Abstract
Life expectancy has been increasing in the last few decades in the Western world and is accompanied by higher occurrence of age-related diseases like metabolic, cardiovascular, and renal diseases and also with a decline in immune functions. In HIV-infected people, due to the use of combination antiretroviral therapy (cART), life expectancy has increased. As a result, non-AIDS conditions which are age-associated have become more prevalent and appear earlier, resulting in accelerated aging in HIV patients. These non-AIDS conditions in HIV patients are associated with CD4+ T cell counts: lower counts are associated with higher rates of liver, cardiovascular, renal, and neurocognitive disorders. The effect of viral load and cART on the earlier occurrence of age-associated diseases is less significant than the CD4 count effect. Thus, the loss of immune functions in HIV-infected patients may enhance aging.
Keywords: Aging, AIDS, HIV, immune senescence
HIV/AIDS came to the world’s awareness over 30 years ago, with the first reports of young homosexual men, considered to be previously healthy, suffering from various types of opportunistic infections and profound cellular immunodeficiency.1 In the relatively short time since then, it has grown in scale to become a worldwide epidemic, with an estimated number of 34 million people living with HIV by 2011.2 The introduction of antiretroviral therapy (ART) in the middle of the 1990s has improved survival with HIV dramatically (Figure 1) and turned HIV infection, in effect, into a chronic condition. The percentage of people living with HIV/AIDS in USA who are over 50 years old is on the rise (Figure 2), and it is estimated that, by 2015, people over 50 will constitute the majority of all people living with HIV/AIDS in USA.5
Figure 1.
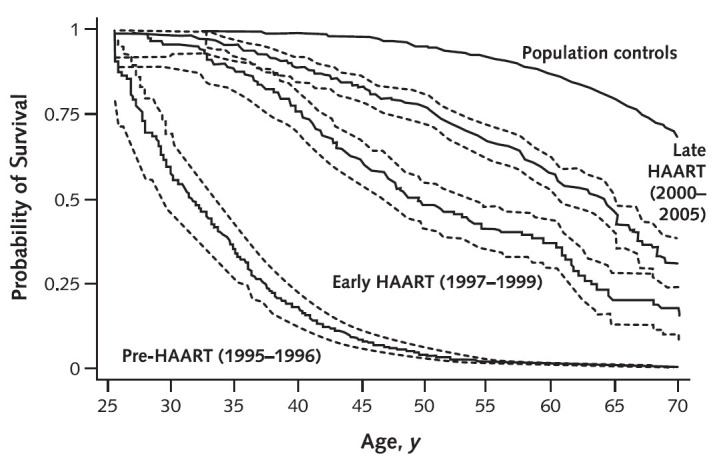
Estimated survival of 25-year-old HIV-infected and non-infected men in Denmark, 1995–2005.
Persons with HIV infection are divided into 3 calendar periods of observation. Dashed lines indicate 95% CIs. HIV = human immunodeficiency virus; HAART = highly active antiretroviral therapy. Reprinted from Lohse et al.3 with permission of the American College of Physicians.
Figure 2.
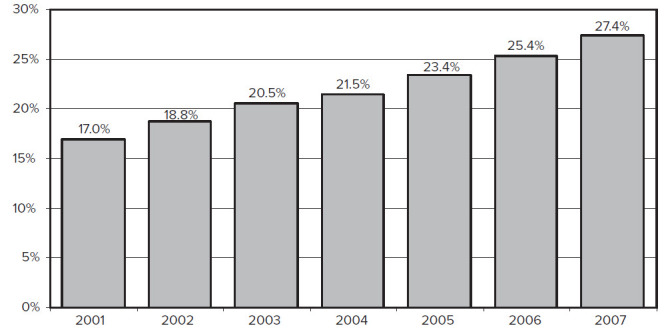
Estimated percentage of persons living with HIV/AIDS in USA who are older than 50, by year, 2001–2007.
Modified from Growing Older with the Epidemic: HIV and Aging,4 with permission.
As a result, we are encountering more chronic diseases typical of aging: cardiovascular disease, diabetes mellitus, dyslipidemia, osteoporosis and bone fractures, malignancies, and neurocognitive impairment.6 In addition, the accelerated aging of the immune system of HIV carriers has been demonstrated,7 and this is accompanied by the parallel process of increased incidence of chronic diseases typical of aging and early signs of physical and functional frailty in this population.8
Accelerated aging may be a result of several factors, including HIV infection itself, ART side-effects, and the aging of the immune system. It is now clear that the function of the immune system declines with age, but is the decline affecting the accelerated aging in HIV patients?
These evolving processes which interact with each other are becoming a major factor in treatment decisions of HIV carriers and shape research and clinical priorities, and they will be discussed further in this review.
IMMUNOSENESCENCE AND HIV INFECTION
Physiological aging of the immune system, termed immune senescence, is associated with a dysfunction in innate and adaptive immunity which diminishes the ability to respond to novel foreign antigens—vaccinations and infections. Similar changes in immune functions occur in people with chronic HIV infection but at a much younger age.
Changes seen in adaptive immune system manifest as lower naïve:memory CD4 ratio and enrichment of CD28−/CD57+/CD8+ effector T cells.9 The latter are senescent cells with shorter telomeres and limited proliferative capacity. In addition, there are putative qualitative and quantitative changes in T regulatory cells10 and a decrease in the diversity of naïve B cells and a qualitative B cell dysfunction.11
In HIV carriers, peripheral blood lymphocytes show a tendency towards T cell senescence with enrichment of CD28−/CD57+/CD8+ T cells and inverted ratio of naïve/memory T cells,9 as seen in normal aging. However, the immunophenotypic changes seen in HIV-infected patients, though similar to the changes seen in HIV-negative individuals, appear 20–30 years earlier.12 Considering the innate immune system, peripheral blood monocytes from young HIV-positive individuals exhibit changes in phenotype, function, and telomere length that closely resemble those observed in elderly controls aged approximately 30 years older. Furthermore, these immune defects are not fully restored by ART.13 Another example of the same accelerated aging process is the telomere length of CD4+ and CD8+ T cells in HIV carriers, which resembles the telomere length of HIV-negative patients 38 years older.14
Chronic activation of the immune system probably contributes to the accelerated aging in HIV-infected patients.15 The chronic inflammatory process caused by the persistent immune activation is associated with the increased release of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α as well as pro-coagulants such as cystatin-C and D-dimer.16 These plasma biomarkers of inflammation decline dramatically with combination antiretroviral therapy (cART) administration, but do not normalize entirely.17 Chronic inflammatory manifestations are also seen in physiological aging and have been implicated in the development of cardiovascular disease in aged people. Chronic inflammation may also serve as a proximate mediator to functional decline,18 and to frailty development in aging.19
NON-AIDS COMPLICATIONS IN AGING HIV-INFECTED PATIENTS
One of the important studies of the ART era was the Strategies for Management of Antiretroviral Therapy (SMART).20 It was designed to compare two treatment strategies: one which was viral suppressive and continuous regardless of CD4 count; the other with treatment interruptions according to CD4 levels. After a mean follow-up period of 16 months, the study review board recommended to stop enrollment to the trial because of a safety risk in the treatment interruption group. The statistical analysis showed that patients in the interruption group had an increased risk of mortality, both from opportunistic infections and from cardiovascular, renal, or hepatic disease. This study demonstrated the health effects of HIV beyond AIDS-defining illnesses.
METABOLIC CHANGES AND CARDIOVASCULAR DISEASE
The HIV-positive population experiences both external and internal metabolic changes. Abnormal fat distribution, also known as lipodystrophy, occurs in both treated21 and untreated22 HIV-positive patients. It includes two different syndromes: lipoatrophy, or subcutaneous fat loss of face, extremities, and buttocks; and lipohypertrophy, or central fat deposition, manifested as intra-abdominal (visceral) fat, buffalo hump, or breast enlargement. The risk factors for the two abnormal fat distribution syndromes are different. According to the Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study, lipoatrophy can be found in almost 40% of HIV-positive men23 and 30% of HIV-positive women.24 Patients at higher risk to develop lipoatrophy are the ones with lower BMI (body mass index), higher nadir HIV load, and use of ART, especially stavudine, zidovudine, and earlier protease inhibitors (PIs).25 Lipohypertrophy is more common in HIV-positive women than HIV-positive men and in individuals with greater body fat levels to begin with.26
HIV-positive patients with abnormal fat distribution have significantly increased prevalence of dyslipidemia and impaired glucose homeostasis in comparison with HIV-negative controls matched for age and BMI.27 The dyslipidemia associated with HIV infection itself includes elevated triglyceride levels and decreased high-density lipoprotein cholesterol (HDL-C) levels. ART is also a major contributor to dyslipidemia, mainly a more profound elevation of triglycerides with ritonavir-based PI regimens.28 Likewise, both decreased subcutaneous leg fat and increased visceral fat are strongly associated with decreased insulin sensitivity in this population.29 In addition, ART may have an effect on insulin sensitivity, mainly the PIs. One of the mechanisms by which PIs induce insulin resistance is through blocking the transport of glucose by the insulin-sensitive glucose transporter GLUT4.30 A prospective 10-year follow-up of 1,046 ART-treated HIV-positive patients demonstrated an increased incidence of diabetes mellitus in comparison to the general population, and the risk factors were older age, adiposity, and short exposure to the PI indinavir and the nucleoside reverse transcriptase inhibitors (NRTIs) stavudine and didanosine,31 which are mostly not used today in the developed world.
The combination of metabolic and immunologic changes are the base of cardiovascular disease (CVD) in HIV-positive patients.32 In addition to the established risk factors for coronary heart disease (CHD) in the general population, which have been shown to be increased in the HIV-positive population,33 there is additional risk that might be explained in part by both antiretroviral medications and novel CHD risk factors including inflammation and immune dysfunction. The effect of ART was assessed in the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study, which demonstrated an association between duration of exposure to combination ART and the risk of myocardial infarction, specifically with exposure to PIs.34 In contrast, a large study from the Veteran Affairs (VA) system showed no connection between any ART class and CHD or cerebrovascular event outcomes.
Several surrogate indices of CVD have been tested in HIV-positive patients. A recent study demonstrated an association between immune activation markers and carotid artery plaque in patients virologically suppressed on ART, and another study demonstrated elevated carotid intima-media thickness in all HIV groups versus controls, including elite-controllers (HIV-infected patients who maintain an undetectable HIV RNA by standard assay in the absence of ART).35 The same trend was demonstrated with increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men in comparison with controls.36
The actual increased risk for CHD and acute myocardial infarction in HIV-positive patients was shown in several studies, which found significantly increased risk ratios up to 1.94 (95% CI 1.58–2.37).37
Renal Complications
The pathogenesis of renal disease in HIV-positive individuals is diverse. It includes: 1) HIV-associated nephropathy (HIVAN), a form of focal segmental glomerulosclerosis that is accompanied by tubuleinterstitial inflammation, and clinically manifests as rapidly progressive renal failure with nephritic range proteinuria. 2) HIV immune complex kidney disease (HIVICK), a collective term that includes IgA nephropathy, membranoproliferative glomerulonephritis, membranous nephropathy, and a lupus-like glomerulonephritis that is serologically negative.38 3) Hypertensive and atherosclerotic renal disease. 4) ART side-effects, mainly tenofovir-induced renal tubular injury39 and indinavir/atazanavir-induced crystaluria and renal calculi formation.40 The first two pathologies are more common in untreated patients, the last two in treated. It has been shown that chronic kidney disease and proteinuria are associated with increased risk of mortality in HIV-positive patients.41
Bone Mineral Density and Osteoporosis
Several population-based studies in the United States showed increased prevalence of osteoporotic fractures in HIV-infected men and women compared with HIV-uninfected individuals.42 The etiology of low bone mineral density (BMD) in HIV-positive patients is multifactorial. It includes both traditional, non-HIV-related risk factors such as smoking, alcohol and opiate use, low body weight, and vitamin D deficiency; and also HIV-related factors such as direct viral and inflammatory effects on bone resorption43,44 and the effects of ART, especially tenofovir.45 Multiple studies have shown a 2%–6% BMD loss after 48–96 weeks of therapy, regardless of the type of ART initiated.46 Several longitudinal studies have shown that, with continued ART use, BMD stabilizes over time.47,48
Neurocognitive Changes
HIV-associated neurocognitive disorder (HAND) is divided into three levels of impairment: asymptomatic neurocognitive impairment, mild neurocognitive disorders, and HIV-associated dementia (HAD). The introduction of ART has reduced significantly the rate of HAD, but unfortunately the effect on less severe forms of impairment is not as impressive. Studies of HAND in treated patients have documented high persisting rates of mild-to-moderate neurocognitive impairment despite effective suppressing antiretroviral treatment,49 especially in individuals with a history of low nadir CD4s.50
Frailty Syndrome in HIV-positive Older Adults
Frailty is defined as a syndrome of decreased physiological reserve, which increases vulnerability to negative outcomes such as loss of independence, nursing home admission, morbidity, and mortality.51 Recent studies demonstrated that HIV-positive individuals are at an increased risk of frailty and that some individuals with HIV manifest frailty characteristics at a much younger age than frail individuals without HIV.6 In the pre-ART era frailty in HIV was connected to the AIDS-wasting syndrome, with advanced immunosuppression and very high viral loads. In contrast, the current risk factors for frailty in the HIV-positive population is high fat mass, particularly trunkal fat, and high BMI.52
CONCLUSION
Accelerated aging of the immune system together with earlier appearance of aging co-morbidities (Figure) in HIV patients point to a potential major contribution of immune system dysfunction to the accelerated aging in HIV-infected patients. This may once again highlight the role of normal immune function as a critical factor in the fight against HIV which, if successful, may both suppress HIV and also attenuate the process of accelerated aging. Successful cART is critical to the recovery of the immune system in HIV-infected individuals. Early initiation of antiretroviral therapy once HIV diagnosis has been established, which will probably keep the normal function of the immune system, may help in alleviating at least some of the morbid conditions related to accelerated aging. We will be able to verify this hypothesis once the results of the on-going international large study, testing the right time to start cART (START study), come out.54
Figure 3.
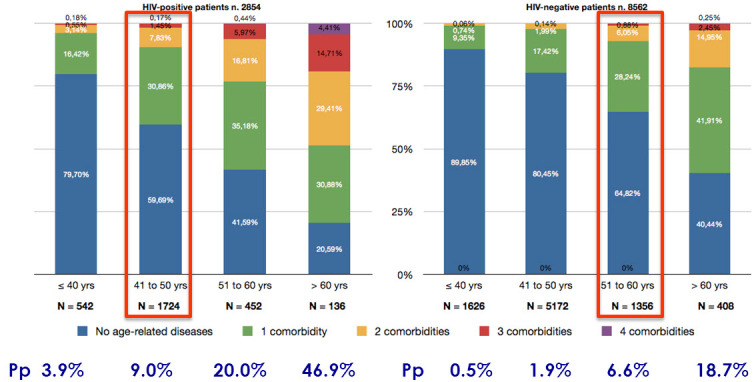
Poly-patology (Pp) prevalence of age-related non-AIDS conditions in HIV-positive versus HIV-negative populations, 2002–2008.
Modified from Guaraldi G et al.,53 with permission.
Abbreviations:
- ART
antiretroviral therapy;
- BMD
bone mineral density;
- BMI
body mass index;
- cART
combination antiretroviral treatment;
- FRAM
Fat Redistribution and Metabolic Change in HIV Infection;
- HAD
HIV-associated dementia;
- HAND
HIV-associated neurocognitive disorder;
- HIVAN
HIV-associated nephropathy;
- HIVICK
HIV immune complex kidney disease;
- NRTIs
nucleoside reverse transcriptase inhibitors;
- SMART
Strategies for Management of Antiretroviral Therapy.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Mildvan D, Mathur U, Enlow RW, et al. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982;96(6 Pt 1):700–4. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- 2. UNAIDS Data Tables | 2011. Published by the Joint United Nations Programme on HIV/AIDS (UNAIDS). Available at: http://tinyurl.com/8qjnw63. Accessed October 2012.
- 3.Lohse N, Eg Hansen A-B, Pedersen G, et al. Survival of persons with and without HIF infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 4.Growing Older with the Epidemic . HIV and Aging. New York, NY: Gay Men’s Health Crisis, Inc; 2010. [Google Scholar]
- 5.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: emerging challenges of growing older in the era of successful antiretroviral therapy. J NeuroVirology. 2012;18:247–55. doi: 10.1007/s13365-011-0073-y. [DOI] [PubMed] [Google Scholar]
- 8.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–86. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 9.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187:1924–33. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 10.Tsaknaridis L, Spencer L, Culbertson N, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- 11.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012 May 3; doi: 10.1016/j.smim.2012.04.004. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Rickabaugh TM, Kilpatrick RD, Hultin LE, et al. The dual impact of HIV-1 infection and aging on naïve CD4 T-cells: additive and distinct patterns of impairment. PLoS One. 2011;6:e16459. doi: 10.1371/journal.pone.0016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hearpsa AC, Maisaa A, Chenga W-J, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–53. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 14.Bestilny LJ, Gill MJ, Mody CH, Riabowol KT. Accelerated replicative senescence of the peripheral immune system induced by HIV infection. AIDS. 2000;14:771–80. doi: 10.1097/00002030-200005050-00002. [DOI] [PubMed] [Google Scholar]
- 15.Van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corsonello A, Garasto S, Abbatecola AM, et al. Targeting inflammation to slow or delay functional decline: where are we? Biogerontology. 2010;11:603–14. doi: 10.1007/s10522-010-9289-0. [DOI] [PubMed] [Google Scholar]
- 19.Bortz WM. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–8. doi: 10.1093/gerona/57.5.M283. [DOI] [PubMed] [Google Scholar]
- 20.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 21.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Madge S, Kinloch-de-Loes S, Mercey D, Johnson MA, Weller IV. Lipodystrophy in patients naive to HIV protease inhibitors. AIDS. 1999;13:735–7. doi: 10.1097/00002030-199904160-00020. [DOI] [PubMed] [Google Scholar]
- 23.Bacchetti P, Gripshover B, Grunfeld C, et al. Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–71. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlay JC, Sharma S, Peng G, Gibert CL, Grunfeld C. The effect of individual antiretroviral drugs on body composition in HIV infected persons initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;51:298–304. doi: 10.1097/QAI.0b013e3181aa1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005;40:1837–45. doi: 10.1086/430379. [DOI] [PubMed] [Google Scholar]
- 27.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–9. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 28.Pao V, Lee GA, Grunfeld C. HIV therapy, metabolic syndrome, and cardiovascular risk. Curr Atheroscler Rep. 2008;10:61–70. doi: 10.1007/s11883-008-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunfeld C, Rimland D, Gibert CL, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–90. doi: 10.1097/QAI.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–4. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 31.Capeau J, Bouteloup V, Katlama C, et al. ANRS CO8 APROCO-COPILOTE Cohort Study Group Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26:303–14. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 32.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205:S375–82. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DAD Study Group. Friis-Møller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 35.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freiberg M, McGinnis K, Butt A, et al. HIV is associated with clinically confirmed myocardial infarction after adjustment for smoking and other risk factors. CROI. 2011 Poster No. 809. [Google Scholar]
- 38.Bruggeman LA, Bark C, Kalayjian RC. HIV and the kidney. Curr Infect Dis Rep. 2009;11:479–85. doi: 10.1007/s11908-009-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. 2009;23:689–96. doi: 10.1097/QAD.0b013e3283262a64. [DOI] [PubMed] [Google Scholar]
- 40.Mocroft A, Kirk O, Reiss P, et al. for the EuroSIDA Study Group Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–78. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 41.Choi A, Scherzer R, Bacchetti P, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV infected persons. Am J Kidney Dis. 2010;56:872–82. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)–infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278:48251–8. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 44.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 46.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–61. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 47.Bolland MJ, Grey AB, Horne AM, et al. Bone mineral density remains stable in HAART-treated HIV-infected men over 2 years. Clin Endocrinol (Oxf) 2007;67:270–5. doi: 10.1111/j.1365-2265.2007.02875.x. [DOI] [PubMed] [Google Scholar]
- 48.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91:2938–45. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 50.Heaton RK, Franklin DR, Ellis RJ, et al. for the CHARTER and HNRC Groups HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med. 2007;120:748–53. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc. 2012;60:545–9. doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guaraldi G, Roverato A, Orlando G, et al. Prevalence of poly-pathology is more common in HIV infected patients than in HIV negative controls in any age strata. CROI. 2010 Poster 727 (O-209). [Google Scholar]
- 54.Babiker AG, Emery S, Fätkenheuer G, et al. for the INSIGHT START Study Group Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2012 Apr 30; doi: 10.1177/1740774512440342. [Epub ahead of print]. http://dx.doi.org/10.1177%2F1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]


