltration of airway epithelial cells by neutrophils is one part of the epithelial cell injury that occurs in cystic fibrosis (CF) [47]. It has also been reported that a TLR2 ligand (PAM3), expressed by a diversity of bacteria that cause infections in patients with CF, increases IL-8 production by airway epithelial cells that harbor the mutation found in CF [116]. This production of IL-8 by tracheal epithelial cells (CFTE29o- line) was inhibited in a dose-dependent way by exposure to nicotine (10 μM), and this effect was reversed by the alpha7 nicotinic receptor antagonist, α-bungarotoxin. These findings suggest that an alpha7 nicotinic receptor agonist could help prevent recurrent bacterial infections that occur in patients with CF.
Parkinson’s disease
Numerous studies have provided evidence suggesting that neuroinflammation plays an important role in the pathogenesis of PD [121]. It has long been known that increased levels of cytokines such as TNF-α, IL-1β, and interferon-γ are present in the substantia nigra of PD patients [122], and PET studies using radiotracers for activated microglia have confirmed that microglial activation occurs in patients with early PD and is closely linked to the degree of dopaminergic neuronal loss [123]. Other studies suggest that the detrimental effects of microglial activation may be compounded by dysfunction of the blood–brain barrier, combined with infiltration of peripheral immune cells, contributing further to the degeneration of dopamine neurons [124]. More recently, it has been proposed that neuromelanin may play a role in propagating neuroinflammation in PD, based on observations that human neuromelanin induced positive chemotactic effects in microglial cell cultures, activated the proinflammatory transcription factor nuclear factor NF-κB via phosphorylation and degradation of the inhibitor protein IκB, and led to an upregulation of TNF-α, IL-6, and NO [125]. Neuromelanin has also been shown to induce neuroinflammation and neurodegeneration in the rat substantia nigra in vivo [126]. Epidemiological studies have reported that smoking is associated with a lower incidence of Parkinson’s disease (PD), leading to theories that smoking in general and nicotine in particular might be neuroprotective [127]. Consistent with these theories, it has been demonstrated that nicotine has a neuroprotective effect on dopaminergic neurons via an anti-inflammatory mechanism mediated by the modulation of microglial activation [93]. In these studies nicotine pretreatment (0.1–1.0 μM) decreased microglial activation, with significant reduction of LPS-induced TNF-α release, and significantly decreased the loss of tyrosine hydroxylase immuno-positive cells in co-cultures of microglia and mesencephalic neurons. Similar effects were observed in rats chronically (4 weeks) pre-treated with nicotine (5 mg kg−1 day−1 i.p.). All of these effects were blocked by the alpha7 nicotinic receptor antagonist α-bungarotoxin, confirming the important role played by alpha7 receptors.
Psoriatic arthritis and psoriasis
In patients with psoriatic arthritis, synovial tissue was studied using immunohistochemical analyses. Perivascular expression of the alpha7 nicotinic receptor was detected in the majority of patients, underscoring a potential role for alpha7 receptors and the cholinergic inflammatory pathway in proliferation of endothelial cells in the inflamed synovium of these patients [128]. As in rheumatoid arthritis, these findings suggest that alpha7 receptor modulation could add to the armamentarium of biologic agents (e.g., etanercept) that are effective in controlling psoriatic lesions [54].
Sepsis
Severe sepsis is a major cause of mortality in developed nations. Drug discovery efforts for conditions associated with sepsis have focused a great deal of energy on targeting TNF-α. Vagal nerve stimulation can reduce excessive inflammation and acute reaction to sepsis by inhibiting TNF-α synthesis, attenuating peak serum TNF-α levels, and preventing the development of shock. Conversely, vagotomy significantly increases the levels of serum TNF-α [129]. However, since sepsis is characterized by the widespread production of multiple proinflammatory cytokines, including TNF-α, INF-γ, IL-1β, HMGB1, and IL-12 [130], it has been hypothesized that inhibition of several or all proinflammatory mediators may be required to treat the condition successfully. For example, it is recognized that HMGB1, a late mediator of sepsis in the disease process, can be inhibited by nicotine [131]. Nicotine attenuates HMGB1 release from stimulated RAW264.7 macrophages in vitro (1 μM) and reduces serum HMGB1 levels in experimental sepsis in vivo (0.4 mg/kg i.p.) [132]. The reduced HMGB1 levels in sepsis correlate with increased survival. Splenectomy also inactivates the cholinergic anti-inflammatory pathway, demonstrating the involvement of the RES [133].
Anti-inflammatory effects of various agonists for alpha7 nicotinic receptors in experimental sepsis have been widely reported. Experimentally induced systemic inflammation using endotoxin in healthy human volunteers can be reduced by pretreatment with nicotine (7 mg patch), as judged by a lowered febrile response and increased circulating levels of IL-10 [134]. Pretreatment of cultured human monocytes with cotinine, a stable metabolite of nicotine, shifts the inflammatory response to LPS to an IL-10-dominated anti-inflammatory profile that is initiated by engagement of the monocytic alpha7 nicotinic receptors [84]. GTS-21, a selective alpha7 agonist, significantly inhibits serum HMGB1 levels in murine cecal ligation-and-puncture (CLP) mice and improves survival. Also, GTS-21 strongly inhibits LPS-induced TNF-α release into the peritoneal cavity and attenuates the influx of neutrophils into peritoneal fluid upon administration of LPS, and it is independent of its effect on TNF-α release. Choline is a precursor in the biosynthesis of acetylcholine and a selective natural alpha7 nicotinic receptor agonist. In a murine model of sepsis, choline suppressed TNF-α release from LPS-activated macrophages through inhibition of NF-κB activation, reduced systemic TNF-α levels during endotoxemia through an alpha7-mediated mechanism, and suppressed HMGB1 release [71]. These effects were not seen in alpha7 knockout mice.
The LPS-stimulated increase in systemic TNF-α production is eliminated in splenectomized animals but, interestingly, nicotine (0.4 mg/kg i.p.) decreases the survival of splenectomized animals with sepsis and bacterial clearance in septic peritonitis [133]. Thus, alpha7 nicotinic receptor activation may have a detrimental effect on host defense against bacteria in septic animals. Pretreatment of mice with nicotine (100 μg/mL p.o.) during septic peritonitis results in a reduction of local and systemic inflammation, but increased lethality due to a decrease in bacterial clearance [74]. Because it is likely that host defense in septic peritonitis is a balance between proinflammatory pathways to eliminate bacteria and anti-inflammatory pathways to prevent systemic inflammation, any imbalance might be harmful. Consistent with this, alpha7 knock-out mice display an accelerated bacterial clearance, reduced numbers of infiltrating neutrophils, and lower circulating cytokine levels as compared with wild-type mice.
Studies have suggested that the inflammatory response in sepsis can also be controlled by activation of the CNS cholinergic system. For example, intravenously administered oxytocin can reduce the neuroendocrine and cytokine response to bacterial LPS by increasing the excitability of central vagal neurons [135]. Similarly, intravenous administration of the orexigenic hormone ghrelin markedly reduces TNF-α and IL-6 levels in sepsis through central activation of the vagus nerve [136]. Cholinesterase inhibitors can also control the inflammatory response in experimental sepsis, presumably by increasing and prolonging acetylcholine release through an inhibitory effect on acetylcholinesterase. Cholinesterase inhibition significantly improves survival if administered immediately after induction of sepsis, but no reduction in mortality is achieved when treatment is delayed [137].
Ulcerative colitis
One of the earliest noted effects of nicotine on a peripheral tissue was in inflammation of the intestine. Early reports discussed patients with ulcerative colitis who upon cessation of smoking experienced more severe disease progression, which was ameliorated by returning to smoking [138]. The reduced incidence of ulcerative colitis among smokers suggests that nicotine may modulate the immune response that leads to this inflammatory bowel disease and that nicotinic receptors may be therapeutic targets for inflammatory diseases [139, 140]. However, the cellular and molecular mechanisms involved in the effects of nicotine are not well understood. In peripheral blood mononuclear cells from ulcerative colitis patients who were challenged with LPS or phytohemagglutinin (PHA), nicotine (1–100 μg/mL) reduced LPS and PHA-stimulated production of IL-1β, IL-10, TGF-β, and TNF-α [141], suggesting direct effects on cytokine production and/or release. Attempts to treat ulcerative colitis with nicotine or other nicotinic ligands have produced conflicting results [139, 140].
Ulcerative colitis is characterized by increases in proinflammatory cytokines, tissue damage, and loss of neurons in the inflamed mucosa. The vagus nerve has been shown to produce a tonic efferent cholinergic anti-inflammatory effect in acute experimental colitis and chronic colitis [142, 143]. Colitis induced by dextran sodium sulfate (DSS) is more severe in vagotomized mice compared with sham-operated mice [143]. Acetylcholinesterase inhibitors have been shown to effectively modulate the acute colonic inflammation associated with dinitrobenzene sulfonic acid (DNBS)-induced colitis [144], presumably by increasing cholinergic tone in the colon. The observation that cholinergic agonists can inhibit colonic inflammatory responses suggests that the cholinergic inflammatory pathway may be compromised. For example, nicotine (20 g/mL p.o.) decreases the levels of proinflammatory mediators in colonic mucosa during experimental colitis [142]. In a TNBS mouse model of colitis, animals treated with anabaseine, an alpha7 nicotinic cholinergic agonist, showed less tissue damage, less myeloperoxidase activity, less TNF-α production in the colon, and inhibition of NF-κB activation in mononuclear cells, compared to untreated mice with colitis [145]. By comparison, mice with colitis treated with the nicotinic antagonist chlorisondamine showed the worst tissue damage, the highest myeloperoxidase activity, the highest TNF-α levels, and increased NF-κB activation in mononuclear cells.
Clinical and experimental evidence indicates that intestinal inflammatory conditions can be exacerbated by behavioral conditions such as depression. Experimental conditions that induce depressive-like behaviors in mice have been shown to increase susceptibility to intestinal inflammation by interfering with the tonic vagal inhibition of proinflammatory macrophages [146]. These effects of depression can be attenuated by choline, a specific alpha7 agonist [146]. In other studies experimental colitis was aggravated in nicotinic receptor α5 subunit deficient mice, suggesting that not only alpha7 nicotinic receptors but also other subunits may participate in the vagus modulation of colitis [147].
Mechanisms of alpha7-mediated anti-inflammatory effects
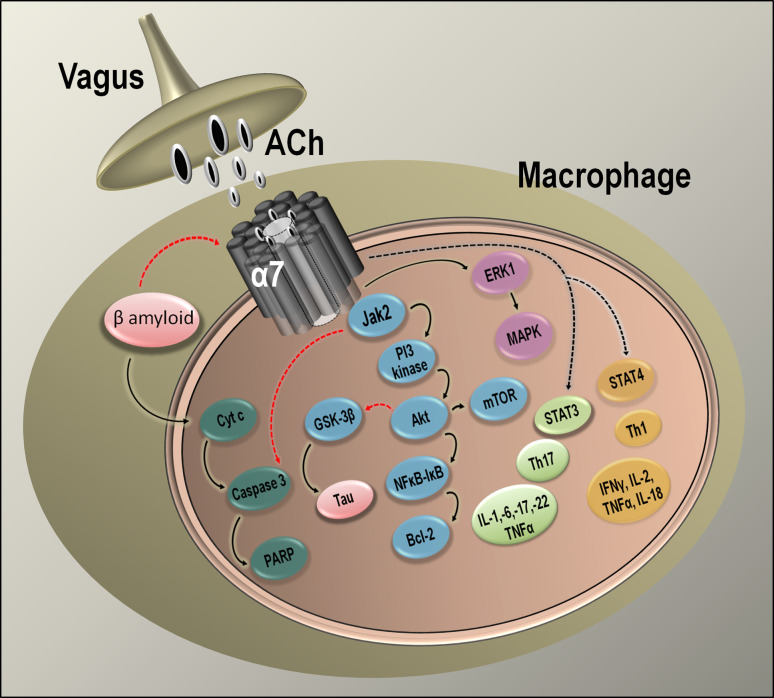
As our understanding of the tissues involved in the cholinergic anti-inflammatory pathway from the CNS to the reticuloendothelial system has unfolded, advances have also been made in understanding the molecular mechanisms involved. For example, the neuroprotective effects of nicotine and alpha7-selective ligands can be traced to alpha7 activation and transduction of signals to PI-3-K (phosphatidylinositol 3-kinase) and AKT (protein kinase B) via JAK2 [148], all of which participate in a key cell survival pathway (see Fig. 5). Immunoprecipitation experiments indicate that the alpha7 receptor and JAK2 interact directly [148]. Additional studies have examined the effects of nicotine on LPS-treated and control peritoneal macrophages and found that nicotine treatment (100 nM) leads to phosphorylation of STAT3 [75], another key component of the cellular antiapoptotic cascade. This nicotine-mediated phosphorylation is inhibited by the alpha7-selective antagonists, α-bungarotoxin and MLA, as well as by AG490, a selective inhibitor of JAK2 phosphorylation. Immunoprecipitation studies support previous findings showing that nicotine exposure recruits JAK2 and leads to an increased association between the kinase and alpha7 receptors [148]. Studies examining mice whose macrophages are deficient in STAT3 (LysM-Stat3fl/−) found that vagus nerve stimulation does not reduce peritoneal cytokine levels or intestinal inflammation as it does in control animals [75]. These data support the interaction of JAK2 and alpha7 and the critical role of STAT3 in the cholinergic anti-inflammatory pathway. LPS-stimulated release of TNF-α and the neutrophil chemoattractant macrophage inflammatory proteins MIP-1a and MIP-1b from human macrophages are inhibited by low concentrations of nicotine (10 nM) and the mRNA levels of these inflammatory mediators are also modulated [149]. Therefore, cholinergic anti-inflammatory regulation occurs upstream of transcription. Nicotine also inhibits LPS-stimulated IκB phosphorylation, thereby preventing NF-κB activation, which is necessary for gene transcription of proinflammatory mediators [149].
Fig. 5.
Relationship of alpha7 nicotinic receptors and anti-inflammatory pathways. Schematic showing alpha7 nicotinic receptor-mediated activation of JAK2 and cross-talk mechanisms between alpha7 receptors and β-amyloid-activated pathways. Alpha7-mediated neuroprotection via the JAK2 pathway intersects with the anti-inflammatory pathway mediated through STAT3/NF-κB. ACh Acetylcholine, Akt protein kinase B, Bcl-2 B cell lymphoma 2 protein, GSK-3β glycogen synthase kinase 3 beta, IκB inhibitor kappa B, JAK2 Janus kinase 2, mTOR mammalian target of rapamycin, NF-κB nuclear factor kappa B and transcription factor complex, STAT signal transducer and activator of transcription, PARP poly (ADP-ribose) polymerase, Thy thymocyte
A role of α4β2 nicotinic receptors in inflammatory processes is also emerging. For example, the α4β2-selective ligand (E)-metanicotine inhibits IL-8 and TNF-α production in human macrophages and in cells of the inflamed mucosa [150]. Conversely, proinflammatory cytokines can shift the subunit assembly of neuronal nicotinic receptors, as evidenced by the impact of IL-1β and TNF-α on assembly of receptors from subunit mixtures of α4, β2, and β4 transiently transfected into HEK-293 cells [151]. In control transfections, approximately 55% of α4 associated preferentially with β4, but less than 15% complexed with β2 and the remainder was associated with both beta subunits. These relative ratios were modified by IL-1β, which strongly enhanced α4/β2 association and decreased α4/β4 association, and by TNF-α, which promoted mixed α4/β2/β4 interactions. These results show that the emerging rules governing assembly of nicotinic receptors are subject to modification by the proinflammatory cytokine environment, suggesting bidirectional regulation of cytokines and nicotinic receptor expression. In addition, recent studies have implicated distinct participation of α4β2 and alpha7 in the regulation of B-lymphocyte development and activation and have suggested that the CD40 pathway contributes to these effects [152]. These studies support a potential role for both α4β2 and alpha7 nicotinic receptors in the regulation of inflammatory/immune processes.
Future directions
Our understanding of the cholinergic anti-inflammatory pathway and its role in mitigating inflammation and disease continues to grow, and it is clear that alpha7 and other nicotinic receptor subtypes play a prominent role. However, disparate and sometimes conflicting reports as to the mechanisms of nicotinic cholinergic mediation of anti-inflammatory effects need to be reconciled before a complete understanding can be achieved. For example, exposure to nicotine has been reported to activate the nuclear factor of activated T cells transcription factor in lymphocytes and endothelial cells, resulting in alterations in cell growth and vascular endothelial growth factor production [153]. Additional studies suggest that nicotine stimulates COX-2 expression via the activation of NF-κB and subsequent release of proinflammatory mediators, such as prostaglandin E2 (PGE2), IL-1β, IL-6, nitric oxide, and TNF-α in human gingival fibroblasts [154, 155]. On the other hand, there are numerous reports that nicotine inhibits LPS-stimulated phosphorylation of the cytoplasmic inhibitory protein I-κB, thereby preventing NF-κB activation, which is necessary for gene transcription of proinflammatory mediators. For example, low-dose nicotine causes inhibition of TNF-α, PGE2, and MIP-1alpha production in lipopolysaccharide (LPS)-activated cultured human monocytes, and these suppressive effects appear to occur at the transcriptional level through alpha7 nicotinic receptor-mediated suppression of the phosphorylation of I-κB, and inhibition of the transcriptional activity of NF-κB [149]. Furthermore, anti-inflammatory effects mediated by alpha7 nicotinic receptors can be synergistic with other signaling systems such as steroids and are mediated through reduction of nuclear translocation of p65 to suppress cytokine expression [156]. Nicotine has also been shown to have paradoxical effects that might alternatively enforce survival or trigger apoptosis, suggesting that depending on timing and context, nicotine might act either as a survival factor or as an inducer of apoptosis in normal or transformed lymphocytes, and possibly other nonneuronal cells [153]. Similar paradoxical effects have been reported for other targets involved in inflammation. Extracellular signal-regulated kinase 1/2 (ERK1/2), one of the most characterized members of the MAPK family, mediates a range of activities from inflammation to cell death and survival. It has been argued that ERK1/2 activity generated by endogenous inflammatory factors may have detrimental effects, whereas ERK1/2 activity produced by exogenous signals (e.g., growth factors) favors neuroprotection [157].
Many of the apparently discordant findings noted above appear to have in common the use of nicotine as a probe of the cholinergic anti-inflammatory pathway. Because nicotine possesses relatively low selectivity for various nicotinic receptor subtypes, most if not all of the conflicting results may simply be due to nicotine’s interactions with multiple receptor subtypes, thus confounding the contribution of alpha7. To resolve the mechanistic complexity surrounding this area of research, it will therefore be necessary to expand the development of new ligands that are more selective than nicotine for the alpha7 target. Indeed, for those novel alpha7-selective ligands that have been tested to date, there appear to be consistent anti-inflammatory effects [73, 83, 96, 101, 111]. It will also be necessary to conduct in-depth mechanistic studies in vivo in order to account for the cross-talk that naturally occurs among the many cells and pathways involved. Unfortunately, this presents a formidable challenge that will require a concerted effort using both the established armamentarium of methods as well as new techniques and approaches. As a starting point it might be reasonable to develop cytokine “fingerprints” for the inflammatory signatures of the pathological conditions of interest. By also including temporal information it might be possible to distinguish early (innate immune) versus late (adaptive immune) responses in the inflammatory cascade and how they differentially interact with nicotinic receptor-mediated effects on cellular pathways. Additional layers of information that will need to be taken into account include species, host, and cell-specific differences in the initiation and propagation of the cytokine storm. All of this information should further refine and enhance the development of highly targeted therapeutic approaches for a variety of inflammatory diseases and disorders.
Acknowledgments
The authors wish to thank Dr. David Hosford and Beth Fordham-Meier for their insightful review and suggestions that contributed significantly to the quality of the manuscript.
References
- 1.Haverslag R, Pasterkamp G, Hoefer IE. Targeting adhesion molecules in cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets. 2008;8:252–260. doi: 10.2174/187152908786786188. [DOI] [PubMed] [Google Scholar]
- 2.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loiarro M, Ruggiero V, Sette C. Targeting TLR/IL-1R signalling in human diseases. Mediators Inflamm. 2010;2010:674363. doi: 10.1155/2010/674363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opal SM. Endotoxins and other sepsis triggers. Contrib Nephrol. 2010;167:14–24. doi: 10.1159/000315915. [DOI] [PubMed] [Google Scholar]
- 5.Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. 2010;125:S53–S72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvnjak L, Duvnjak M. The metabolic syndrome—an ongoing story. J Physiol Pharmacol. 2009;60(Suppl 7):19–24. [PubMed] [Google Scholar]
- 8.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 9.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145–156. doi: 10.1159/000289203. [DOI] [PubMed] [Google Scholar]
- 10.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes. 2009;58:104–115. doi: 10.2337/db07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filkova M, Haluzik M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Folco EJ, Rocha VZ, Lopez-Ilasaca M, Libby P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem. 2009;284:25569–25575. doi: 10.1074/jbc.M109.019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20:182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan ZS, Seshadri S. Inflammation in the Alzheimer’s disease cascade: culprit or innocent bystander? Alzheimers Res Ther. 2010;2:6. doi: 10.1186/alzrt29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res. 2008;39:1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jazayeri JA, Carroll GJ, Vernallis AB. Interleukin-6 subfamily cytokines and rheumatoid arthritis: role of antagonists. Int Immunopharmacol. 2010;10:1–8. doi: 10.1016/j.intimp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Toussirot E, Wendling D. The use of TNF-alpha blocking agents in rheumatoid arthritis: an update. Expert Opin Pharmacother. 2007;8:2089–2107. doi: 10.1517/14656566.8.13.2089. [DOI] [PubMed] [Google Scholar]
- 22.Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32:43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol. 2010;125:814–820. doi: 10.1016/j.jaci.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 25.Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, Benoist C, Mathis D, Lee DM. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci USA. 2007;104:2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uysal H, Nandakumar KS, Kessel C, Haag S, Carlsen S, Burkhardt H, Holmdahl R. Antibodies to citrullinated proteins: molecular interactions and arthritogenicity. Immunol Rev. 2010;233:9–33. doi: 10.1111/j.0105-2896.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- 28.Dua B, Watson RM, Gauvreau GM, O’Byrne PM. Myeloid and plasmacytoid dendritic cells in induced sputum after allergen inhalation in subjects with asthma. J Allergy Clin Immunol. 2010;126:133–139. doi: 10.1016/j.jaci.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Holt PG, Strickland DH. Interactions between innate and adaptive immunity in asthma pathogenesis: new perspectives from studies on acute exacerbations. J Allergy Clin Immunol. 2010;125:963–972. doi: 10.1016/j.jaci.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol. 2007;119:1055–1062. doi: 10.1016/j.jaci.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Tavakkol AJ, Farid HR, Hosseini FS, Heydarian F, Boskabady MH, Khoshnavaz R, Razavi A, Ghayoor KE, Ghasemi G. Association of the expression of IL-4 and IL-13 genes, IL-4 and IgE serum levels with allergic asthma. Iran J Allergy Asthma Immunol. 2007;6:67–72. [PubMed] [Google Scholar]
- 33.Busse WW, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. J Allergy Clin Immunol. 2010;125:803–813. doi: 10.1016/j.jaci.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro F, Alves AJ, Teixeira M, Ribeiro V, Duarte JA, Oliveira J. Endothelial function and atherosclerosis: circulatory markers with clinical usefulness. Rev Port Cardiol. 2009;28:1121–1151. [PubMed] [Google Scholar]
- 35.Lubos E, Handy DE, Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2008;13:5323–5344. doi: 10.2741/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez G, Mago N, Rosa F. Role of inflammation in atherogenesis. Invest Clin. 2009;50:109–129. [PubMed] [Google Scholar]
- 37.Puddu P, Puddu GM, Cravero E, De Pascalis S, Muscari A. The emerging role of cardiovascular risk factor-induced mitochondrial dysfunction in atherogenesis. J Biomed Sci. 2009;16:112. doi: 10.1186/1423-0127-16-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeill E, Channon KM, Greaves DR. Inflammatory cell recruitment in cardiovascular disease: murine models and potential clinical applications. Clin Sci (Lond) 2010;118:641–655. doi: 10.1042/CS20090488. [DOI] [PubMed] [Google Scholar]
- 39.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sgambato A, Cittadini A. Inflammation and cancer: a multifaceted link. Eur Rev Med Pharmacol Sci. 2010;14:263–268. [PubMed] [Google Scholar]
- 41.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 43.Wang CS, Sun CF. C-reactive protein and malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med J. 2009;32:471–482. [PubMed] [Google Scholar]
- 44.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2010;126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 46.Kreindler JL. Cystic fibrosis: exploiting its genetic basis in the hunt for new therapies. Pharmacol Ther. 2010;125:219–229. doi: 10.1016/j.pharmthera.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koller B, Kappler M, Latzin P, Gaggar A, Schreiner M, Takyar S, Kormann M, Kabesch M, Roos D, Griese M, Hartl D. TLR expression on neutrophils at the pulmonary site of infection: TLR1/TLR2-mediated up-regulation of TLR5 expression in cystic fibrosis lung disease. J Immunol. 2008;181:2753–2763. doi: 10.4049/jimmunol.181.4.2753. [DOI] [PubMed] [Google Scholar]
- 48.Matricon J. Immunopathogenesis of inflammatory bowel disease. Med Sci (Paris) 2010;26:405–410. doi: 10.1051/medsci/2010264405. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei J, Feng J. Signaling pathways associated with inflammatory bowel disease. Recent Pat Inflamm Allergy Drug Discov. 2010;4:105–117. doi: 10.2174/187221310791163071. [DOI] [PubMed] [Google Scholar]
- 51.Polinska B, Matowicka-Karna J, Kemona H. The cytokines in inflammatory bowel disease. Postepy Hig Med Dosw (Online) 2009;63:389–394. [PubMed] [Google Scholar]
- 52.Tonel G, Conrad C. Interplay between keratinocytes and immune cells—recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009;41:963–968. doi: 10.1016/j.biocel.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 53.Spah F. Inflammation in atherosclerosis and psoriasis: common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol. 2008;159(Suppl 2):10–17. doi: 10.1111/j.1365-2133.2008.08780.x. [DOI] [PubMed] [Google Scholar]
- 54.Mazza J, Rossi A, Weinberg JM. Innovative uses of tumor necrosis factor alpha inhibitors. Dermatol Clin. 2010;28:559–575. doi: 10.1016/j.det.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461–467. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii M, Kurachi Y. Muscarinic acetylcholine receptors. Curr Pharm Des. 2006;12:3573–3581. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- 57.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 58.Sharma G, Vijayaraghavan S. Nicotinic receptors containing the alpha7 subunit: a model for rational drug design. Curr Med Chem. 2008;15:2921–2932. doi: 10.2174/092986708786848703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30:673–680. doi: 10.1038/aps.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther. 2009;122:302–311. doi: 10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 61.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawashima K, Fujii T. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J Pharmacol Sci. 2008;106:167–173. doi: 10.1254/jphs.FM0070073. [DOI] [PubMed] [Google Scholar]
- 63.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang DW, Zhou RB, Yao YM. Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin J Traumatol. 2009;12:355–364. [PubMed] [Google Scholar]
- 65.van Westerloo DJ. The vagal immune reflex: a blessing from above. Wien Med Wochenschr. 2010;160:112–117. doi: 10.1007/s10354-010-0761-x. [DOI] [PubMed] [Google Scholar]
- 66.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 67.Dowling O, Rochelson B, Way K, Al-Abed Y, Metz CN. Nicotine inhibits cytokine production by placenta cells via NFkappaB: potential role in pregnancy-induced hypertension. Mol Med. 2007;13:576–583. doi: 10.2119/2007-00067.Dowling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 70.Moon JH, Kim SY, Lee HG, Kim SU, Lee YB. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar beta amyloid peptide (1-42)-stimulated microglia. Exp Mol Med. 2008;40:11–18. doi: 10.3858/emm.2008.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 74.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 75.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 76.Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS J. 2005;7:E885–E894. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies BD, Hoss W, Lin JP, Lionetti F. Evidence for a noncholinergic nicotine receptor on human phagocytic leukocytes. Mol Cell Biochem. 1982;44:23–31. doi: 10.1007/BF00573842. [DOI] [PubMed] [Google Scholar]
- 78.Benhammou K, Lee M, Strook M, Sullivan B, Logel J, Raschen K, Gotti C, Leonard S. [(3)H]Nicotine binding in peripheral blood cells of smokers is correlated with the number of cigarettes smoked per day. Neuropharmacology. 2000;39:2818–2829. doi: 10.1016/S0028-3908(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 79.De Rosa MJ, Dionisio L, Agriello E, Bouzat C, Esandi MC. Alpha 7 nicotinic acetylcholine receptor modulates lymphocyte activation. Life Sci. 2009;85:444–449. doi: 10.1016/j.lfs.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 81.Chernyavsky AI, Arredondo J, Skok M, Grando SA. Auto/paracrine control of inflammatory cytokines by acetylcholine in macrophage-like U937 cells through nicotinic receptors. Int Immunopharmacol. 2010;10:308–315. doi: 10.1016/j.intimp.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183:6681–6688. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- 83.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rehani K, Scott DA, Renaud D, Hamza H, Williams LR, Wang H, Martin M. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim Biophys Acta. 2008;1783:375–382. doi: 10.1016/j.bbamcr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Maasen JA. Mitochondria, body fat and type 2 diabetes: what is the connection? Minerva Med. 2008;99:241–251. [PubMed] [Google Scholar]
- 88.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/S1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 89.Liu RH, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- 90.Carnevale D, De Simone R, Minghetti L. Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Disord Drug Targets. 2007;6:388–397. doi: 10.2174/187152707783399193. [DOI] [PubMed] [Google Scholar]
- 91.Nizri E, Irony-Tur-Sinai M, Faranesh N, Lavon I, Lavi E, Weinstock M, Brenner T. Suppression of neuroinflammation and immunomodulation by the acetylcholinesterase inhibitor rivastigmine. J Neuroimmunol. 2008;203:12–22. doi: 10.1016/j.jneuroim.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 92.Guseva MV, Hopkins DM, Scheff SW, Pauly JR. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25:975–983. doi: 10.1089/neu.2008.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park HJ, Lee PH, Ahn YW, Choi YJ, Lee G, Lee DY, Chung ES, Jin BK. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26:79–89. doi: 10.1111/j.1460-9568.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, Andra M, Matsubayashi H, Sakai N, Kohsaka S, Inoue K, Nakata Y. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- 95.van Maanen MA, Stoof SP, van der Zanden EP, de Jonge WJ, Janssen RA, Fischer DF, Vandeghinste N, Brys R, Vervoordeldonk MJ, Tak PP. The alpha7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: a possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 2009;60:1272–1281. doi: 10.1002/art.24470. [DOI] [PubMed] [Google Scholar]
- 96.Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum. 2008;58:3439–3449. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, Albuquerque EX, Conti-Fine BM. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:1201–1209. doi: 10.1124/mol.60.6.1201. [DOI] [PubMed] [Google Scholar]
- 98.Blanchet MR, Langlois A, Israel-Assayag E, Beaulieu MJ, Ferland C, Laviolette M, Cormier Y. Modulation of eosinophil activation in vitro by a nicotinic receptor agonist. J Leukoc Biol. 2007;81:1245–1251. doi: 10.1189/jlb.0906548. [DOI] [PubMed] [Google Scholar]
- 99.Maouche K, Polette M, Jolly T, Medjber K, Cloez-Tayarani I, Changeux JP, Burlet H, Terryn C, Coraux C, Zahm JM, Birembaut P, Tournier JM. Alpha7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am J Pathol. 2009;175:1868–1882. doi: 10.2353/ajpath.2009.090212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J Immunol. 2010;184:401–410. doi: 10.4049/jimmunol.0901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giebelen IA, van Westerloo DJ, Larosa GJ, de Vos AF, van der Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007;28:700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- 102.Xu B, Makris A, Thornton C, Hennessy A. Glucocorticoids inhibit placental cytokines from cultured normal and preeclamptic placental explants. Placenta. 2005;26:654–660. doi: 10.1016/j.placenta.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Kwon JY, Kim YH, Kim SH, Kang MH, Maeng YS, Lee KY, Park YW. Difference in the expression of alpha 7 nicotinic receptors in the placenta in normal versus severe preeclampsia pregnancies. Eur J Obstet Gynecol Reprod Biol. 2007;132:35–39. doi: 10.1016/j.ejogrb.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 104.Lips KS, Bruggmann D, Pfeil U, Vollerthun R, Grando SA, Kummer W. Nicotinic acetylcholine receptors in rat and human placenta. Placenta. 2005;26:735–746. doi: 10.1016/j.placenta.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 105.Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- 106.Moriwaki Y, Yoshikawa K, Fukuda H, Fujii YX, Misawa H, Kawashima K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. 2007;80:2365–2368. doi: 10.1016/j.lfs.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 107.Favre B, Plantard L, Aeschbach L, Brakch N, Christen-Zaech S, de Viragh PA, Sergeant A, Huber M, Hohl D. SLURP1 is a late marker of epidermal differentiation and is absent in Mal de Meleda. J Invest Dermatol. 2007;127:301–308. doi: 10.1038/sj.jid.5700551. [DOI] [PubMed] [Google Scholar]
- 108.Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, Huber M, Bertrand D, Hohl D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–3024. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- 109.Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 110.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 111.Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, Bencherif M. An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332:173–180. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 112.Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther. 2007;115:208–222. doi: 10.1016/j.pharmthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 113.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Smoking and the development of allergic sensitization to aeroallergens in adults: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2001;56:328–332. doi: 10.1034/j.1398-9995.2000.00509.x-i1. [DOI] [PubMed] [Google Scholar]
- 114.Chen LY, Liu ZG, Li YH, Feng YZ, Wang JR. Expression of neuronal acetylcholine receptor alpha 7 (nAChRalpha7) in peripheral blood CD(4)(+) T lymphocytes from asthmatic children. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31:803–805. [PubMed] [Google Scholar]
- 115.Mishra NC, Rir-Sima-Ah J, Boyd RT, Singh SP, Gundavarapu S, Langley RJ, Razani-Boroujerdi S, Sopori ML. Nicotine inhibits fcφ RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through alpha7/alpha9/alpha10-nicotinic receptors. J Immunol. 2010;185:588–596. doi: 10.4049/jimmunol.0902227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Greene CM, Ramsay H, Wells RJ, O’Neill SJ, McElvaney NG. Inhibition of Toll-like receptor 2-mediated interleukin-8 production in cystic fibrosis airway epithelial cells via the alpha7-nicotinic acetylcholine receptor. Mediators Inflamm. 2010;2010:423241. doi: 10.1155/2010/423241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dorion G, Israel-Assayag E, Beaulieu MJ, Cormier Y. Effect of 1, 1-dimethylphenyl 1,4-piperazinium on mouse tracheal smooth muscle responsiveness. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1139–L1145. doi: 10.1152/ajplung.00406.2004. [DOI] [PubMed] [Google Scholar]
- 118.Zhang P, Han D, Tang T, Zhang X, Dai K. Inhibition of the development of collagen-induced arthritis in Wistar rats through vagus nerve suspension: a 3-month observation. Inflamm Res. 2008;57:322–328. doi: 10.1007/s00011-008-8070-1. [DOI] [PubMed] [Google Scholar]
- 119.van Maanen MA, Lebre MC, van der Poll T, Larosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 120.van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. Ann Rheum Dis. 2010;69:1717–1723. doi: 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 121.Chung YC, Ko HW, Bok E, Park ES, Huh SH, Nam JH, Jin BK. The role of neuroinflammation on the pathogenesis of Parkinson’s disease. BMB Rep. 2010;43:225–232. doi: 10.5483/bmbrep.2010.43.4.225. [DOI] [PubMed] [Google Scholar]
- 122.Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- 123.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 124.Ajonuma LC, Chan PK, Ng EH, Fok KL, Wong CH, Tsang LL, Tang XX, Ho LS, Lau MC, Chung CM, He Q, Huang HY, Yang DZ, Rowlands DK, Chung YW, Chan HC. Involvement of cystic fibrosis transmembrane conductance regulator (CFTR) in the pathogenesis of hydrosalpinx induced by Chlamydia trachomatis infection. J Obstet Gynaecol Res. 2008;34:923–930. doi: 10.1111/j.1447-0756.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- 125.Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R. Inflammation in Parkinson’s diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des. 2007;13:1925–1928. doi: 10.2174/138161207780858429. [DOI] [PubMed] [Google Scholar]
- 126.Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta Neuropathol. 2008;116:47–55. doi: 10.1007/s00401-008-0361-7. [DOI] [PubMed] [Google Scholar]
- 127.Quik M, O’Leary K, Tanner CM. Nicotine and Parkinson’s disease: implications for therapy. Mov Disord. 2008;23:1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Westman M, Engstrom M, Catrina AI, Lampa J. Cell specific synovial expression of nicotinic alpha 7 acetylcholine receptor in rheumatoid arthritis and psoriatic arthritis. Scand J Immunol. 2009;70:136–140. doi: 10.1111/j.1365-3083.2009.02266.x. [DOI] [PubMed] [Google Scholar]
- 129.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 130.Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009;32:348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 132.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 133.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol. 2007;147:28–34. doi: 10.1111/j.1365-2249.2006.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- 136.Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–486. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, Mautner S, Wente MN, Encke J, Werner J, Dalpke AH, Stremmel W, Nawroth PP, Martin E, Krammer PH, Bierhaus A, Weigand MA. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med. 2008;36:404–408. doi: 10.1097/01.CCM.0B013E31816208B3. [DOI] [PubMed] [Google Scholar]
- 138.Wolf JM, Lashner BA. Inflammatory bowel disease: sorting out the treatment options. Cleve Clin J Med. 2002;69:621–631. doi: 10.3949/ccjm.69.8.621. [DOI] [PubMed] [Google Scholar]
- 139.Scott DA, Martin M. Exploitation of the nicotinic anti-inflammatory pathway for the treatment of epithelial inflammatory diseases. World J Gastroenterol. 2006;12:7451–7459. doi: 10.3748/wjg.v12.i46.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McGilligan VE, Wallace JM, Heavey PM, Ridley DL, Rowland IR. Hypothesis about mechanisms through which nicotine might exert its effect on the interdependence of inflammation and gut barrier function in ulcerative colitis. Inflamm Bowel Dis. 2007;13:108–115. doi: 10.1002/ibd.20020. [DOI] [PubMed] [Google Scholar]
- 141.Aldhous MC, Prescott RJ, Roberts S, Samuel K, Waterfall M, Satsangi J. Does nicotine influence cytokine profile and subsequent cell cycling/apoptotic responses in inflammatory bowel disease? Inflamm Bowel Dis. 2008;14:1469–1482. doi: 10.1002/ibd.20523. [DOI] [PubMed] [Google Scholar]
- 142.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 143.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G560–G567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- 144.Miceli PC, Jacobson K. Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton Neurosci. 2003;105:16–24. doi: 10.1016/S1566-0702(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 145.Bai A, Guo Y, Lu N. The effect of the cholinergic anti-inflammatory pathway on experimental colitis. Scand J Immunol. 2007;66:538–545. doi: 10.1111/j.1365-3083.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 146.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–2218. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Orr-Urtreger A, Kedmi M, Rosner S, Karmeli F, Rachmilewitz D. Increased severity of experimental colitis in alpha 5 nicotinic acetylcholine receptor subunit-deficient mice. Neuroreport. 2005;16:1123–1127. doi: 10.1097/00001756-200507130-00018. [DOI] [PubMed] [Google Scholar]
- 148.Shaw S, Bencherif M, Marrero MB. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1–42) amyloid. J Biol Chem. 2002;277:44920–44924. doi: 10.1074/jbc.M204610200. [DOI] [PubMed] [Google Scholar]
- 149.Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spoettl T, Paetzel C, Herfarth H, Bencherif M, Schoelmerich J, Greinwald R, Gatto GJ, Rogler G. (E)-metanicotine hemigalactarate (TC-2403–12) inhibits IL-8 production in cells of the inflamed mucosa. Int J Colorectal Dis. 2007;22:303–312. doi: 10.1007/s00384-006-0135-4. [DOI] [PubMed] [Google Scholar]
- 151.Gahring LC, Days EL, Kaasch T, de Gonzalez MM, Owen L, Persiyanov K, Rogers SW. Pro-inflammatory cytokines modify neuronal nicotinic acetylcholine receptor assembly. J Neuroimmunol. 2005;166:88–101. doi: 10.1016/j.jneuroim.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 152.Skok MV, Grailhe R, Agenes F, Changeux JP. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007;80:2334–2336. doi: 10.1016/j.lfs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 153.Oloris SC, Frazer-Abel AA, Jubala CM, Fosmire SP, Helm KM, Robinson SR, Korpela DM, Duckett MM, Baksh S, Modiano JF. Nicotine-mediated signals modulate cell death and survival of T lymphocytes. Toxicol Appl Pharmacol. 2010;242:299–309. doi: 10.1016/j.taap.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakao S, Ogata Y, Sugiya H. Nicotine stimulates the expression of cyclooxygenase-2 mRNA via NFkappaB activation in human gingival fibroblasts. Arch Oral Biol. 2009;54:251–257. doi: 10.1016/j.archoralbio.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 155.Xiao F, Zhu J, Zhao L, Zheng G, Yang A, Tao J, Zhang B, Huang Z, Xiong F. Involvement of pro-inflammatory and anti-inflammatory cytokines in the anti-inflammatory activity of Rubus idaeus L. on LPS-treated RAW 264.7 cells. J Chin Pharm Sci. 2010;19:201–208. [Google Scholar]
- 156.Martinez-Ferrer M, Iturregui JM, Uwamariya C, Starkman J, Sharif-Afshar AR, Suzuki K, Visedsindh W, Matusik RJ, Dmochowski RR, Bhowmick NA. Role of nicotinic and estrogen signaling during experimental acute and chronic bladder inflammation. Am J Pathol. 2008;172:59–67. doi: 10.2353/ajpath.2008.070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]