Abstract
Objective
To evaluate vascular dysfunction using both physiologic measures and biochemical markers, longitudinally, prior to and during pregnancy, in nulliparous women who had uncomplicated pregnancies compared to those who developed complicated hypertension during pregnancy.
Methods
Twenty healthy nulliparous women were studied during the follicular phase and in early (EP) and late (LP) pregnancy. All had singleton conceptions and delivered at term, seventeen with uncomplicated pregnancies (NP) and three who developed complicated hypertension (HP) after the LP evaluation. We compared prepregnancy, EP and LP pulse wave velocity (PWV) and soluble vascular cell adhesion molecule (sVCAM-1) between the NP and HP groups. PWV was measured using ultrasound and simultaneous echocardiogram tracing then calculated as the estimated distance divided by interval between EKG r-wave peak and peak brachial artery flow. SVCAM-1 was measured using a commercially available kit. Data are means ± SE, significance accepted as p < 0.05.
Results
The NP group had significantly lower prepregnant PWV (NP: 2.66 ± 0.06 m/s, HP: 3.00 ± 0.04, p=.02), but PWV was not different at the EP or LP time points. SVCAM-1 was significantly lower prior to pregnancy and during EP and LP in the NP group (Prepregnancy: NP: 712 ± 32 ng/mL, HP: 1058 ± 107, p < .001; EP: NP: 695 ± 31 ng/mL, HP: 924 ± 52, p = .004; LP: NP: 663 ± 25 ng/mL, HP: 946 ± 36, p < .001).
Conclusions
PWV and sVCAM-1 may be important prepregnancy discriminators useful in assessing risk for preeclampsia prior to pregnancy.
Keywords: arterial stiffness, hypertension, pregnancy, preeclampsia, endothelial dysfunction
Introduction
Preeclampsia (PE) is a multi-organ disease that affects 3–8% of first pregnancies and is a major contributor to perinatal morbidity and mortality. Significant evidence points to a primary cardiovascular component in PE development. Vascular compliance, an index of vascular function, underlies the vascular adaptations of pregnancy which accommodates large increases in plasma volume with limited changes in intravascular pressure. Arterial stiffness, an index of compliance, is an established risk factor for cardiovascular disease and is positively associated with heart failure, stroke, coronary heart disease and hypertension [1, 2]. Pulse wave velocity (PWV) is considered the gold-standard for measuring arterial stiffness and is correlated with risk for cardiovascular disease including hypertension [1, 3].
Both inflammation and endothelial dysfunction are regarded as contributors to the etiology of PE and, likely associated with the vascular dysfunction that accompanies PE [4, 5]. Measurements of flow-mediated vasodilation (FMD), a physiologic assessment of endothelial function, suggests endothelial dysfunction during PE [6, 7]. Increased peripheral endothelial cell surface markers, such as soluble intracellular cell adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), von Willebrand factor (vWF), and inflammatory cytokines, such as tumor necrosis factor (TNFα), have been used to gauge endothelial and vascular health [8–10].
Our laboratory has hypothesized that some women are destined to develop PE because of an intolerance to the normal volume expansion of pregnancy [11]. We believe this intolerance results from subclinically decreased arterial compliance which is present prior to pregnancy [11]. Here, we examined the hypothesis that arterial stiffness and markers of vascular dysfunction are elevated prior to pregnancy in those destined to develop PE. Specifically, we longitudinally evaluated arterial stiffness, endothelial function and inflammation prior to, and during, pregnancy in women who subsequently experienced normal pregnancies and those who developed PE.
Materials and Methods
This study is a prospective longitudinal cohort study. Thirty four nulligravid women interested in conception were enrolled in this research study through an open advertisement. Women were provided with ovulation detection kits (Quidel Corporation, San Diego, CA) to assist with achieving a successful conception. All subjects were young (18–40) healthy, nonsmokers with regular menstrual cycles at the time of enrollment. None of the women had a history of hypertension, autoimmune disease, diabetes or other disorders known to affect blood pressure. Thirty women subsequently conceived. Eight subjects conceived before baseline prepregnancy studies were performed; one subject had a first trimester miscarriage; one subject was lost to follow-up. The remaining 20 subjects, all of whom conceived singleton pregnancies, had complete prepregnancy assessments and successful pregnancy outcomes comprise the current report. Of the twenty subjects, seventeen women had uncomplicated, nonhypertensive and normal, pregnancies (NP). One NP woman missed her first trimester assessment and was therefore not included in the early pregnancy study day data. Three women developed complicated hypertension (HP) during pregnancy. Two of which had classically defined preeclampsia with 24 hour urine collections demonstrating proteinuria >300 mg/dl and blood pressure >140/90 mmHg. The third woman had new onset third trimester, elevated blood pressure >140/90 mmHg, elevated liver enzymes, elevated uric acid concentration (>5mg/dl) and fetal growth restriction with iatrogenic delivery at 37 weeks. Women were enrolled consecutively over a 33 month period, from May 2004 through February 2007. Prior to each study visit subjects were provided with a 3500-mg sodium-balanced diet for 72 hours. Each subject was asked to abstain from alcohol and caffeine beginning at least 24 hours before the study and to avoid the use of decongestants and nonsteroidal medications beginning at least 48 hours before the study. All prepregnancy assessments were performed during the follicular phase. Assessments during early pregnancy were performed between 11 and 15 menstrual weeks. Assessments during late pregnancy were performed between 30 and 34 menstrual weeks. All late pregnancy assessments were conducted prior to the clinical recognition of hypertensive complications of pregnancy and all women were normotensive at all study visits. Ovulation detection and early pregnancy ultrasound assessments were used to calculate gestational age.
The research protocols were approved by the University of Vermont Human Investigational Committees. All women studied provided written informed consent.
Each periodic assessment was conducted between 8 AM and 10 AM. Subjects were admitted to the University of Vermont Clinical Research Center on the day of the study after an overnight fast. For subjects' prepregnancy visit, first-void urine was obtained to confirm nonpregnant state. Following height and weight determination, subjects rested in the supine position for the remainder of the study with a minimum of 30 minutes before blood collection.
Blood samples were collected into EDTA or sodium citrate tubes, depending on the assay, from the antecubital fossa with the use of an indwelling venous saline lock and following a 2.5 mL discard. They were centrifuged within 60 minutes for 15 minutes at 3000 × g to isolate plasma. Plasma was then aliquoted and stored at −70°C until analysis.
Biochemical Analysis
TNFα was measured using the human cytokine/chemokine milliplex kit (Millipore, Billerica, MA). The technology involves a proprietary process that dyes latex microbeads with two fluorophores. A Luminex 100 instrument captures the color signals and translates these signals into real time quantitative data for each assay. The assay range is 3.2–10,000 pg/ml. Intra- and inter-assay CVs range from 4.8–9.0% and 3.1–18.4%, respectively.
Human soluble intercellular adhesion molecule-1 (sICAM-1) and human soluble vascular adhesion molecule-1 (sVCAM-1) were measured using commercially available ELISA assays (Parameter Human sICAM-1 Immunoassay and Parameter Human sVCAM-1 Immunoassay, R&D Systems, Minneapolis, MN) following manufacturer's instructions. The laboratory CV for the sICAM-1 assay is 5.0% and the minimum detectable level is < 30 ng/ml. Inter-assay CVs range from 5 to 10%. The minimum detectable level of sVCAM-1 is typically < 2.0 ng/ml. The intra- and inter-assay CVs range from 4.3–5.9% and 8.5–10.2%, respectively. von Willebrand factor was measured by an immunoturbidimetric assay (liatest vWF, Stago, Parsippany, NJ) on the Sta-R analyzer (Stago). The assay utilizes latex particles to which specific antibodies have been attached. In the presence of vWF the particles agglutinate to form aggregates, which absorb more light. This increase in absorbance is proportional to the vWF present in the test sample. The results are presented as percent vWF, with an expected normal range of 50–160%. The inter-assay CV is generally less than 5%.
Flow-mediated vasodilation studies
These studies are functional in-vivo assessments of endothelial health as reflected by the ability of the endothelium to generate a shear stress mediated vasodilatory signal in response to an acute increase in volumetric flow. These studies were conducted by establishing baseline vessel diameter under direct visualization with a 10 MHz transducer employing a Vivid 7 General Electric ultrasound unit (Milwaukee, WI). All diameter measurements were made in m-mode function and the mean of 3 measurements was accepted as the best estimate of diameter. Visualization of vessels was made 2 finger breadths above the antecubital fossae of the arm. A blood pressure cuff was then placed across the distal extremity and inflated to 50 mmHg above systolic pressure for a period of 3 minutes. Following deflation, vessel diameter (3 estimates at each time point) was measured at 50, 60, and 70 seconds and the mean of these measurements was accepted as the post-restriction maximal diameter. The vasodilatory response was calculated as the difference in diameters, pre and post restriction, divided by the baseline diameter resulting in flow-mediated dilation percent (FMD%).
Shear stress measurements
Blood samples were collected in 8 ml EDTA tubes and sent to the laboratory of Dr. Ron Magness at University of Wisconsin for analysis. Blood viscosities were determined using a cylindrical spindle digital Torque viscometer (Brookfield Engineering Labs, Inc., Middleboro, MA). Shear stress (SS) was calculated by the following formula: 4 * UBF * viscosity/ π * r3. FMD% was divided by SS to determine FMD/SS.
Pulse wave velocity
Brachial pulse wave forms were obtained by Doppler ultrasound using a 10 MHz transducer. Time from EKG R wave to peak systolic flow in the brachial artery was used to determine PWV, relative to the distance from the heart to brachial artery (distance from heart to brachial artery was calculated post hoc as height*0.33).
Statistical Methods
Baseline characteristics were compared between non-hypertensive (NP) and women who developed complicated hypertension (HP) using two sample t-tests. F-tests corresponding to simple effects derived from repeated measures analyses of variance were used to compare physiologic and biochemical measures between within each assessment. Spearman's rank correlation was used to examine the association between prepregnancy measures and those obtained during pregnancy.
Results
Subject characteristics
All pregnancies were singletons, and the majority of the subjects were Caucasian, (90%, 18/20). Clinical and demographic characteristics are presented in Table 1. There were no significant differences between NP and HP in age, body mass index (BMI), prepregnancy cycle day, early pregnancy study day and gestational age at delivery (Table 1). There was a significant difference between NP and HP third trimester study day where NPs were studied sixteen days later than the HP group. Birth weights and birth weight percentiles of the newborns were significantly higher in NP subjects compared with HP (birth weight: p = .001 and birth weight percentile: p = .01). Two of the HP newborns were small for gestational age (1st and 5th birth weight percentiles) whereas the third HP newborn was in the 30th percentile. NP newborn birth weights ranged from the 15th to 95th percentile.
Table 1.
Maternal demographic characteristics and pregnancy outcomes
| Characteristic | NP (n = 17) Mean ± SE | HP (n = 3)a Mean ± SE | p-valueb |
|---|---|---|---|
| Maternal age (years) | 28.9 ± 0.7 | 31.3 ± 1.2 | .21 |
| BMI (kg/m2) | 23.6 ± 0.8 | 21.5 ± 0.9 | .31 |
| Prepregnancy cycle day | 8.6 ± 1.1 | 7.7 ± 1.2 | .71 |
| First trimester study dayc | 93.3 ± 2.6 | 97 ± 7.6 | .60 |
| Third trimester study day | 227.8 ± 2.0 | 212.0 ± 0.6 | .005 |
| Gestational age at delivery (weeks) | 39.7 ± 0.3 | 38 ± 1.0 | .13 |
| Birth weight (g) | 3582 ± 96 | 2514 ± 421 | .001 |
| Birth weight percentile | 55.2 ± 6.0 | 12 ± 9.1 | .01 |
Three women developed complicated hypertension (HP) during pregnancy. Two of which had classically defined preeclampsia, the third woman had elevated blood pressure, elevated liver enzymes, elevated uric acid concentration and fetal growth restriction in the third trimester. The remaining 17 women are designated as uncomplicated or normal pregnancies (NP).
Significance based on two sample t-tests
One control subject missed her first trimester study day. The demographic and outcome data presented in this table include all 17 women.
Prepregnancy physiologic and biochemical assessments
PWV was significantly lower in the NP group (p = .02) and segregated the HP group from the NP (Table 2 and Fig 1). SVCAM-1 was significantly lower in the NP group as compared to the HP group (p<.001). There were no significant differences between groups in TNFα, IL-4, sICAM-1, vWF, CRP and FMD/SS.
Table 2.
Numerical values and statistical comparisons of physiologic measures and biochemical markers of vascular dysfunction before pregnancy and during pregnancy in those with normal pregnancy (NP) and those who developed hypertension during pregnancy (HP).
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prepregnancy | Early Pregnancyb | Late Pregnancy | |||||||
|
| |||||||||
| Variable | NP | HP | p-valuea | NP | HP | p-valuea | NP | HP | p-valuea |
| Physiologic Measures | |||||||||
| PWV (m/s) | 2.66 ± .06 | 3.00 ± .04 | 0.02 | 2.73 ± 0.05 | 2.90 ± 0.20 | 0.21 | 2.57 ± 0.06 | 2.61 ± 0.06 | 0.77 |
| FMD/SS | 0.32 ± 0.08 | 0.20 ± 0.004 | 0.53 | 0.17 ± 0.05 | 0.13 ± 0.04 | 0.85 | −0.20 ± 0.08 | −0.09 ± 0.03 | 0.54 |
|
| |||||||||
| Biochemical Markers | |||||||||
| sVCAM-1 (ng/mL) | 712 ± 32 | 1058 ± 107 | <.001 | 695 ± 31 | 924 ± 52 | 0.004 | 663 ± 25 | 946 ± 36 | <.001 |
| sICAM-1 (ng/mL) | 158 ± 11 | 170 ± 14 | 0.47 | 150 ± 11 | 191 ± 12 | 0.15 | 158 ± 42 | 178 ± 11 | 0.44 |
| TNFα (pg/mL) | 3.94 ± 0.34 | 4.9 ± 1.1 | 0.22 | 3.39 ± 0.23 | 4.71 ± 0.84 | 0.08 | 4.41 ± 0.26 | 5.05 ± 0.95 | 0.42 |
| IL-4 (pg/mL) | 15.3 ± 0.7 | 15.3 ± 1.7 | 0.97 | 16.8 ± 0.7 | 17.9 ± 1.5 | 0.76 | 20.7 ± 2.5 | 18.7 ± 0.78 | 0.60 |
| vWF % | 85.4 ± 8.0 | 78.0 ± 13.7 | 0.87 | 118.3 ± 14.3 | 195.0 ± 46.3 | 0.08 | 179.5 ± 22.7 | 202.0 ± 77.5 | 0.61 |
| CRPc (μg/ml) | 0.51 ± 0.13 | 0.26 ± 0.13 | 0.40 | 2.80 ± 0.67 | 1.31 ± 0.73 | 0.24 | 2.29 ± 0.48 | 1.35 ± 0.38 | 0.37 |
PWV: pulse wave velocity; FMD/SS: flow-mediated vasodilation/shear stress; sVCAM-1: soluble vascular cellular adhesion molecule-1; sICAM-1: soluble intercellular cell adhesion molecule-1; TNFα: tumor necrosis factor alpha; IL-4: interleukin-4; vWF: von Willebrand factor; CRP: C-reactive peptide. Data is presented as mean ± SE.
Significance associated with F-test corresponding to difference between groups within assessment.
One control subject missed her first trimester study day. Therefore, the first trimester measurements only include 16 women.
Data was log transformed prior to analysis, tabled values represent geometric means and associated standard error.
Figure 1. Distribution of brachial pulse wave velocity (bPWV), prior to pregnancy, in women who developed complicated hypertension (HP) compared to those with normal pregnancies (NP).
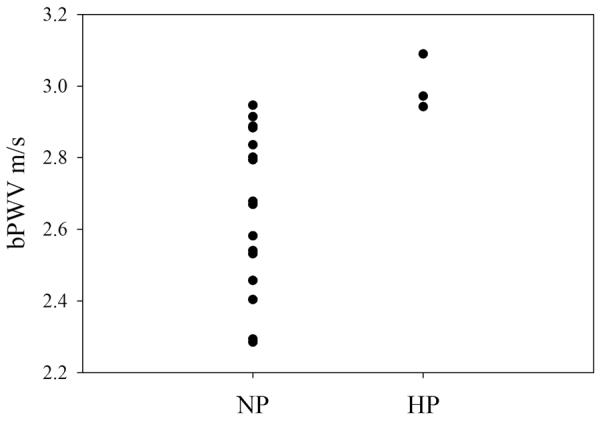
Brachial pulse wave velocity was measured prior to pregnancy in twenty nulliparous women and compared between those women developed complicated hypertension in a subsequent pregnancy (HP) and those with a normal pregnancy outcome (NP). Brachial PWV was significantly lower in NP than in those who developed HP (p = .02).
Early pregnancy physiologic and biochemical assessments
At the early pregnancy assessment, PWV was not significantly different between the two groups (p = .24, Table 2). Soluble VCAM-1 and TNFα were significantly lower in the NP group as compared to HP (p = .01 and p = 0.05, respectively). VWF tended to be lower in NP group (p = .06, Table 2). IL-4, sICAM, CRP and FMD/SS were not significantly different between NP and HP.
Late pregnancy physiologic and biochemical assessments
PWV was not significantly different at the late pregnancy assessment (p = .53, Table 2). As with the other time points, sVCAM-1 was significantly lower in the NP group compared to HP (p<.001). Soluble ICAM-1, TNFα, IL-4, vWF, LogCRP and FMD/SS were not significantly different.
Correlation Analyses
As one of the central hypotheses of our laboratory is that prepregnancy physiology is a large contributor to pregnancy physiology, we evaluated the association between prepregnancy physiology and pregnancy physiology. Correlation analyses were performed on all physiologic and biochemical markers, matching the prepregnancy assessment with each of the two subsequent pregnancy visits. PWV, sVCAM-1, sICAM-1, TNFα, IL-4, vWF and LogCRP prepregnant measurements were significantly and positively correlated with their corresponding early or late pregnant assessment (Table 3). Prepregnant FMD/SS was not significantly correlated with either early or late pregnancy measurements of FMD/SS.
Table 3.
Evaluation of correlations between prepregnant and subsequent visit measurements.
| Characteristic | Pre vs. Early Pregnancy | Pre vs. Late Pregnancy | ||
|---|---|---|---|---|
| r | p-valuea | r | p-valuea | |
| PWV | 0.55 | 0.02 | 0.49 | 0.03 |
| FMD/SS | −0.39 | 0.10 | −0.06 | 0.81 |
| sVCAM-1 | 0.57 | 0.01 | 0.63 | 0.003 |
| sICAM-1 | 0.82 | <.001 | 0.73 | <.001 |
| TNFα | 0.80 | <.001 | 0.82 | <.001 |
| IL-4 | 0.68 | 0.002 | 0.54 | 0.02 |
| vWF | 0.544 | 0.02 | 0.63 | 0.003 |
| CRPb | 0.81 | <.001 | 0.89 | <.001 |
PWV: pulse wave velocity; FMD/SS: flow-mediated vasodilation/shear stress; sVCAM-1: soluble vascular cellular adhesion molecule -1; sICAM-1: soluble intercellular cell adhesion molecule-1; TNFα: tumor necrosis factor alpha; IL-4: interleukin-4; vWF: von Willebrand factor; CRP: C-reactive peptide
Tabled values are Spearman's rank correlation coefficients and associated significance levels.
Data was log transformed prior to analysis.
Discussion
The current study evaluates prepregnancy markers of vascular dysfunction and inflammation in relation to changes in these factors that are identified during pregnancy in women who had subsequent normal or hypertensive pregnancies. Longitudinal studies including prepregnancy evaluation with subsequent pregnancy evaluation and outcome are scant as most investigations include observations exclusive to pregnancy and lack prepregnancy/baseline observations. While our observations include a small number of women who ultimately developed HP, we have complete prepregnancy characterization of these women, at our prepregnant/baseline time point, a unique and strengthening element of our study.
Arterial stiffness is most often associated with decreased elasticity stemming from increased extracellular matrix deposition and fibrosis that contributes to the inward eutropic remodeling characteristic of small arteries isolated from hypertensive individuals [12]. We observed increased PWV, a direct, though noninvasive, measure of arterial stiffness, prior to pregnancy in nulliparous women who subsequently developed preeclampsia. These observations suggest that women who are predisposed to preeclampsia may have subclinical arterial changes prior to pregnancy. While most often this process is associated with ageing, our work is not the first to identify increased PWV in relation to PE. Numerous studies show increased PWV after clinical onset of PE, with no difference between early and late onset PE [13–15]. Unlike previous studies, we did not observe a difference between the NP and HP groups in PWV during pregnancy, however, this may be due to our small numbers.
Arterial stiffness is often associated with evidence of endothelial damage and inflammation indicating a concurrent mechanism in the development of cardiovascular disease [16, 17]. Similarly, both human and animal studies demonstrate a role for endothelial dysfunction in PE [18]. Numerous studies performed prior to the clinical onset of PE, but during pregnancy, show evidence of endothelial dysfunction apparent in either flow-mediated vasodilatory response or increases in biochemical markers associated with endothelial dysfunction [19, 20]. Our observations showing a significant increase in sVCAM-1, in the women who developed HP, prior to pregnancy, that persisted throughout pregnancy, echoes other studies evaluating sVCAM-1 after clinical onset of PE and further supporting existence of a global subclinical arteriosclerotic phenotype prior to pregnancy [21]. Increased expression of sVCAM-1 has been linked to atherosclerotic changes by increasing recruitment of monocytes that subsequently produce inflammatory cytokines such as TNFα and IL-6 [22]. Both TNFα and sVCAM-1 are known to be increased after clinical onset of PE [23, 24]. The trend towards increased TNFα during early pregnancy, coupled with increased arterial stiffness and increased endothelial activation, strongly suggests presence of a cardiovascular disease process that is established prior to pregnancy, and eventually manifests as PE. Increased arterial stiffness and monocyte recruitment, via sVCAM-1 expression, coupled with the superimposed cardiovascular challenge of pregnancy, specifically adaptations related to volume expansion during pregnancy, likely leads to increased TNFα and damage to the endothelium. This hypothesis is further supported by our data indicating a tendency towards increases in vWF, a biochemical marker often used to evaluate endothelial remodeling or damage [25]. VWF is associated with PE and is affiliated with changes in endothelial function [25, 26]. However, we did not find any differences in endothelial function as measured by FMD/SS. This is contrary to other studies demonstrating a decreased FMD in those women who develop PE [20]. Our sample size of three is likely too small to detect a difference in FMD/SS between NP and HP pregnancies. Interestingly, FMD/SS was the only measure that was not significantly correlated with early and late pregnancy FMD/SS suggesting that pregnancy-induced changes in FMD/SS may be independent of prepregnancy values. Of note, PWV, TNFα, sVCAM-1 and vWF were significantly, and positively associated with their prepregnancy correlate. This finding strongly supports the hypothesis that prepregnancy phenotype is a major contributor to pregnancy physiology.
All differences observed prior to pregnancy and in early pregnancy were nonexistent during late pregnancy, except sVCAM-1, which remained elevated. This is surprising as our third trimester assessment occurred between 30 – 34 weeks, however, the HP group was studied significantly earlier at 30 weeks, 7 weeks prior to the first HP delivery. It might be expected that inflammation and endothelial dysfunction would be at its height immediately prior to clinical development of hypertension, however, our data did not identify this pattern.
Often pregnancy is referred to as a stress test for life suggesting that pregnancy induces cardiovascular strain that may temporarily unmask latent cardiovascular abnormalities manifesting as PE. The longitudinal nature of our studies, and evidence of prepregnancy vascular dysfunction suggest that evaluating prepregnancy phenotype may contribute to the assessment of risk for hypertensive disorders of pregnancy. Though we have a small sample size, data presented in the current study supports the hypothesis that women who are destined to develop PE have a subclinical phenotype that, with superimposed pregnancy, is revealed as PE. Increased arterial stiffness prior to pregnancy likely impairs the vascular adaptations necessary to accommodate pregnancy-induced plasma volume expansion that is crucial to successful pregnancy outcome. Most importantly, the finding that PWV and sVCAM-1 are significantly and distinctly elevated prior to pregnancy suggests that both PWV and sVCAM-1 may be valuable prepregnancy discriminators for PE risk, with respective independent value as a noninvasive measure, PWV, and sVCAM-1 as a biochemical marker, easily measured from peripheral blood.
Acknowledgements
The authors wish to thank Jason Austin at the University of Wisconsin, Madison, for his help in evaluating shear stress.
Grant support: NIH R01 HL 071944 (IMB), M01 RR000109 (UVM GCRC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- [2].Mitchell GF. Arterial Stiffness and Wave Reflection: Biomarkers of Cardiovascular Risk. Artery Res. 2009;3(2):56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sonoda H, Takase H, Dohi Y, Kimura G. Factors associated with brachial-ankle pulse wave velocity in the general population. J Hum Hypertens. 2011 doi: 10.1038/jhh.2011.100. [DOI] [PubMed] [Google Scholar]
- [4].Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- [5].Bernardi F, Guolo F, Bortolin T, Petronilho F, Dal-Pizzol F. Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. J Obstet Gynaecol Res. 2008;34(6):948–51. doi: 10.1111/j.1447-0756.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- [6].Dørup I, Skajaa K, Sørensen KE. Normal pregnancy is associated with enhanced endothelium-dependent flow-mediated vasodilation. Am J Physiol Heart Circ Physiol. 1999;276(3):H821–H5. doi: 10.1152/ajpheart.1999.276.3.H821. [DOI] [PubMed] [Google Scholar]
- [7].Yamamoto T, Suzuki Y, Kojima K, Suzumori K. Reduced flow-mediated vasodilation is not due to a decrease in production of nitric oxide in preeclampsia. Am J Obstet Gynecol. 2005;192(2):558–63. doi: 10.1016/j.ajog.2004.08.031. [DOI] [PubMed] [Google Scholar]
- [8].Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- [9].Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost. 2006;4(6):1186–93. doi: 10.1111/j.1538-7836.2006.01949.x. [DOI] [PubMed] [Google Scholar]
- [10].Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116(3):219–30. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bernstein IM, Meyer MC, Osol G, Ward K. Intolerance to volume expansion: a theorized mechanism for the development of preeclampsia. Obstet Gynecol. 1998;92(2):306–8. doi: 10.1016/s0029-7844(98)00207-5. [DOI] [PubMed] [Google Scholar]
- [12].Mulvany MJ. Small Artery Remodelling in Hypertension. Basic Clin Pharmacol Toxicol. 2012;110(1):49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- [13].Savvidou MD, Kaihura C, Anderson JM, Nicolaides KH. Maternal arterial stiffness in women who subsequently develop pre-eclampsia. PLoS One. 2011;6(5):e18703. doi: 10.1371/journal.pone.0018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaihura C, Savvidou MD, Anderson JM, McEniery CM, Nicolaides KH. Maternal arterial stiffness in pregnancies affected by preeclampsia. Am J Physiol Heart Circ Physiol. 2009;297(2):H759–H64. doi: 10.1152/ajpheart.01106.2008. [DOI] [PubMed] [Google Scholar]
- [15].Robb AO, Mills NL, Din JN, Smith IB, Paterson F, Newby DE, et al. Influence of the menstrual cycle, pregnancy, and preeclampsia on arterial stiffness. Hypertension. 2009;53(6):952–8. doi: 10.1161/HYPERTENSIONAHA.109.130898. [DOI] [PubMed] [Google Scholar]
- [16].van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, et al. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011;58(4):588–95. doi: 10.1161/HYPERTENSIONAHA.111.174557. [DOI] [PubMed] [Google Scholar]
- [17].Tousoulis D, Koutsogiannis M, Papageorgiou N, Siasos G, Antoniades C, Tsiamis E, et al. Endothelial dysfunction: potential clinical implications. Minerva Med. 2010;101(4):271–84. [PubMed] [Google Scholar]
- [18].Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294(2):H541–50. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- [19].Mori T, Shinohara K, Wakatsuki A, Watanabe K, Fujimaki A. Adipocytokines and endothelial function in preeclamptic women. Hypertens Res. 2010;33(3):250–4. doi: 10.1038/hr.2009.222. [DOI] [PubMed] [Google Scholar]
- [20].Yoshida A, Nakao S, Kobayashi M, Kobayashi H. Flow-Mediated Vasodilation and Plasma Fibronectin Levels in Preeclampsia. Hypertension. 2000;36(3):400–4. doi: 10.1161/01.hyp.36.3.400. [DOI] [PubMed] [Google Scholar]
- [21].Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–14. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- [22].Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2010;47(2):168–80. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Szarka A, Rigo J, Jr., Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioglu E. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med. 2010;23(8):880–6. doi: 10.3109/14767051003774942. [DOI] [PubMed] [Google Scholar]
- [25].Parra-Cordero M, Bosco C, González J, Gutiérrez R, Barja P, Rodrigo R. Immunohistochemical expression of von Willebrand factor in the preeclamptic placenta. J Mol Histol. 2011;42(5):459–65. doi: 10.1007/s10735-011-9351-5. [DOI] [PubMed] [Google Scholar]
- [26].Karthikeyan V, Blann A, Baghdadi S, Lane D, Gareth Beevers D, Lip G. Endothelial dysfunction in hypertension in pregnancy: associations between circulating endothelial cells, circulating progenitor cells and plasma von Willebrand factor. Clin Res Cardiology. 2011;100(6):531–7. doi: 10.1007/s00392-010-0277-9. [DOI] [PubMed] [Google Scholar]


